Social Enrichment Improves Affective State and Foraging Behavior Compared to Physical Enrichment, While Maintaining Growth Performance in Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Management
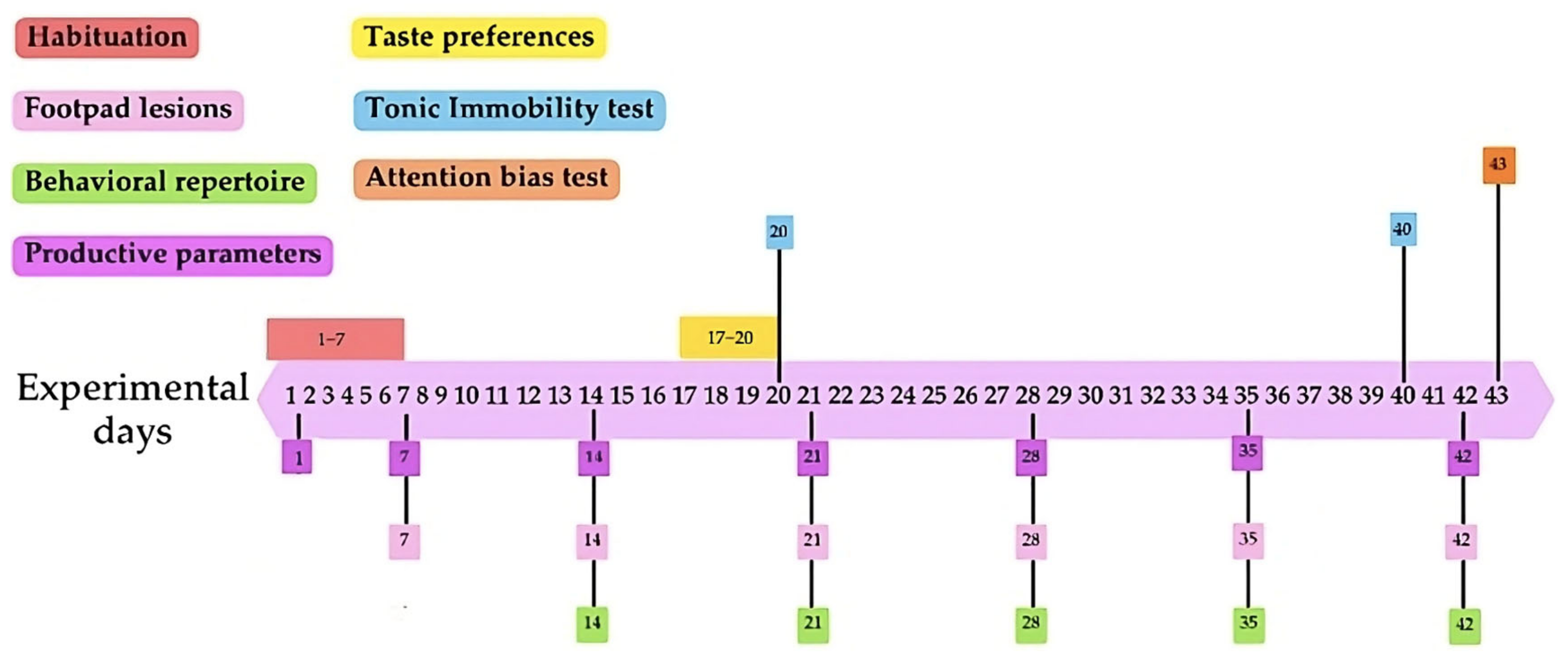
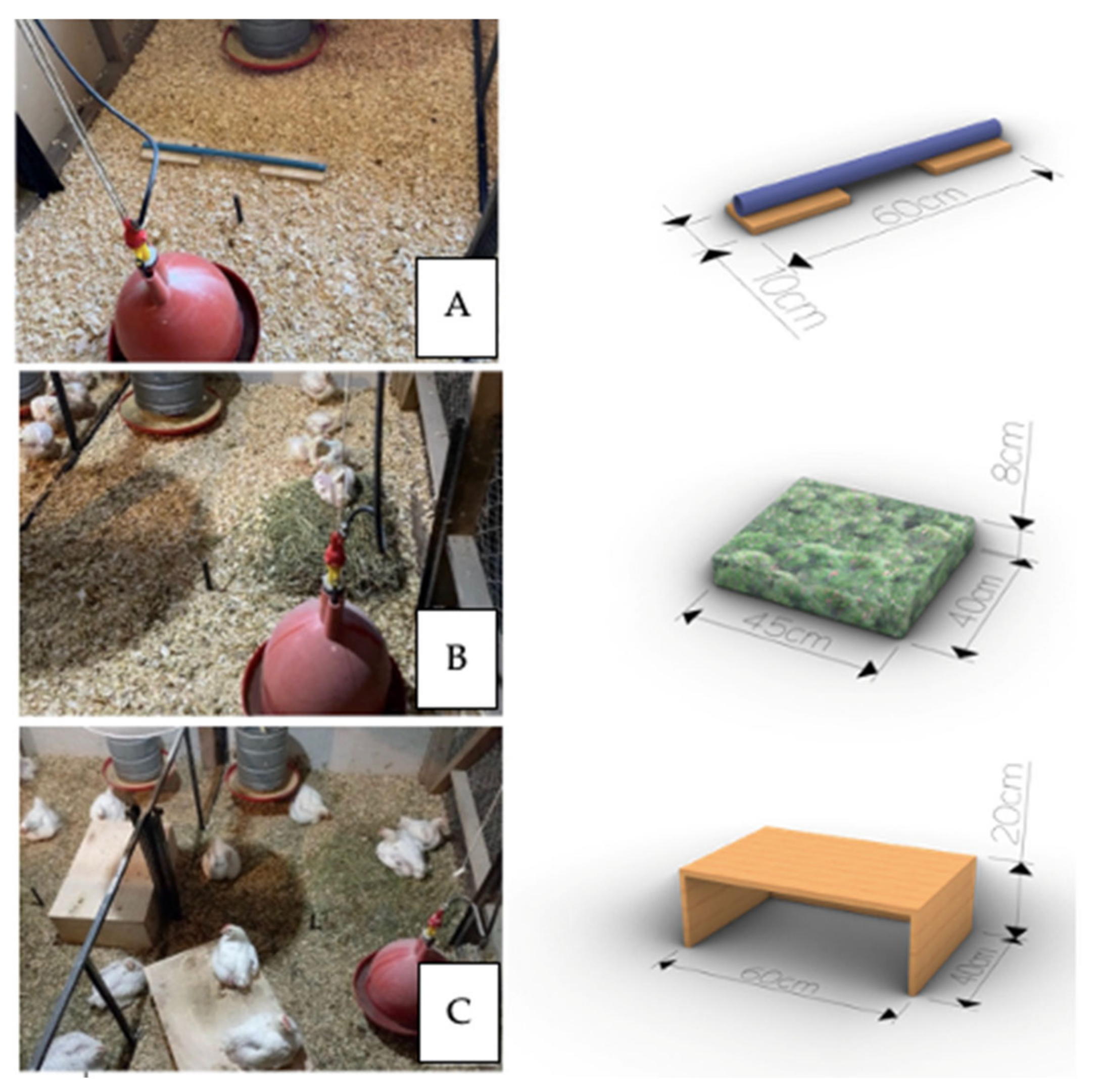
2.2. Experimental Design
2.3. Behavioral Analysis
2.4. Footpad Lesions
2.5. Sweet and Umami Taste Preference Analysis
2.6. Tonic Immobility Test
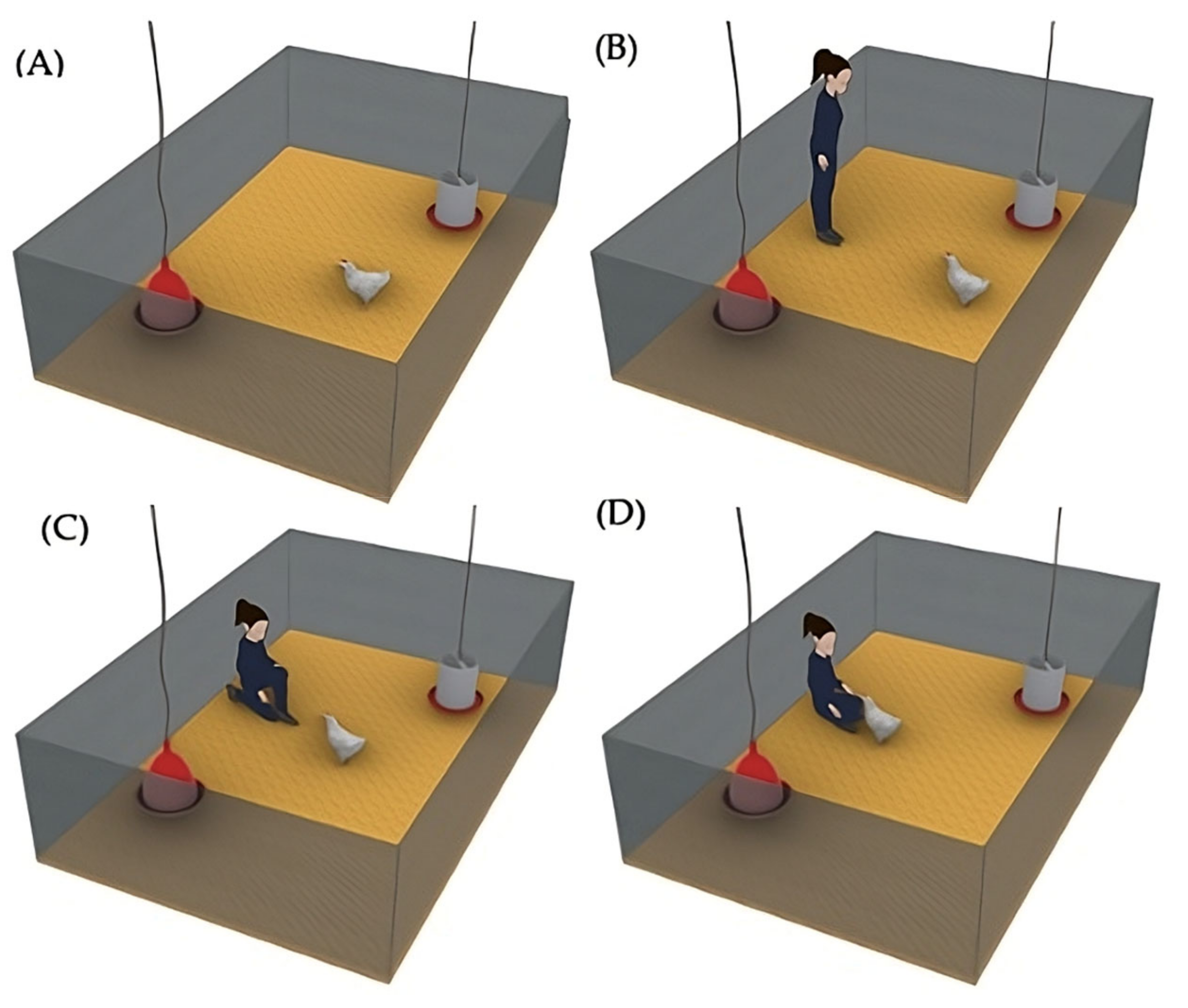
2.7. Attention Bias Test
2.8. Growth Performance
2.9. Statistical Analysis
3. Results
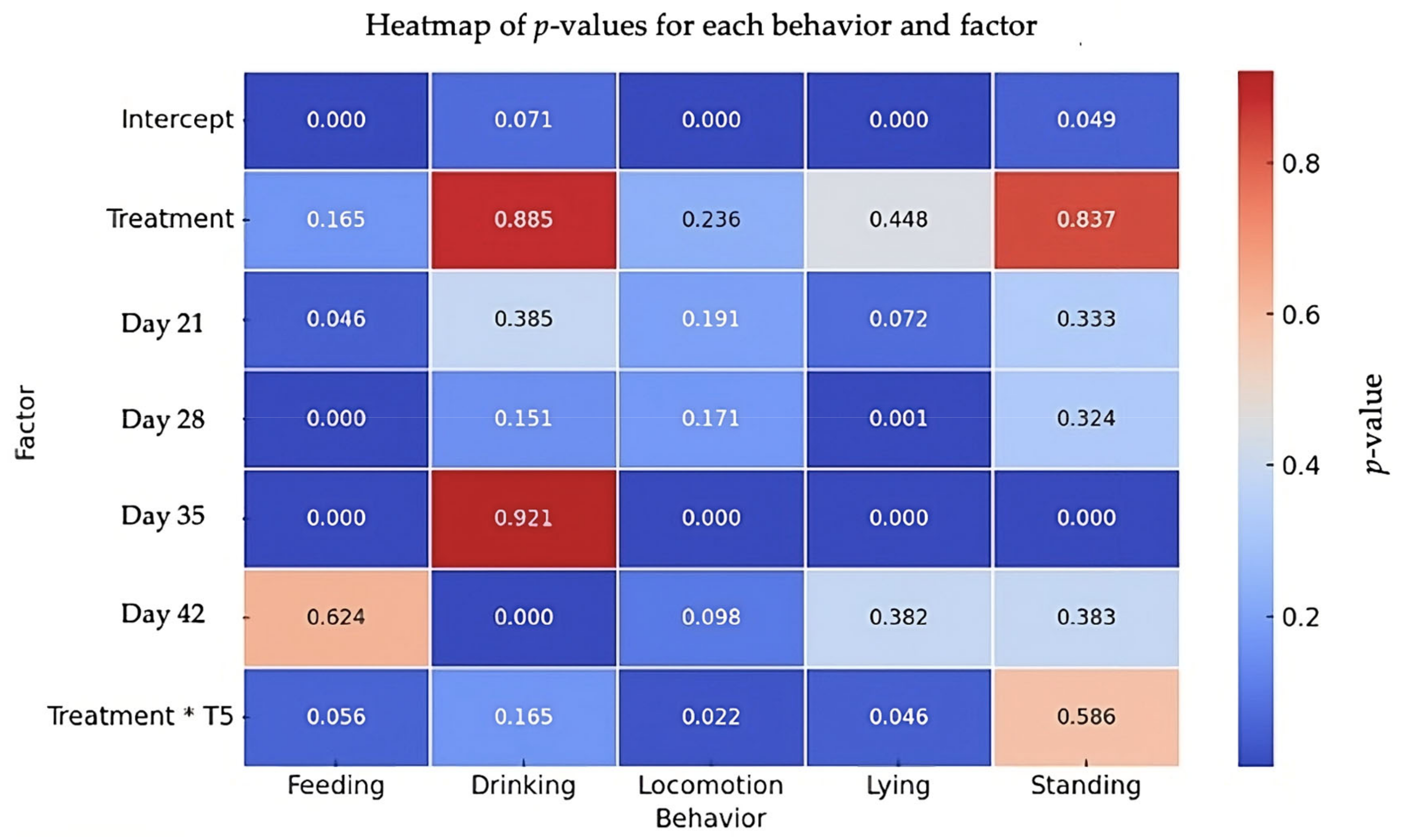
3.1. Behavioral Analysis
3.2. Footpad Lesions
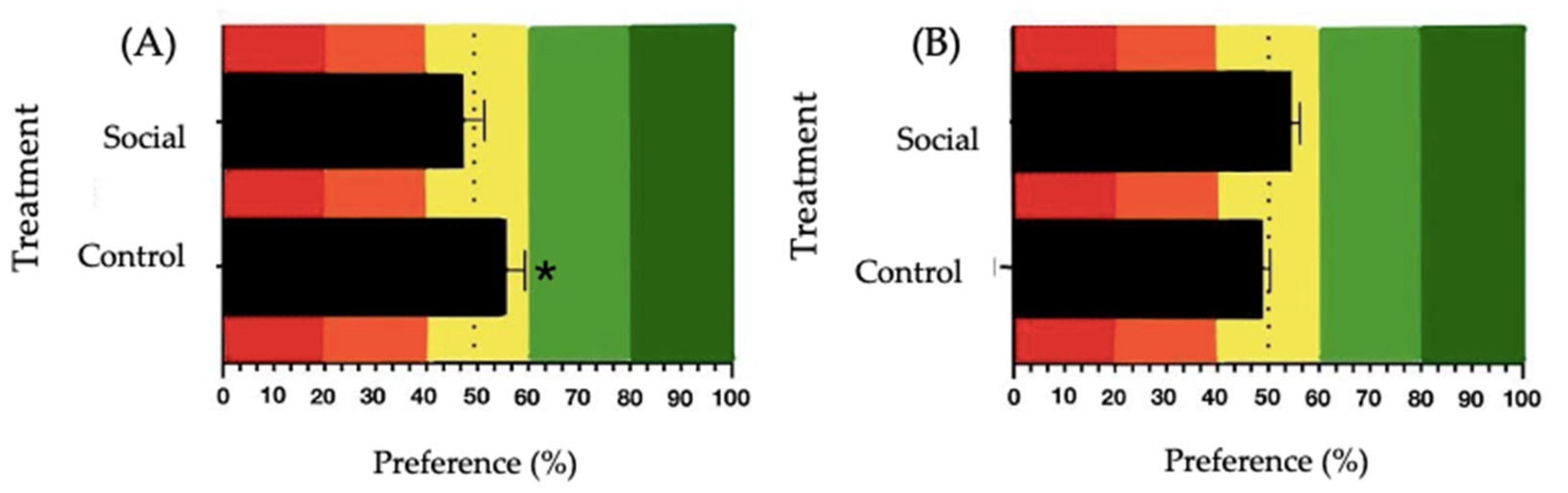
3.3. Sweet and Umami Taste Preference Analysis
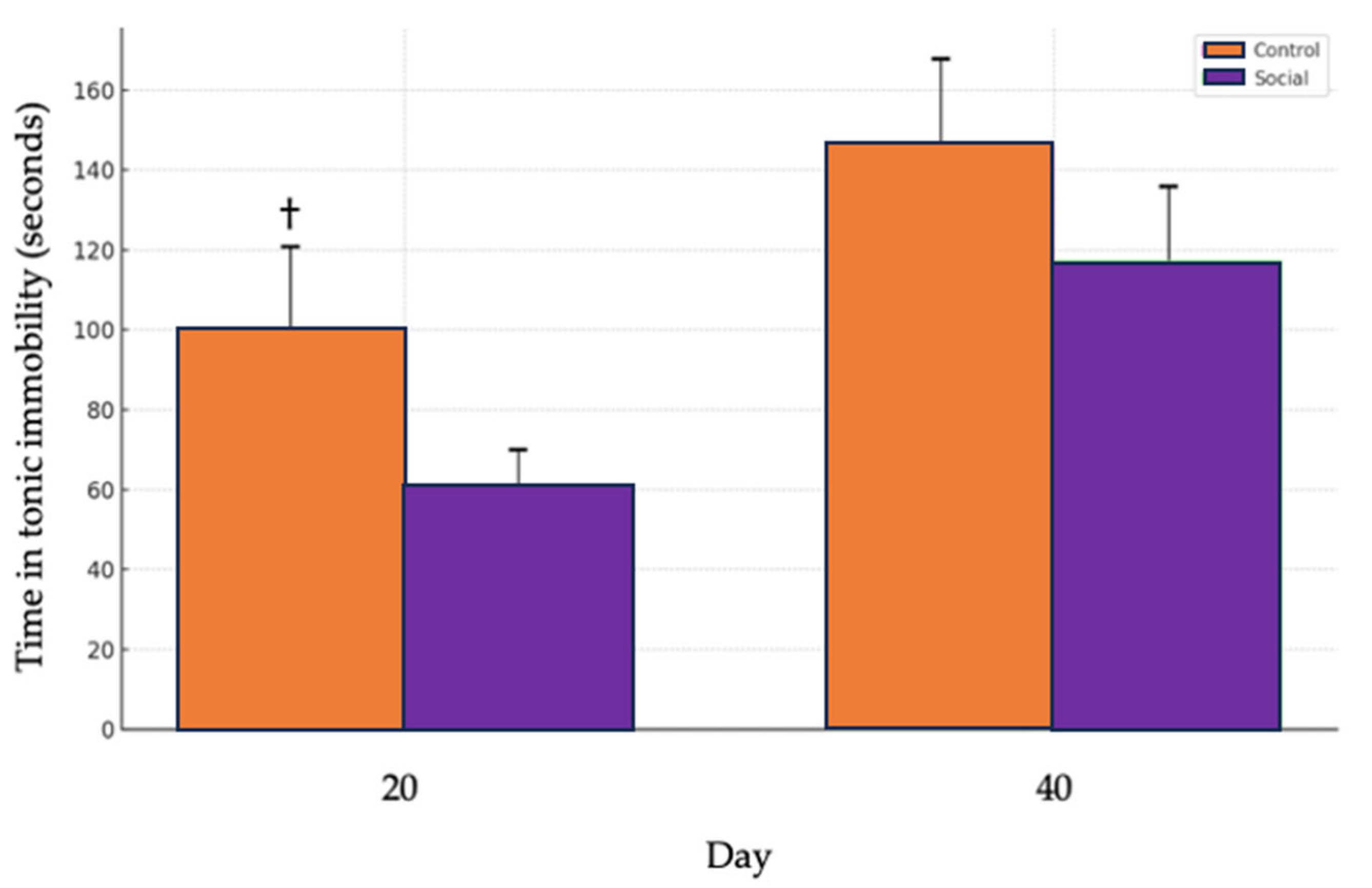
3.4. Tonic Immobility Test
3.5. Attention Bias Test
3.6. Growth Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradshaw, R.; Kirkden, R.; Broom, D. A review of the aetiology and pathology of leg weakness in broilers in relation to welfare. Avian Poult. Biol. Rev. 2002, 13, 45–103. [Google Scholar] [CrossRef]
- Giersberg, M.; Hartung, J.; Kemper, N.; Spindler, B. Floor space covered by broiler chickens kept at stocking densities according to Council Directive 2007/43/EC. Vet. Rec. 2016, 179, 124. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, M.; Donnelly, C.; Jones, T. Chicken welfare is influenced more by housing conditions than by stocking density. Nature 2004, 427, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Bessei, W. Welfare of broilers: A review. World's Poult. Sci. J. 2006, 62, 455–466. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on the influence of genetic parameters on the welfare and the resistance to stress of commercial broilers. EFSA J. 2010, 8, 1666. [Google Scholar] [CrossRef]
- Riber, A.B.; van de Weerd, H.A.; de Jong, I.C.; Steenfeldt, S. Environmental Enrichment for Broiler Chickens: A Review. Poult. Sci. 2018, 97, 378–396. [Google Scholar] [CrossRef]
- Newberry, R. Environmental enrichment: Increasing the biological relevance of captive environments. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Young, R. Environmental Enrichment for Captive Animals; Blackwell Science Ltd.: Oxford, UK, 2003. [Google Scholar]
- Van de Weerd, H.; Day, J. A review of environmental enrichment for pigs housed in intensive housing systems. Appl. Anim. Behav. Sci. 2009, 116, 1–20. [Google Scholar] [CrossRef]
- Zahoor, M.S.; Ahmad, S.; Usman, M.; Dawood, M.; El-Sabrout, K.; Hashmi, S.G.M.D.; Khan, E.U.; Hussain, M.; Maqsood, M.A.; Latif, H.R.A. Effects of mirror and coloured balls as environmental enrichment tools on performance, welfare and meat quality traits of commercial broiler. Trop. Anim. Health Prod. 2022, 54, 151. [Google Scholar] [CrossRef] [PubMed]
- Bloomsmith, M.A.; Brent, L.Y.; Schapiro, S.J. Guidelines for developing and managing an environmental enrichment program for nonhuman primates. Lab. Anim. Sci. 1991, 41, 372–377. [Google Scholar]
- Reiter, K.; Bessei, W. Effect of locomotor activity on leg disorder in fattening chicken. Berl. Munch. Tierarztl. Wochenschr. 2009, 122, 264–270. [Google Scholar] [PubMed]
- Ruiz, C.; Arroyo, J.; Pro, A.; Bautista, J.; Cortes, A.; Narciso, C. Effects of distance and barriers between resources on bone and tendon strength and productive performance of broiler chickens. Poult. Sci. 2014, 93, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Bailie, C.; O’Connell, N.E. The Influence of Providing Perches and String on Activity Levels, Fearfulness, and Leg Health in Commercial Broiler Chickens. Animals 2015, 9, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Bailie, C.L.; Baxter, M.; O’Connell, N.E. Exploring perch provision options for commercial broiler chickens. Appl. Anim. Behav. Sci. 2018, 200, 114–122. [Google Scholar] [CrossRef]
- Spieß, F.; Reckels, B.; Abd-El Wahab, A.; Ahmed, M.; Sürie, C.; Auerbach, M.; Raurenschlein, S.; Distl, O.; Hartung, J.; Visscher, C. The influence of different types of environmental enrichment on the performance and welfare of broiler chickens and the possibilities of real-time monitoring via a farmer-assistant system. Sustainability 2022, 14, 5727. [Google Scholar] [CrossRef]
- Ohara, A.; Oyakawa, C.; Yoshihara, Y.; Ninomiya, S.; Sato, S. Effect of environmental enrichment on the behavior and welfare of Japanese broilers at a commercial farm. J. Poult. Sci. 2015, 52, 323–330. [Google Scholar] [CrossRef]
- Vasdal, G.; Vas, J.; Newberry, R.C.; Moe, R.O. Effects of environmental enrichment on activity and lameness in commercial broiler production. J. Appl. Anim. Welf. Sci. 2019, 22, 197–205. [Google Scholar] [CrossRef]
- Bach, M.H.; Tahamtani, F.M.; Pedersen, I.H.; Riber, A.B. Effects of environmental complexity on behaviour in fast-growing broiler chickens. Appl. Anim. Behav. Sci. 2019, 219, 104840. [Google Scholar] [CrossRef]
- Baxter, M.; Bailie, C.L.; O’Connell, N.E. Play behaviour, fear responses and activity levels in commercial broiler chickens provided with preferred environmental enrichments. Animals 2019, 13, 171–179. [Google Scholar] [CrossRef]
- Mocz, F.; Michel, V.; Janvrot, M.; Moysán, J.; Keita, A.; Riber, A.B.; Guinebretiere, M. Positive effects of elevated platforms and straw bales on the welfare of fast-growing broiler chickens reared at two different stocking densities. Animals 2022, 12, 542. [Google Scholar] [CrossRef]
- Al-Aqil, A.; Zulkifli, I.; Hair Bejo, M.; Sazili, A.Q.; Rajion, M.A.; Somchit, M.N. Changes in heat shock protein 70, blood parameters and fear-related behavior in broiler chickens as affected by pleasant and unpleasant human contact. Poult. Sci. 2013, 93, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Cransberg, P.H.; Hemsworth, P.H.; Coleman, G.J. Human factors affecting the behaviour and productivity of commercial broiler chickens. Poult. Sci. 2000, 79, 1374–1381. [Google Scholar] [CrossRef]
- Calderón-Amor, J.; Zuleta, B.; Ceballos, M.C.; Cartes, D.; Byrd, C.J.; Lecorps, B.; Palomo, R.; Guzmán-Pino, S.A.; Siel, D.; Luna, D. Affective Implications of Human–Animal Relationship on Pig Welfare: Integrating Non-Linear Heart Rate Variability Measures. Animals 2024, 14, 2217. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M. Voluntary Food Intake and Diet Selection in Farm Animals, 2nd ed.; CABI: Wallingford, UK, 2007. [Google Scholar] [CrossRef]
- Cordero, P.; Herrera-Alcaíno, S.; Philp, V.; Muñoz, G.; Luna, D.; Guzmán-Pino, S. Taste preferences in broilers: Effect of age, delivery matrix, and number of chickens per pen on selection and consumption behaviour. Animals 2024, 14, 1507. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Campbell, A.; Crump, A.; Arnott, G.; Jacobs, L. Effect of Environmental Complexity and Stocking Density on Fear and Anxiety in Broiler Chickens. Animals 2021, 11, 2383. [Google Scholar] [CrossRef]
- Vas, J.; BenSassi, N.; Vasdal, G.; Newberry, R.C. Better Welfare for Broiler Chickens Given More Types of Environmental Enrichments and More Space to Enjoy Them. Appl. Anim. Behav. Sci. 2023, 261, 105901. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Poultry, 9th ed.; The National Academies Press: Washington, DC, USA, 1994; 176p. [Google Scholar]
- Aviagen. Ross 308 Broiler: Nutrition Specifications. 2022. Available online: https://aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerNutritionSpecifications2022-EN.pdf (accessed on 30 June 2024).
- Aviagen. Ross 308 Broiler: Management Pocket Guide. 2018. Available online: https://aviagen.com/assets/Tech_Center/BB_Foreign_Language_Docs/Spanish_TechDocs/Ross-BroilerHandbook2018-ES.pdf (accessed on 30 June 2024).
- Rosa-Salva, O.; Daisley, J.N.; Regolin, L.; Vallortigara, G. Time-dependent lateralization of social learning in the domestic chick (Gallus gallus domesticus): Effects of retention delays in the observed lateralization pattern. Behav. Brain Res. 2010, 212, 152–158. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. BORIS: A free versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Sanchez-Casanova, R.; Sarmiento-Franco, L.; Segura-Correa, J.; Phillips, C.J.C. Effects of outdoor access and indoor stocking density on behaviour and stress in broilers in the subhumid tropics. Animals 2019, 9, 1016. [Google Scholar] [CrossRef]
- Butterworth, A.; Arnould, C.; Fiks van Niekerk, T.; Veissier, I.; Keeling, L.; van Overbeke, G.; Bedaux, V. Welfare Quality® Assessment for Poultry (Broilers, Laying Hens); Welfare Quality® Consortium: Lelystad, The Netherlands, 2009. [Google Scholar]
- Wang, S.; Ni, Y.; Guo, F.; Sun, Z.; Ahmed, A.; Zhao, R. Differential expression of hypothalamic fear- and stress-related genes in broiler chickens showing short or long tonic immobility. Domest. Anim. Endocrinol. 2014, 47, 65–72. [Google Scholar] [CrossRef]
- Jones, R. Open-field responses of domestic chicks in the presence of a cagemate or a strange chick. IRCS Med. Sci. Psychol. Psychiatry 1984, 12, 482–483. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 30 September 2024).
- Tahamtani, F.; Pedersen, I.; Toinon, C.; Riber, A. Effects of environmental complexity on fearfulness and learning ability in fast growing broiler chickens. Appl. Anim. Behav. Sci. 2018, 207, 49–56. [Google Scholar] [CrossRef]
- Rowe, E.; Dawkins, M.; Gebhardt, H. A systematic review of precision livestock farming in the poultry sector: Is technology focused on improving bird welfare? Animals 2019, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.S.; Hemsworth, P.H.; Rault, J.L. Environmental complexity: Additional human visual contact reduced meat chickens’ fear of humans and physical items altered pecking behavior. Animals 2022, 12, 310. [Google Scholar] [CrossRef] [PubMed]
- Edgar, J.L.; Held, S.; Paul, E.S.; Pettersson, I.; Price, R.I.; Nicol, C.J. Social buffering in a bird. Anim. Behav. 2015, 105, 11–19. [Google Scholar] [CrossRef]
- Altan, O.; Seremet, C.; Bayraktar, H. The Effects of Early Environmental Enrichment on Performance, Fear and Physiological Responses to Acute Stress of Broiler. Arch. Geflugelkd. 2013, 77, 23–28. [Google Scholar]
| Category | Behavior * | Description |
|---|---|---|
| Individual | Feeding | Eating from a food hopper, whilst standing, sitting, or resting. |
| Drinking | Drinking from the water trough, whilst standing, sitting, or resting. | |
| Locomotion | Moving by walking or running. | |
| Lying | Main part of the body touching the ground, either chest or side. | |
| Standing | The abdomen not touching the litter or ground, and the bird is motionless with no apparent movement of the legs. | |
| Preening | Moving beak along the plumage. | |
| Interaction with the environment | Dustbathing | While lying with fluffed feathers, the bird simultaneously and rapidly lifts its wings up and down multiple times, while scooping loose substrate material up into the feathers. |
| Use of enrichments | Interaction with environmental enrichments such as perches, platforms, or straw bales, including climbing, perching, or pecking at the enrichments. | |
| Foraging | Scratching at the ground, with intermittent bouts of ground pecking items (visible or not). Usually followed by one or two steps backwards after a bout of ground scratching. | |
| Social Interaction | Mock fighting | After running toward each other, birds stop and stare at each other. The interaction is brief, harmless to the birds, and not directed persistently at any one bird. |
| Allopreening | The birds use their beaks to arrange the feathers of another bird. |
| Fitness Assessment | Score * | Descriptions |
|---|---|---|
| Footpad condition | 0 | Feet intact, no or minimal proliferation of the epithelium. |
| 1 | Necrosis or proliferation of the epithelium or chronic bumble foot with no or moderate swelling. | |
| 2 | Swollen (dorsally visible). | |
| 3 | Moderate footpad dermatitis. | |
| 4 | Severe footpad dermatitis. |
| Behaviors | Occurrence (%) | p-Value (Regression) |
|---|---|---|
| Mock fighting | 3.7% | - |
| Allopreening | 1.7% | - |
| Dustbathing | 1% | - |
| Enrichment Use | 3.5% | - |
| Preening | 20.4% | 0.233 |
| Foraging | 17.4% | 0.027 * |
| Latencies (s) | Intercept (p-Value) | Treatment (p-Value) | Pen (p-Value) |
|---|---|---|---|
| First step | 0.05 * | 0.776 | 0.269 |
| Begin feeding | <0.001 * | 0.006 * | 0.059 |
| Time spent feeding | <0.001 * | 0.108 | 0.027 * |
| Resume feeding | <0.001 * | 0.108 | 0.027 * |
| Behaviors | Social Treatment | Control Treatment | Pen (Random Effect) |
|---|---|---|---|
| Erect posture | −0.577 ± 0.752 | Ref | −0.220 ± 0.080 * (p = 0.006) |
| Neck stretching | −0.029 ± 0.555 | Ref | 0.088 ± 0.083 |
| Looking around | −0.577 ± 0.752 | Ref | 0.088 ± 0.083 |
| Freezing | 4.556 ± 1.867 * (p = 0.015) | Ref | −1.053 ± 0.403 * (p = 0.009) |
| Days 1 to 42 | Control | Social | SEM 5 | p-Value |
|---|---|---|---|---|
| (n = 48) | (n = 48) | |||
| Initial BW 1 (g) | 41.0 | 40.7 | 0.396 | 0.542 |
| ADFI 2 (g) | 92.4 | 92.5 | 0.728 | 0.924 |
| ADG 3 (g) | 49.9 | 50.0 | 1.169 | 0.941 |
| FCR 4 | 0.5 | 0.6 | 0.019 | 0.642 |
| Final BW (g) | 2136.6 | 2142.1 | 49.041 | 0.938 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Alcaíno, S.; Luna, D.; González-Pavez, J.; Cordero, P.; Guzmán-Pino, S.A. Social Enrichment Improves Affective State and Foraging Behavior Compared to Physical Enrichment, While Maintaining Growth Performance in Broiler Chickens. Animals 2024, 14, 3186. https://doi.org/10.3390/ani14223186
Herrera-Alcaíno S, Luna D, González-Pavez J, Cordero P, Guzmán-Pino SA. Social Enrichment Improves Affective State and Foraging Behavior Compared to Physical Enrichment, While Maintaining Growth Performance in Broiler Chickens. Animals. 2024; 14(22):3186. https://doi.org/10.3390/ani14223186
Chicago/Turabian StyleHerrera-Alcaíno, Sofía, Daniela Luna, Jorge González-Pavez, Paloma Cordero, and Sergio A. Guzmán-Pino. 2024. "Social Enrichment Improves Affective State and Foraging Behavior Compared to Physical Enrichment, While Maintaining Growth Performance in Broiler Chickens" Animals 14, no. 22: 3186. https://doi.org/10.3390/ani14223186
APA StyleHerrera-Alcaíno, S., Luna, D., González-Pavez, J., Cordero, P., & Guzmán-Pino, S. A. (2024). Social Enrichment Improves Affective State and Foraging Behavior Compared to Physical Enrichment, While Maintaining Growth Performance in Broiler Chickens. Animals, 14(22), 3186. https://doi.org/10.3390/ani14223186








