Genome-Based Analysis of Genetic Diversity, Antimicrobial Susceptibility, and Virulence Gene Distribution in Salmonella Pullorum Isolates from Poultry in China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Antigenic Type Analysis of S. Pullorum Isolates
2.2. Antimicrobial Susceptibility Test
2.3. DNA Extraction and WGS
2.4. Bioinformatic Analysis
2.5. Correlation Analysis of Susceptibility Phenotypes and Genotypes
2.6. Identification of Gtr Operons within S. Pullorum Genomic Sequences
3. Results
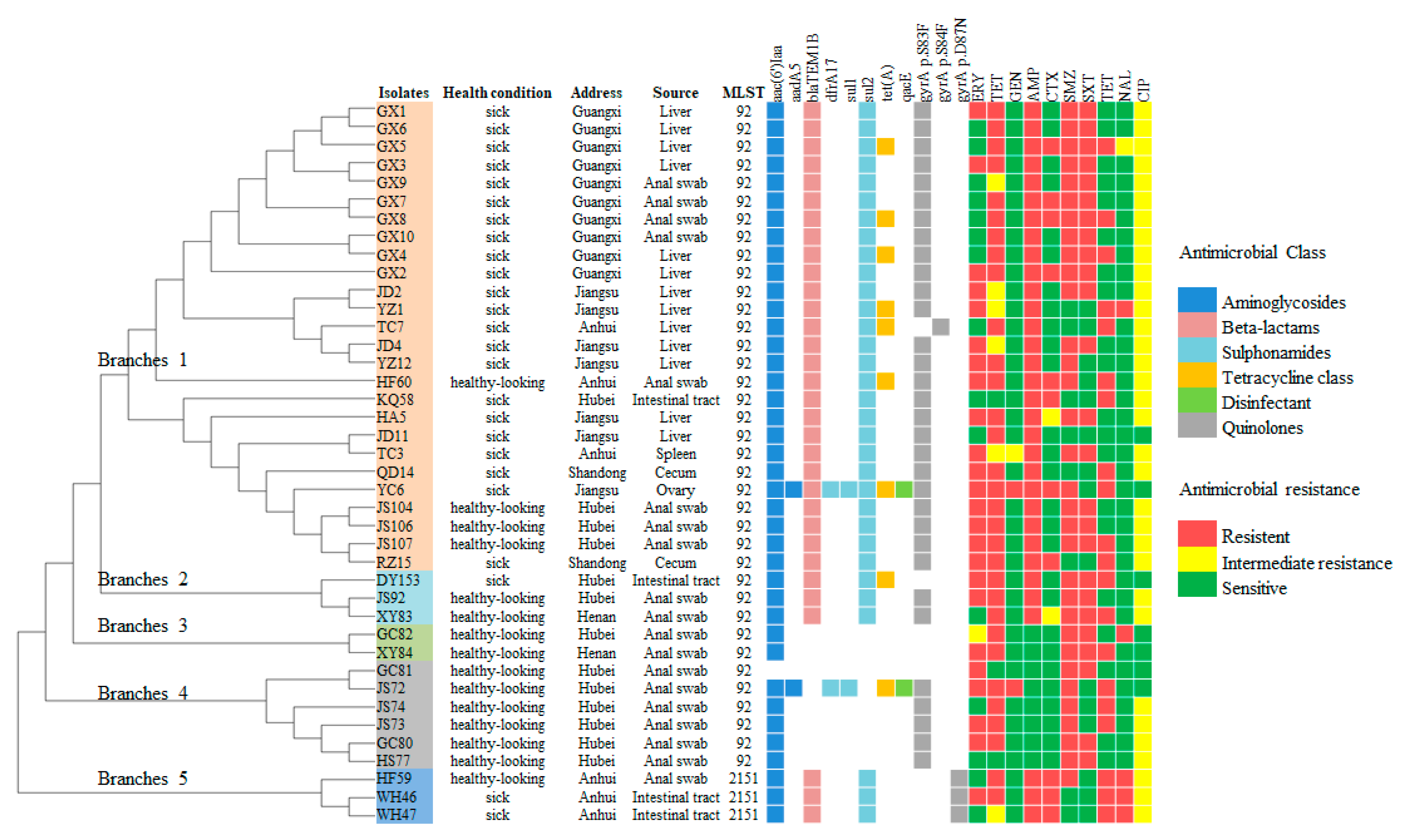
3.1. Genetic Diversity and Antigenic Type Analysis
3.2. Antimicrobial Susceptibility Analysis
3.3. Antibiotic-Resistance Genes and Resistance Mutations
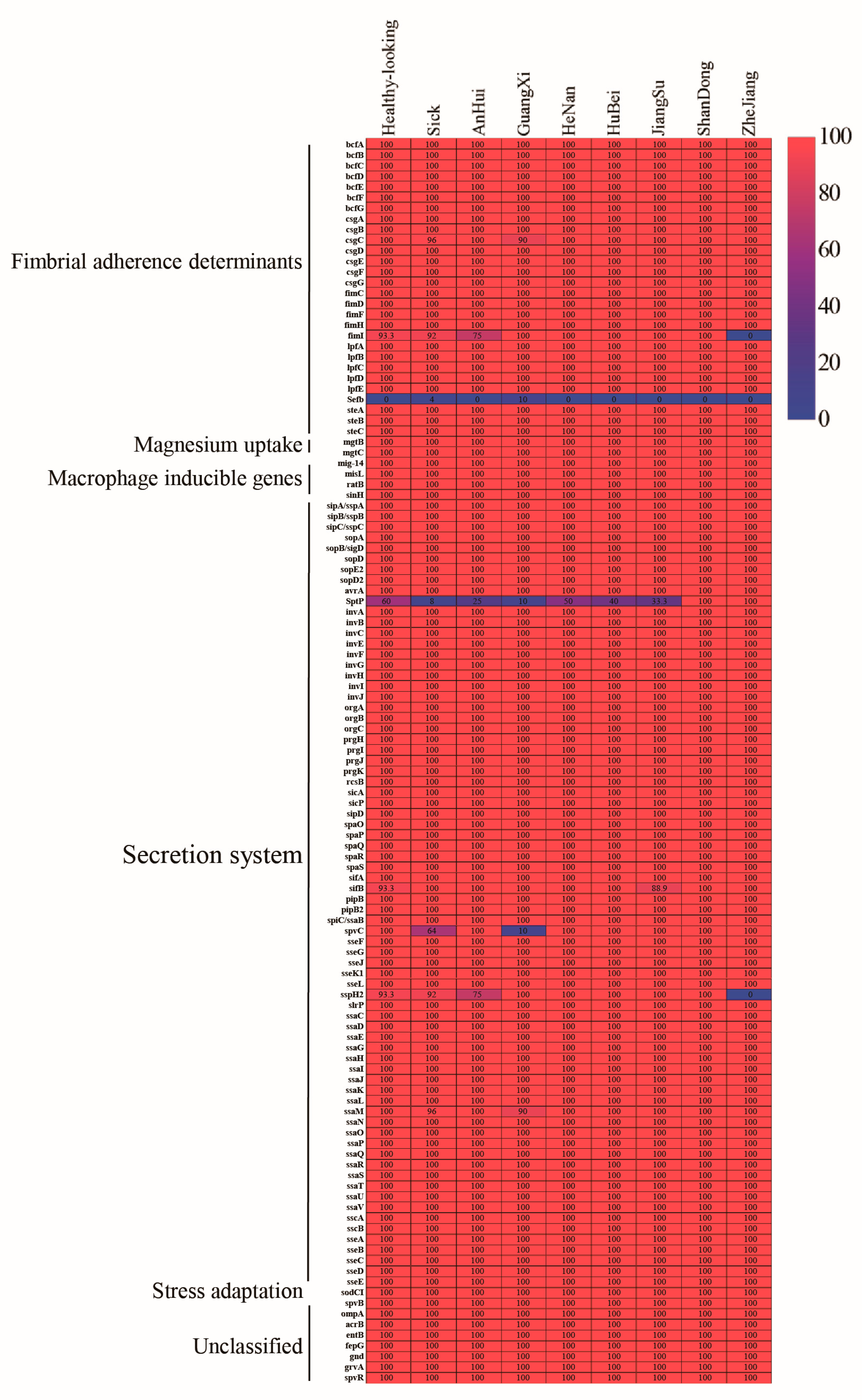
3.4. Detection of Virulence Genes
3.5. Gtr Operon Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrow, P.A.; Neto, O.C.F. Pullorum disease and fowl typhoid—New thoughts on old diseases: A review. Avian Pathol. 2011, 40, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shivaprasad, H.L. Fowl typhoid and pullorum disease. Rev. Sci. Et Tech. (Int. Off. Epizoot.) 2000, 19, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Rettger, L.F.; Harvey, S.C. Fatal Septicemia in Young Chickens, or “White Diarrhea”. J. Med. Res. 1908, 18, 277–290. [Google Scholar] [PubMed]
- Schat, K.A.; Nagaraja, K.V.; Saif, Y.M. Pullorum Disease: Evolution of the Eradication Strategy. Avian Dis. 2021, 65, 227–236. [Google Scholar] [CrossRef]
- Guo, X.; Wang, H.; Cheng, Y.; Zhang, W.; Luo, Q.; Wen, G.; Wang, G.; Shao, H.; Zhang, T. Quinolone resistance phenotype and genetic characterization of Salmonella enterica serovar Pullorum isolates in China, during 2011 to 2016. BMC Microbiol. 2018, 18, 1–7. [Google Scholar] [CrossRef]
- Zhang, D.; Zhuang, L.; Wang, C.; Zhang, P.; Zhang, T.; Shao, H.; Han, X.; Gong, J. Virulence Gene Distribution of Salmonella Pullorum Isolates Recovered from Chickens in China (1953–2015). Avian Dis. 2018, 62, 431–437. [Google Scholar] [CrossRef]
- Christensen, J.P.; Olsen, J.E.; Bisgaard, M. Ribotypes of Salmonella enterica serovar Gallinarum biovars gallinarum and pullorum. AvianPathol. J. WVPA 1993, 22, 725–738. [Google Scholar] [CrossRef]
- Snoeyenbos, G.H.; Crotty, A.M.; Van Roekel, H. Some antigenic characteristics of Salmonella pullorum. Am. J. Vet. Res. 1950, 11, 221–225. [Google Scholar]
- Williams, J.E.; Macüonald, A.D. The Past, Present, and Future of Salmonella Antigens for Poultry. 1956, pp. 333–339. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19562203384 (accessed on 22 August 2024).
- Wilson, M.A.; Nordholm, G.E. DNA fingerprint analysis of standard, intermediate, and variant antigenic types of Salmonella enterica subspecies enterica serovar Gallinarum biovar pullorum. Avian Dis. 1995, 39, 594–598. [Google Scholar] [CrossRef]
- Song, Y.; Wang, F.; Liu, Y.; Song, Y.; Zhang, L.; Zhang, F.; Gu, X.; Sun, S. Occurrence and Characterization of Salmonella Isolated From Chicken Breeder Flocks in Nine Chinese Provinces. Front. Vet. Sci. 2020, 7, 479. [Google Scholar] [CrossRef]
- Schwarz, S.; Chaslus-Dancla, E. Use of antimicrobials in veterinary medicine and mechanisms of resistance. Vet. Res. 2001, 32, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kang, X.; Ed-Dra, A.; Zhou, X.; Jia, C.; Müller, A.; Liu, Y.; Kehrenberg, C.; Yue, M. Genome-Based Assessment of Antimicrobial Resistance and Virulence Potential of Isolates of Non-Pullorum/Gallinarum Salmonella Serovars Recovered from Dead Poultry in China. Microbiol. Spectr. 2022, 10, e0096522. [Google Scholar] [CrossRef]
- Ahsan, S.; Rahman, S. Azithromycin Resistance in Clinical Isolates of Salmonella enterica Serovars Typhi and Paratyphi in Bangladesh. Microb. Drug Resist. 2019, 25, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Satola, S.W.; Read, T.D. Genome-Based Prediction of Bacterial Antibiotic Resistance. J. Clin. Microbiol. 2019, 57, 1–15. [Google Scholar] [CrossRef] [PubMed]
- McDermott, P.F.; Tyson, G.H.; Kabera, C.; Chen, Y.; Li, C.; Folster, J.P.; Ayers, S.L.; Lam, C.; Tate, H.P.; Zhao, S. Whole-Genome Sequencing for Detecting Antimicrobial Resistance in Nontyphoidal Salmonella. Antimicrob. Agents Chemother. 2016, 60, 5515–5520. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Niu, Q.; Yang, Y.; Dai, P.; Yuan, T.; Xu, S.; Pan, X.; Zhu, G. Self-made Salmonella Pullorum agglutination antigen development and its potential practical application. Poult. Sci. 2019, 98, 6326–6332. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef]
- Davies, M.R.; Broadbent, S.E.; Harris, S.R.; Thomson, N.R.; van der Woude, M.W. Horizontally acquired glycosyltransferase operons drive salmonellae lipopolysaccharide diversity. PLoS Genet. 2013, 9, e1003568. [Google Scholar] [CrossRef]
- Byl, C.V.; Kropinski, A.M. Sequence of the genome of Salmonella bacteriophage P22. J. Bacteriol. 2000, 182, 6472–6481. [Google Scholar] [CrossRef]
- Kotetishvili, M.; Stine, O.C.; Kreger, A.; Morris, J.J.G.; Sulakvelidze, A. Multilocus Sequence Typing for Characterization of Clinical and Environmental Salmonella Strains. J. Clin. Microbiol. 2002, 40, 1626–1635. [Google Scholar] [CrossRef]
- Zou, Q.-H.; Li, R.-Q.; Liu, G.-R.; Liu, S.-L. Genotyping of Salmonella with lineage-specific genes: Correlation with serotyping. Int. J. Infect. Dis. 2016, 49, 134–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kintz, E.; Heiss, C.; Black, I.; Donohue, N.; Brown, N.; Davies, M.R.; Azadi, P.; Baker, S.; Kaye, P.M.; van der Woude, M. Salmonella enterica Serovar Typhi Lipopolysaccharide O-Antigen Modification Impact on Serum Resistance and Antibody Recognition. Infect. Immun. 2017, 85, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2015, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Wasyl, D.; Hoszowski, A.; Zając, M. Prevalence and characterisation of quinolone resistance mechanisms in Salmonella spp. Vet. Microbiol. 2014, 171, 307–314. [Google Scholar] [CrossRef] [PubMed]
- de Jong, A.; Smet, A.; Ludwig, C.; Stephan, B.; De Graef, E.; Vanrobaeys, M.; Haesebrouck, F. Antimicrobial susceptibility of Salmonella isolates from healthy pigs and chickens (2008–2011). Vet. Microbiol. 2014, 171, 298–306. [Google Scholar] [CrossRef]
- Xu, J.; Sangthong, R.; McNeil, E.; Tang, R.; Chongsuvivatwong, V. Antibiotic use in chicken farms in northwestern China. Antimicrob. Resist. Infect. Control 2020, 9, 10. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Liu, F.; Cheng, Y.; Su, J. Characterization of Salmonella enterica Isolates from Diseased Poultry in Northern China between 2014 and 2018. Pathogens 2020, 9, 95. [Google Scholar] [CrossRef]
- Zhao, X.; Ju, Z.; Wang, G.; Yang, J.; Wang, F.; Tang, H.; Zhao, X.; Sun, S. Prevalence and Antimicrobial Resistance of Salmonella Isolated From Dead-in-Shell Chicken Embryos in Shandong, China. Front. Vet. Sci. 2021, 8, 581946. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, J.; Ed-Dra, A.; Li, X.; Peng, X.; Xia, L.; Guo, Q.; Yao, G.; Yue, M. Prevalence and genomic investigation of Salmonella isolates recovered from animal food-chain in Xinjiang, China. Food Res. Int. 2021, 142, 110198. [Google Scholar] [CrossRef]
- Hyeon, J.-Y.; Li, S.; Mann, D.A.; Zhang, S.; Kim, K.-J.; Lee, D.-H.; Deng, X.; Song, C.-S. Whole-Genome Sequencing Analysis of Salmonella Enterica Serotype Enteritidis Isolated from Poultry Sources in South Korea, 2010–2017. Pathogens 2021, 10, 45. [Google Scholar] [CrossRef]
- Xiao, J.; Cheng, Y.; Zhang, W.; Lu, Q.; Guo, Y.; Hu, Q.; Wen, G.; Shao, H.; Luo, Q.; Zhang, T. Genetic characteristics, antimicrobial susceptibility, and virulence genes distribution of Campylobacter isolated from local dual-purpose chickens in central China. Front. Cell. Infect. Microbiol. 2023, 13, 1236777. [Google Scholar] [CrossRef]
- Neuert, S.; Nair, S.; Day, M.R.; Doumith, M.; Ashton, P.M.; Mellor, K.C.; Jenkins, C.; Hopkins, K.L.; Woodford, N.; de Pinna, E.; et al. Prediction of Phenotypic Antimicrobial Resistance Profiles From Whole Genome Sequences of Non-typhoidal Salmonella enterica. Front. Microbiol. 2018, 9, 592. [Google Scholar] [CrossRef] [PubMed]
- Imran; Das, K.R.; Naik, M.M. Co-selection of multi-antibiotic resistance in bacterial pathogens in metal and microplastic contaminated environments: An emerging health threat. Chemosphere. 2018, 215, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Byrne, A.; Berger, C.N.; Klemm, E.; Crepin, V.F.; Dougan, G.; Frankel, G. The Type III Secretion System Effector SptP of Salmonella enterica Serovar Typhi. J. Bacteriol. 2017, 199, 10–1128. [Google Scholar] [CrossRef]
- Pavlova, B.; Volf, J.; Ondrackova, P.; Matiasovic, J.; Stepanova, H.; Crhanova, M.; Karasova, D.; Faldyna, M.; Rychlik, I. SPI-1-encoded type III secretion system of Salmonella enterica is required for the suppression of porcine alveolar macrophage cytokine expression. Vet. Res. 2011, 42, 16. [Google Scholar] [CrossRef]
- Gong, H.; Su, J.; Bai, Y.; Miao, L.; Kim, K.; Yang, Y.; Liu, F.; Lu, S. Characterization of the expression of Salmonella Type III secretion system factor PrgI, SipA, SipB, SopE2, SpaO, and SptP in cultures and in mice. BMC Microbiol. 2009, 9, 1–14. [Google Scholar] [CrossRef]
- Edwards, R.A.; Schifferli, D.M.; Maloy, S.R. A role for Salmonella fimbriae in intraperitoneal infections. Proc. Natl. Acad. Sci. USA 2000, 97, 1258–1262. [Google Scholar] [CrossRef]
- Zhou, X.; Kang, X.; Chen, J.; Song, Y.; Jia, C.; Teng, L.; Tang, Y.; Jiang, Z.; Peng, X.; Tao, X.; et al. Genome degradation promotes Salmonella pathoadaptation by remodeling fimbriae-mediated proinflammatory response. Natl. Sci. Rev. 2023, 10, nwad228. [Google Scholar] [CrossRef]
- Freeman, J.A.; Ohl, M.E.; Miller, S.I. The Salmonella enterica Serovar Typhimurium Translocated Effectors SseJ and SifB Are Targeted to the Salmonella -Containing Vacuole. Infect. Immun. 2003, 71, 418–427. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, L.; Hernandez, L.; Shears, S.B.; Galán, J.E. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol. Microbiol. 2001, 39, 248–260. [Google Scholar] [CrossRef]
- Kuban-Jankowska, A.; Kostrzewa, T.; Gorska-Ponikowska, M. Bacterial Protein Tyrosine Phosphatases as Possible Targets for Antimicrobial Therapies in Response to Antibiotic Resistance. Antioxidants 2022, 11, 2397. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.-A.; Fink, M.R.; Perkins, T.T.; Sousa, M.C. Type III secretion system effector proteins are mechanically labile. Proc. Natl. Acad. Sci. USA 2021, 118, e2019566118. [Google Scholar] [CrossRef] [PubMed]
| Antigenic Type | MLST Pattern (No. of Isolates) | arcO | dnaN | hemD | hisD | purE | sucA | thrA |
|---|---|---|---|---|---|---|---|---|
| Standard | ST92 (37) | 5 | 2 | 3 | 7 | 31 | 41 | 11 |
| Standard | ST2151 (3) | 5 | 2 | 361 | 7 | 31 | 41 | 11 |
| Antibiotic Category | Antimicrobial Agents | Healthy-Looking (n = 15) | Sick (n = 25) | Total (n = 40) | |||
|---|---|---|---|---|---|---|---|
| No. of Resistant Isolates a | Resistance Rates (%) | No. of Resistant Isolates | Resistance Rates (%) | No. of Resistant Isolates | Resistance Rates (%) | ||
| Aminoglycoside | STR | 13 | 86.7% | 24 | 96.0% | 37 | 92.5% |
| GEN | 1 | 6.7% | 3 | 12.0% | 4 | 10.0% | |
| β-lactams | AMP | 7 | 46.7% | 25 | 100.0% | 32 | 80.0% |
| CTX | 3 | 20.0% | 10 | 40.0% | 13 | 32.5% | |
| Sulphonamide | SMZ | 15 | 100.0% | 17 | 68.0% | 32 | 80.0% |
| SXT | 10 | 66.7% | 15 | 60.0% | 25 | 62.5% | |
| Tetracyclines | TET | 8 | 53.3% | 12 | 48.0% | 20 | 50.0% |
| Quinolones | CIP | 11 | 73.3% | 22 | 88.0% | 33 | 82.5% |
| Macrolides | ERY | 11 | 73.3% | 14 | 56.0% | 25 | 62.5% |
| Resistance Patterns | Number |
|---|---|
| STR-AMP | 1 |
| ERY-SXT-SMZ | 1 |
| SXT-SMZ-CIP | 1 |
| AMP-CTX-TET-CIP | 1 |
| ERY-STR-SXT-SMZ | 1 |
| STR-AMP-TET-CIP | 1 |
| STR-SMZ-TET-CIP | 1 |
| ERY-STR-AMP-SMZ-CIP | 1 |
| ERY-STR-AMP-TET-CIP | 2 |
| ERY-STR-SXT-SMZ-CIP | 1 |
| ERY-STR-SXT-SMZ-TET | 1 |
| ERY-STR-SMZ-TET-CIP | 1 |
| STR-AMP-CTX-TET-CIP | 1 |
| STR-AMP-SXT-SMZ-CIP | 3 |
| ERY-STR-GEN-SMZ-TET | 1 |
| ERY-STR-AMP-CTX-TET-CIP | 2 |
| ERY-STR-AMP-SXT-SMZ-CIP | 6 |
| ERY-STR-AMP-SXT-SMZ-TET | 1 |
| STR-AMP-CTX-SXT-SMZ-CIP | 1 |
| STR-AMP-CTX-SMZ-TET-CIP | 1 |
| STR-AMP-SXT-SMZ-TET-CIP | 1 |
| ERY-STR-AMP-CTX-SXT-SMZ-CIP | 2 |
| ERY-STR-AMP-CTX-SMZ-TET-CIP | 1 |
| ERY-STR-AMP-SXT-SMZ-TET-CIP | 1 |
| ERY-STR-GEN-AMP-SXT-SMZ-CIP | 2 |
| STR-AMP-CTX-SXT-SMZ-TET-CIP | 3 |
| ERY-STR-GEN-AMP-CTX-SMZ-TET | 1 |
| Antimicrobial Agent | Genotype(+) Phenotype(−) | Genotype(−) Phenotype(+) | Genotype(−) Phenotype(−) | Genotype(+) Phenotype(+) |
|---|---|---|---|---|
| STR | 5% (2) | 0% (0) | 2.5% (1) | 92.5% (37) |
| GEN | 87.5% (35) | 0% (0) | 2.5% (1) | 10% (4) |
| AMP | 0% (0) | 0% (0) | 20% (8) | 80% (32) |
| CTX | 47.5% (19) | 0% (0) | 20% (8) | 32.5% (13) |
| SMZ | 20% (8) | 17.5% (7) | 0% (0) | 62.5% (25) |
| SXT | 32.5% (13) | 12.5% (5) | 5% (2) | 50% (20) |
| TET | 0% (0) | 27.5% (11) | 50% (20) | 22.5% (9) |
| CIP | 7.5% (3) | 0% (0) | 10% (4) | 82.5% (33) |
| ERY | 0% (0) | 62.5% (25) | 37.5% (15) | 0% (0) |
| Sources of Strains | Antigenic Types | No. | Sequence Types | gtrABC1 (2552 bp) | gtrABC2 (2739 bp) | gtrABC3 (2919 bp) |
|---|---|---|---|---|---|---|
| Isolates | Standard | 36 | ST 92 | √ | √ | √ |
| Standard | 1 | ST 92 | √ | × | √ | |
| Standard | 3 | ST 2151 | √ | × | √ | |
| CVCC 519 | Standard | 1 | ST 92 | √ | √ | √ |
| CVCC 530 | Variant | 1 | ST 92 | √ | × | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Zhang, J.; Huang, Q.; Luo, Q.; Zhang, T.; Zhou, R. Genome-Based Analysis of Genetic Diversity, Antimicrobial Susceptibility, and Virulence Gene Distribution in Salmonella Pullorum Isolates from Poultry in China. Animals 2024, 14, 2675. https://doi.org/10.3390/ani14182675
Cheng Y, Zhang J, Huang Q, Luo Q, Zhang T, Zhou R. Genome-Based Analysis of Genetic Diversity, Antimicrobial Susceptibility, and Virulence Gene Distribution in Salmonella Pullorum Isolates from Poultry in China. Animals. 2024; 14(18):2675. https://doi.org/10.3390/ani14182675
Chicago/Turabian StyleCheng, Yiluo, Jigao Zhang, Qi Huang, Qingping Luo, Tengfei Zhang, and Rui Zhou. 2024. "Genome-Based Analysis of Genetic Diversity, Antimicrobial Susceptibility, and Virulence Gene Distribution in Salmonella Pullorum Isolates from Poultry in China" Animals 14, no. 18: 2675. https://doi.org/10.3390/ani14182675
APA StyleCheng, Y., Zhang, J., Huang, Q., Luo, Q., Zhang, T., & Zhou, R. (2024). Genome-Based Analysis of Genetic Diversity, Antimicrobial Susceptibility, and Virulence Gene Distribution in Salmonella Pullorum Isolates from Poultry in China. Animals, 14(18), 2675. https://doi.org/10.3390/ani14182675






