Generation of RAG2 Knockout Immune-Deficient Miniature Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Animals and Cell Lines
2.2. Somatic Cell Nuclear Transfer and Embryo Transfer
2.3. Genotyping of RAG2 KO Piglets
2.4. Routine Blood Tests
2.5. Morphological Analysis of Immune Organs
2.6. Immune Cell Flow Cytometry
2.7. Statistical Analysis
3. Results
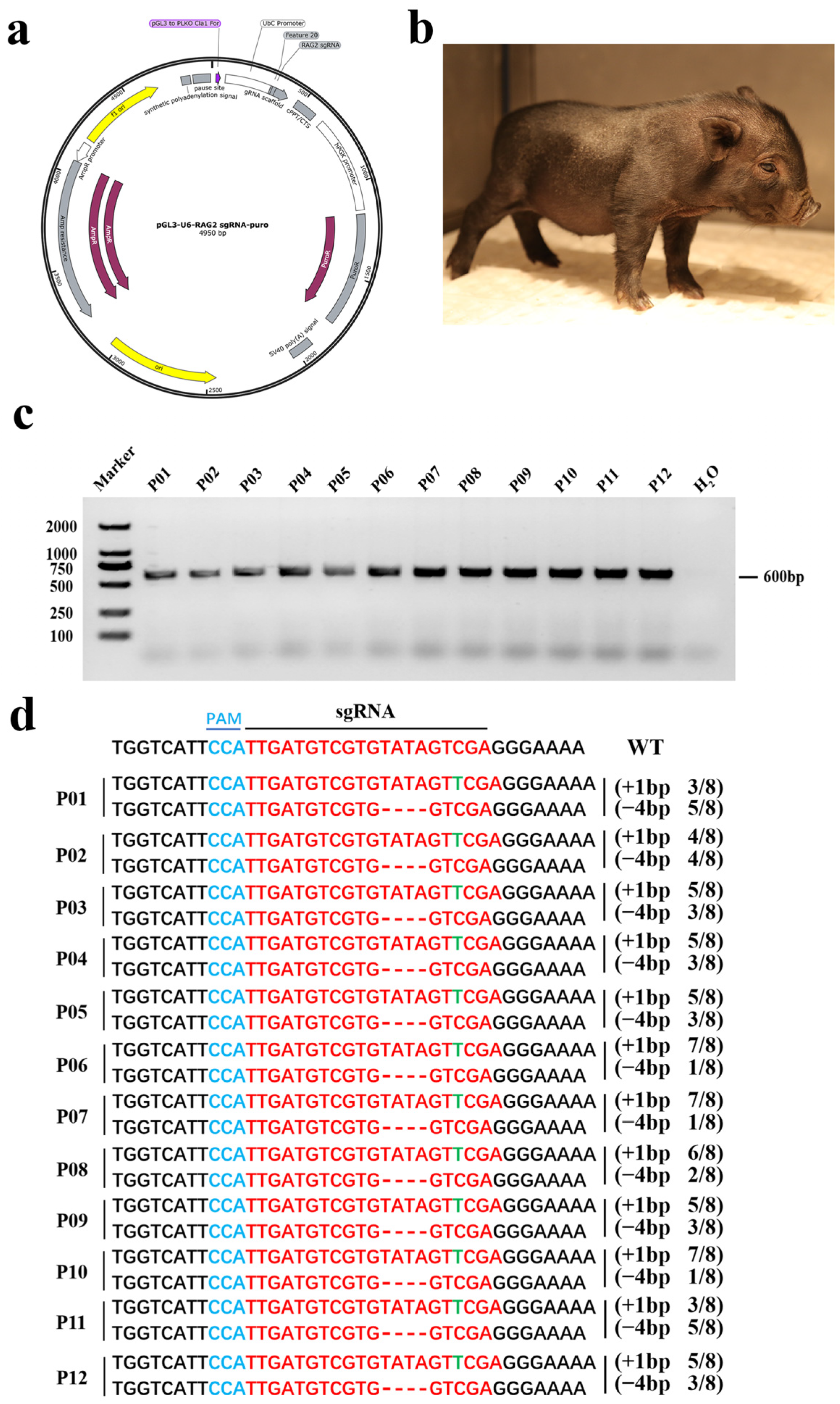
3.1. Generation of RAG2 KO Pigs
3.2. Genotyping of RAG2 KO Piglets
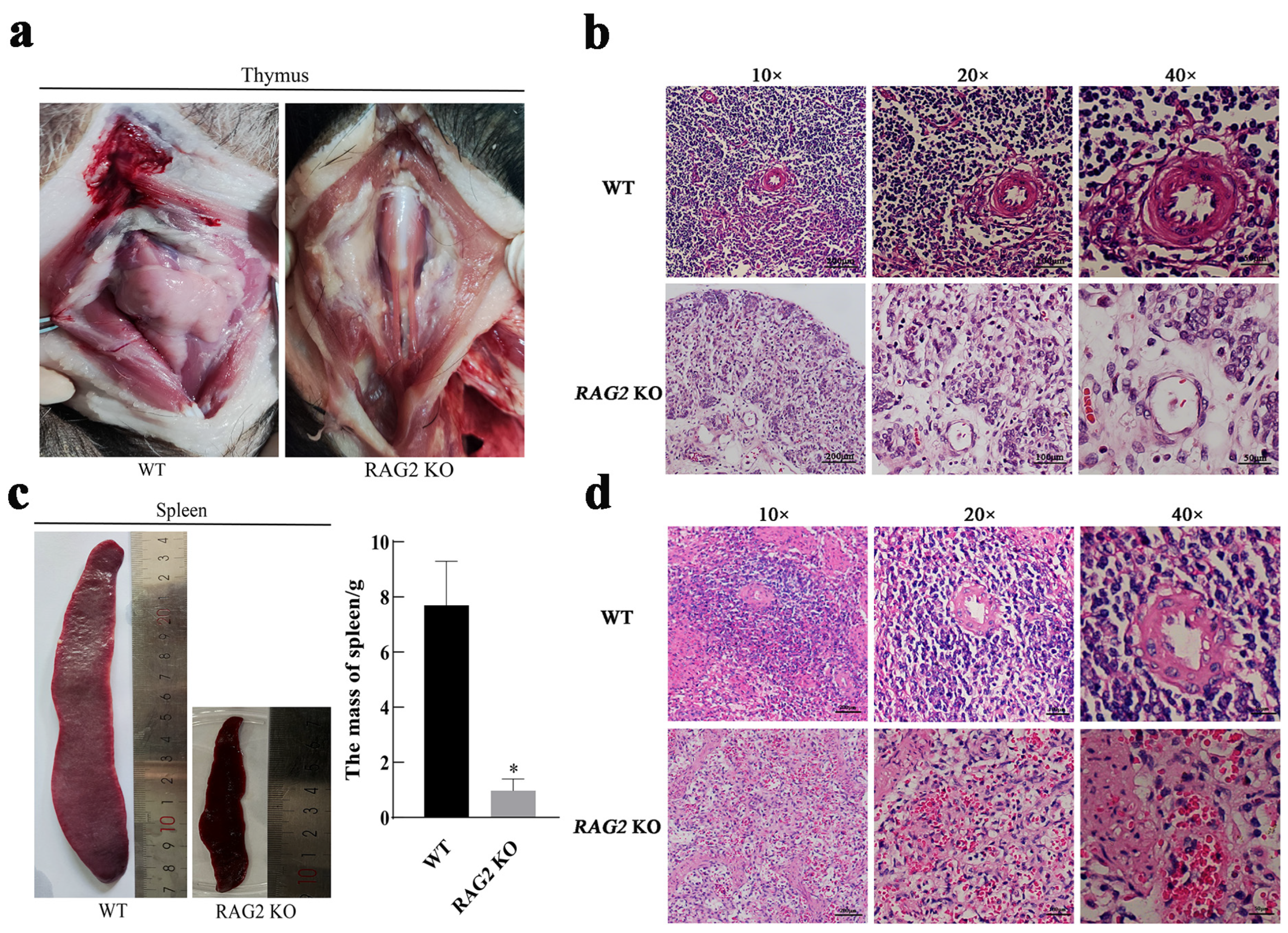
3.3. RAG2 KO Affected the Development of the Thymus and the Spleen
3.4. Effects of RAG2 Gene Knockout on the Development of Immune Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cossu, F. Genetics of SCID. Ital. J. Pediatr. 2010, 36, 76. [Google Scholar] [CrossRef]
- Ochs, H.D.; Hagin, D. Primary immunodeficiency disorders: General classification, new molecular insights, and practical approach to diagnosis and treatment. Ann. Allergy Asthma Immunol. 2014, 112, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Dagley, J.L.; DiCosty, U.; Fricks, C.; Mansour, A.; McCall, S.; McCall, J.W.; Taylor, M.J.; Turner, J.D. Current status of immunodeficient mouse models as substitutes to reduce cat and dog use in heartworm preclinical research. F1000Research 2024, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Fugmann, S.D.; Lee, A.I.; Shockett, P.E.; Villey, I.J.; Schatz, D.G. The RAG proteins and V(D)J recombination: Complexes, ends, and transposition. Annu. Rev. Immunol. 2000, 18, 495–527. [Google Scholar] [CrossRef]
- Agrawal, A.; Schatz, D.G. RAG1 and RAG2 form a stable postcleavage synaptic complex with DNA containing signal ends in V(D)J recombination. Cell 1997, 89, 43–53. [Google Scholar] [CrossRef]
- Ru, H.; Chambers, M.G.; Fu, T.M.; Tong, A.B.; Liao, M.; Wu, H. Molecular Mechanism of V(D)J Recombination from Synaptic RAG1-RAG2 Complex Structures. Cell 2015, 163, 1138–1152. [Google Scholar] [CrossRef]
- Mombaerts, P.; Iacomini, J.; Johnson, R.S.; Herrup, K.; Tonegawa, S.; Papaioannou, V.E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 1992, 68, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, Y.; Rathbun, G.; Lam, K.P.; Oltz, E.M.; Stewart, V.; Mendelsohn, M.; Charron, J.; Datta, M.; Young, F.; Stall, A.M.; et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 1992, 68, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Martignoni, M.; Groothuis, G.M.; de Kanter, R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert. Opin. Drug Metab. Toxicol. 2006, 2, 875–894. [Google Scholar] [CrossRef]
- Anderson, S.; Luffer-Atlas, D.; Knadler, M.P. Predicting circulating human metabolites: How good are we? Chem. Res. Toxicol. 2009, 22, 243–256. [Google Scholar] [CrossRef]
- Hutchinson, L.; Kirk, R. High drug attrition rates—Where are we going wrong? Nat. Rev. Clin. Oncol. 2011, 8, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar]
- Day, C.P.; Merlino, G.; Van Dyke, T. Preclinical mouse cancer models: A maze of opportunities and challenges. Cell 2015, 163, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.A.; Tuggle, C.K. Livestock models in translational medicine. ILAR J. 2015, 56, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, N.H.; Fan, T.M.; Schachtschneider, K.M.; Principe, D.R.; Schook, L.B.; Jungersen, G. Of Mice, Dogs, Pigs, and Men: Choosing the Appropriate Model for Immuno-Oncology Research. ILAR J. 2018, 59, 247–262. [Google Scholar] [CrossRef]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef]
- Dawson, H.D.; Loveland, J.E.; Pascal, G.; Gilbert, J.G.; Uenishi, H.; Mann, K.M.; Sang, Y.; Zhang, J.; Carvalho-Silva, D.; Hunt, T.; et al. Structural and functional annotation of the porcine immunome. BMC Genom. 2013, 14, 332. [Google Scholar] [CrossRef]
- Schachtschneider, K.M.; Madsen, O.; Park, C.; Rund, L.A.; Groenen, M.A.; Schook, L.B. Adult porcine genome-wide DNA methylation patterns support pigs as a biomedical model. BMC Genom. 2015, 16, 743. [Google Scholar] [CrossRef]
- Suzuki, S.; Iwamoto, M.; Saito, Y.; Fuchimoto, D.; Sembon, S.; Suzuki, M.; Mikawa, S.; Hashimoto, M.; Aoki, Y.; Najima, Y.; et al. Il2rg gene-targeted severe combined immunodeficiency pigs. Cell Stem Cell 2012, 10, 753–758. [Google Scholar] [CrossRef]
- Suzuki, S.; Iwamoto, M.; Hashimoto, M.; Suzuki, M.; Nakai, M.; Fuchimoto, D.; Sembon, S.; Eguchi-Ogawa, T.; Uenishi, H.; Onishi, A. Generation and characterization of RAG2 knockout pigs as animal model for severe combined immunodeficiency. Vet. Immunol. Immunopathol. 2016, 178, 37–49. [Google Scholar] [CrossRef]
- Lei, S.; Ryu, J.; Wen, K.; Twitchell, E.; Bui, T.; Ramesh, A.; Weiss, M.; Li, G.; Samuel, H.; Clark-Deener, S.; et al. Increased and prolonged human norovirus infection in RAG2/IL2RG deficient gnotobiotic pigs with severe combined immunodeficiency. Sci. Rep. 2016, 6, 25222. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, A.N.; Li, Y.; Ahrens, A.P.; Kiupel, M.; Byrne, K.A.; Loving, C.L.; Cino-Ozuna, A.G.; Wiarda, J.E.; Adur, M.; Schultz, B.; et al. Novel Engraftment and T Cell Differentiation of Human Hematopoietic Cells in ART(−/−)IL2RG(−/Y) SCID Pigs. Front. Immunol. 2020, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yu, D.; Fu, R.; An, P.; Sun, R.; Wang, Z.; Guo, R.; Li, H.; Zhang, Y.; Li, Z.; et al. IL2RG-deficient minipigs generated via CRISPR/Cas9 technology support the growth of human melanoma-derived tumours. Cell Prolif. 2020, 53, e12863. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, A.N.; Kiupel, M.; Adur, M.K.; Cocco, E.; Santin, A.D.; Bellone, S.; Charley, S.E.; Blanco-Fernandez, B.; Risinger, J.I.; Ross, J.W.; et al. Human Ovarian Cancer Tumor Formation in Severe Combined Immunodeficient (SCID) Pigs. Front. Oncol. 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, E.; Reza, A.; Hong, K.; Song, H.; Park, C.; Cho, S.K.; Lee, K.; Prather, R.S.; Kim, J.H. Recombination activating gene-2(null) severe combined immunodeficient pigs and mice engraft human induced pluripotent stem cells differently. Oncotarget 2017, 8, 69398–69407. [Google Scholar] [CrossRef][Green Version]
- Kim, J.T.; Bresson-Tan, G.; Zack, J.A. Current Advances in Humanized Mouse Models for Studying NK Cells and HIV Infection. Microorganisms 2023, 11, 1984. [Google Scholar] [CrossRef]
- Ito, R.; Takahashi, T.; Katano, I.; Ito, M. Current advances in humanized mouse models. Cell Mol. Immunol. 2012, 9, 208–214. [Google Scholar] [CrossRef]
- Zhao, H.; Ye, W.; Guo, J.; Wang, J.; Jiao, D.; Xu, K.; Yang, C.; Chen, S.; Jamal, M.A.; Bai, Z.; et al. Development of RAG2 (−/−) IL2Rγ (−/Y) immune deficient FAH-knockout miniature pig. Front. Immunol. 2022, 13, 950194. [Google Scholar] [CrossRef]
- Wei, H.; Qing, Y.; Pan, W.; Zhao, H.; Li, H.; Cheng, W.; Zhao, L.; Xu, C.; Li, H.; Li, S.; et al. Comparison of the efficiency of Banna miniature inbred pig somatic cell nuclear transfer among different donor cells. PLoS ONE 2013, 8, e57728. [Google Scholar] [CrossRef]
- Watanabe, M.; Nakano, K.; Matsunari, H.; Matsuda, T.; Maehara, M.; Kanai, T.; Kobayashi, M.; Matsumura, Y.; Sakai, R.; Kuramoto, M.; et al. Generation of interleukin-2 receptor gamma gene knockout pigs from somatic cells genetically modified by zinc finger nuclease-encoding mRNA. PLoS ONE 2013, 8, e76478. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kwon, D.N.; Ezashi, T.; Choi, Y.J.; Park, C.; Ericsson, A.C.; Brown, A.N.; Samuel, M.S.; Park, K.W.; Walters, E.M.; et al. Engraftment of human iPS cells and allogeneic porcine cells into pigs with inactivated RAG2 and accompanying severe combined immunodeficiency. Proc. Natl. Acad. Sci. USA 2014, 111, 7260–7265. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Fan, N.; Song, J.; Zhao, B.; Ouyang, Z.; Liu, Z.; Zhao, Y.; Yan, Q.; Yi, X.; et al. RAG1/2 knockout pigs with severe combined immunodeficiency. J. Immunol. 2014, 193, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Rodewald, H.R. Thymus organogenesis. Annu. Rev. Immunol. 2008, 26, 355–388. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Sun, K.; Luo, Z.; Zhang, J.; Zhou, H.; Yin, H.; Liang, Z.; You, J. Spleen-targeted delivery systems and strategies for spleen-related diseases. J. Control. Release 2024, 370, 773–797. [Google Scholar] [CrossRef]
- Aranda, C.S.; Gouveia-Pereira, M.P.; da Silva, C.J.M.; Rizzo, M.; Ishizuka, E.; de Oliveira, E.B.; Condino-Neto, A. Severe combined immunodeficiency diagnosis and genetic defects. Immunol. Rev. 2024, 322, 138–147. [Google Scholar] [CrossRef]
- Liu, Q.; Fan, C.; Zhou, S.; Guo, Y.; Zuo, Q.; Ma, J.; Liu, S.; Wu, X.; Peng, Z.; Fan, T.; et al. Bioluminescent imaging of vaccinia virus infection in immunocompetent and immunodeficient rats as a model for human smallpox. Sci. Rep. 2015, 5, 11397. [Google Scholar] [CrossRef]
- Noto, F.K.; Adjan-Steffey, V.; Tong, M.; Ravichandran, K.; Zhang, W.; Arey, A.; McClain, C.B.; Ostertag, E.; Mazhar, S.; Sangodkar, J.; et al. Sprague Dawley Rag2-Null Rats Created from Engineered Spermatogonial Stem Cells Are Immunodeficient and Permissive to Human Xenografts. Mol. Cancer Ther. 2018, 17, 2481–2489. [Google Scholar] [CrossRef]
- Fugmann, S.D. RAG1 and RAG2 in V(D)J recombination and transposition. Immunol. Res. 2001, 23, 23–39. [Google Scholar] [CrossRef]
- Roth, K.; Oehme, L.; Zehentmeier, S.; Zhang, Y.; Niesner, R.; Hauser, A.E. Tracking plasma cell differentiation and survival. Cytom. A 2014, 85, 15–24. [Google Scholar] [CrossRef]

| Recipients | No. of Transferred Embryos | Pregnancy (%) | Duration of Pregnancy (d) | No. of Offspring (Piglets) |
|---|---|---|---|---|
| 1 | 660 | + | 116 | 6 |
| 2 | 660 | - | - | - |
| 3 | 680 | + | 116 | 6 |
| 4 | 680 | - | - | - |
| Total | 2680 | 2 (50%) | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhu, F.; Jiao, D.; Yang, C.; Wang, J.; Wang, F.; Zhao, H.; Wei, H.-J.; Zhao, H.-Y. Generation of RAG2 Knockout Immune-Deficient Miniature Pigs. Animals 2024, 14, 2597. https://doi.org/10.3390/ani14172597
Wang J, Zhu F, Jiao D, Yang C, Wang J, Wang F, Zhao H, Wei H-J, Zhao H-Y. Generation of RAG2 Knockout Immune-Deficient Miniature Pigs. Animals. 2024; 14(17):2597. https://doi.org/10.3390/ani14172597
Chicago/Turabian StyleWang, Jing, Feiyan Zhu, Deling Jiao, Chang Yang, Junqi Wang, Fengchong Wang, Heng Zhao, Hong-Jiang Wei, and Hong-Ye Zhao. 2024. "Generation of RAG2 Knockout Immune-Deficient Miniature Pigs" Animals 14, no. 17: 2597. https://doi.org/10.3390/ani14172597
APA StyleWang, J., Zhu, F., Jiao, D., Yang, C., Wang, J., Wang, F., Zhao, H., Wei, H.-J., & Zhao, H.-Y. (2024). Generation of RAG2 Knockout Immune-Deficient Miniature Pigs. Animals, 14(17), 2597. https://doi.org/10.3390/ani14172597






