Using an Automated Operant Conditioning Procedure to Test Colour Discrimination in Two Juvenile Piranhas, Pygocentrus nattereri: A Lesson on Failures and Pitfalls and How to Avoid Them
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Preliminary Assessment of Colour Preference
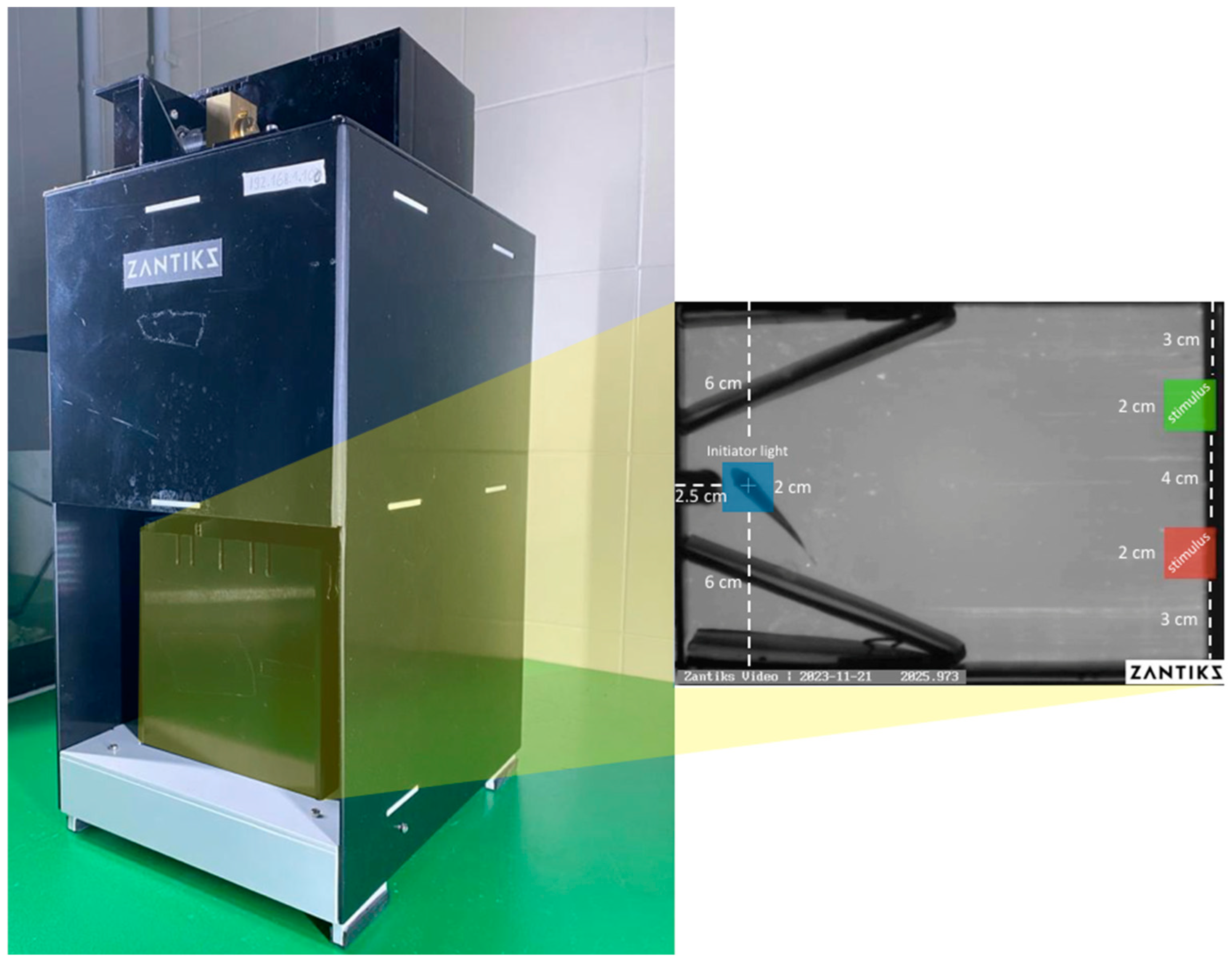
2.2.1. Stimuli and Apparatus
2.2.2. Procedure
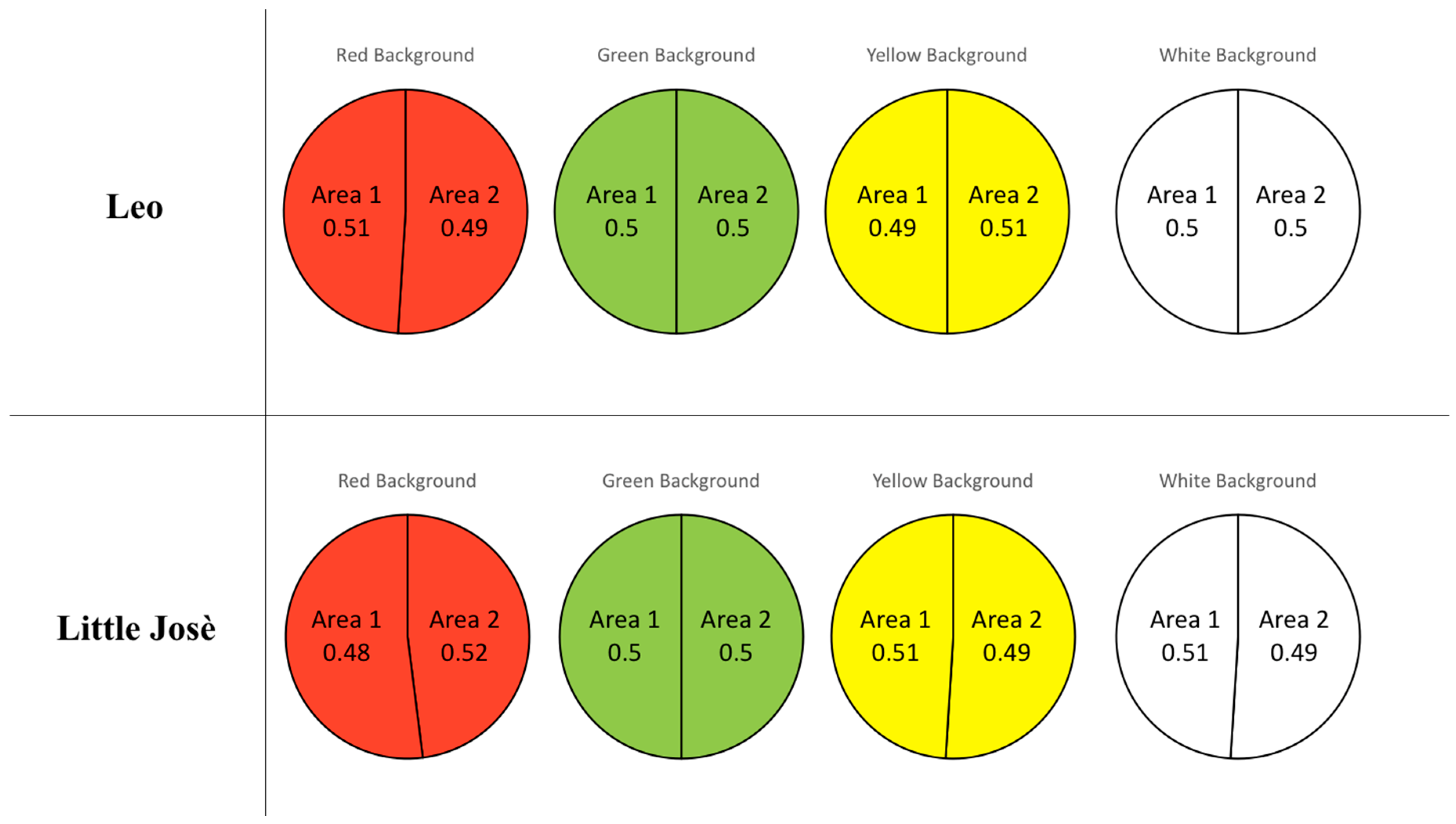
2.2.3. Data Analysis
2.3. Experiment 1 and 2: Response Learning with Coloured Stimuli
2.3.1. Stimuli and Apparatus
2.3.2. Procedure
2.4. Experiment 3: Response Learning (Achromatic Stimuli) + Place Learning Task
2.5. Data Analysis for Experiment 1, 2, and 3.
3. Results and Discussion
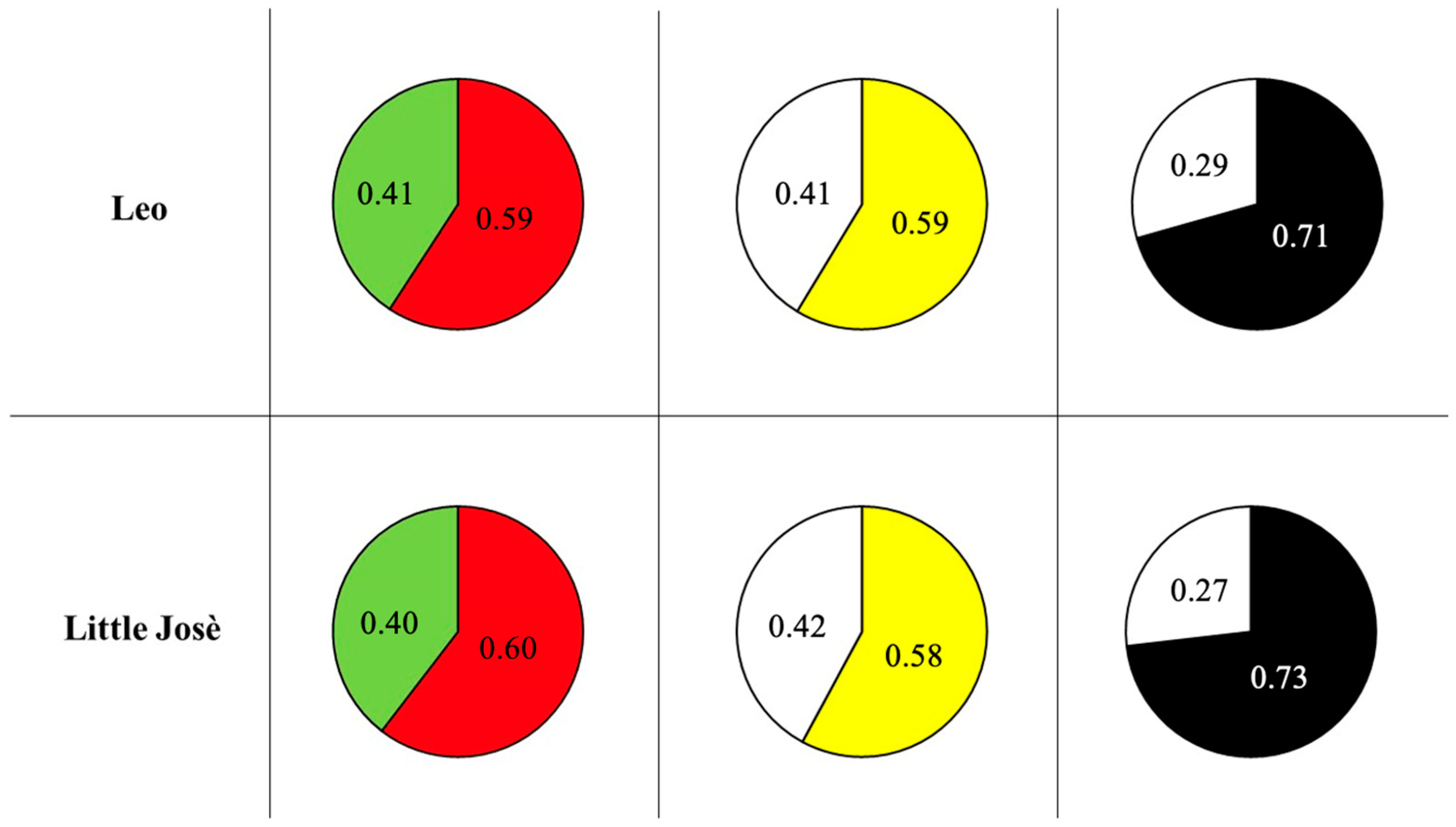
3.1. Preliminary Assessment of Colour Preference
3.2. Response Learning with Coloured Stimuli
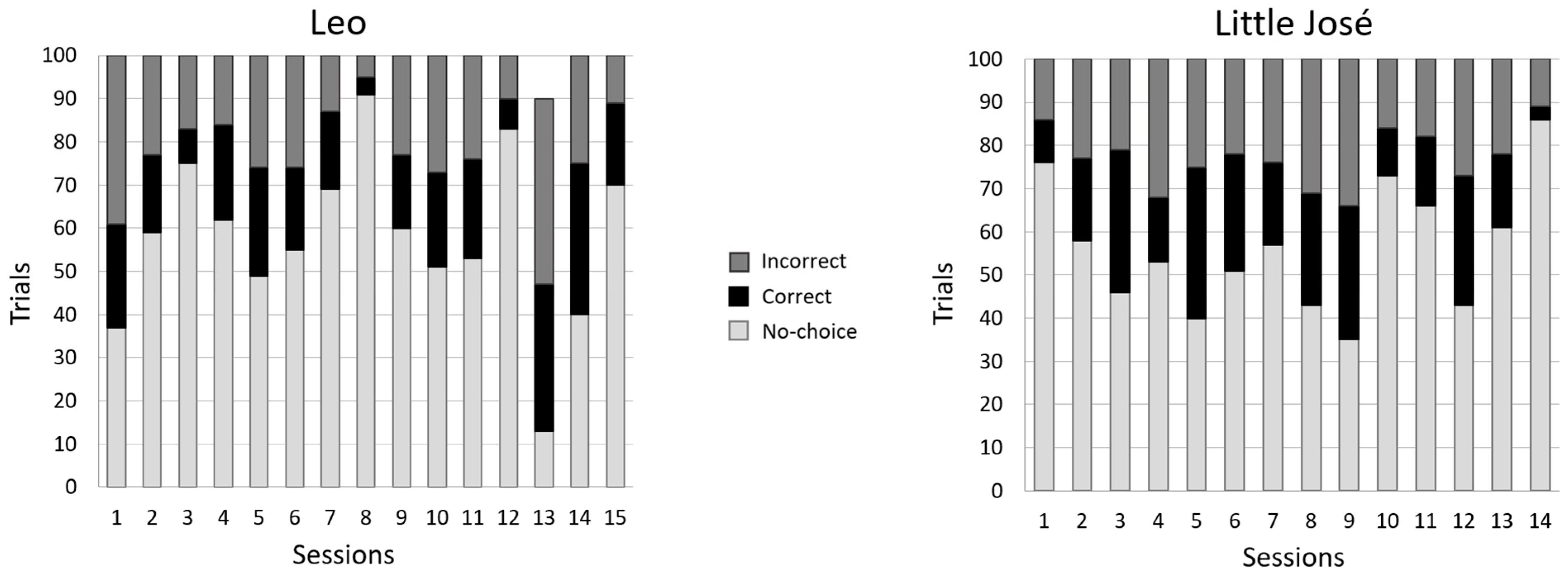
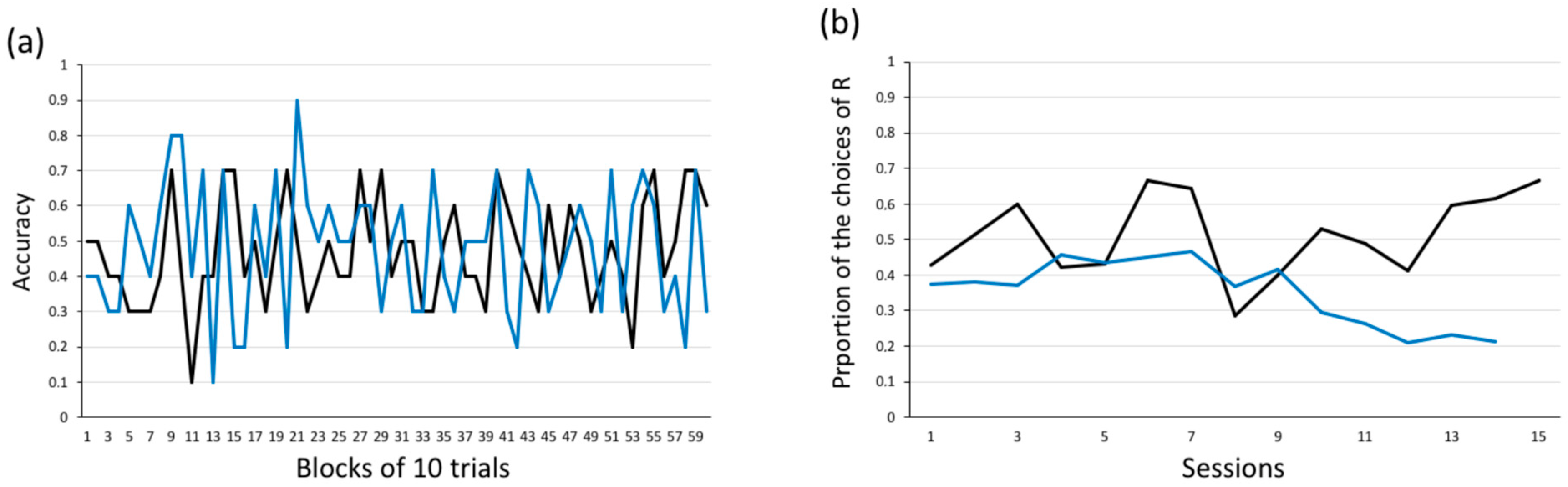
3.2.1. Experiment 1 (Red Vs. Green)
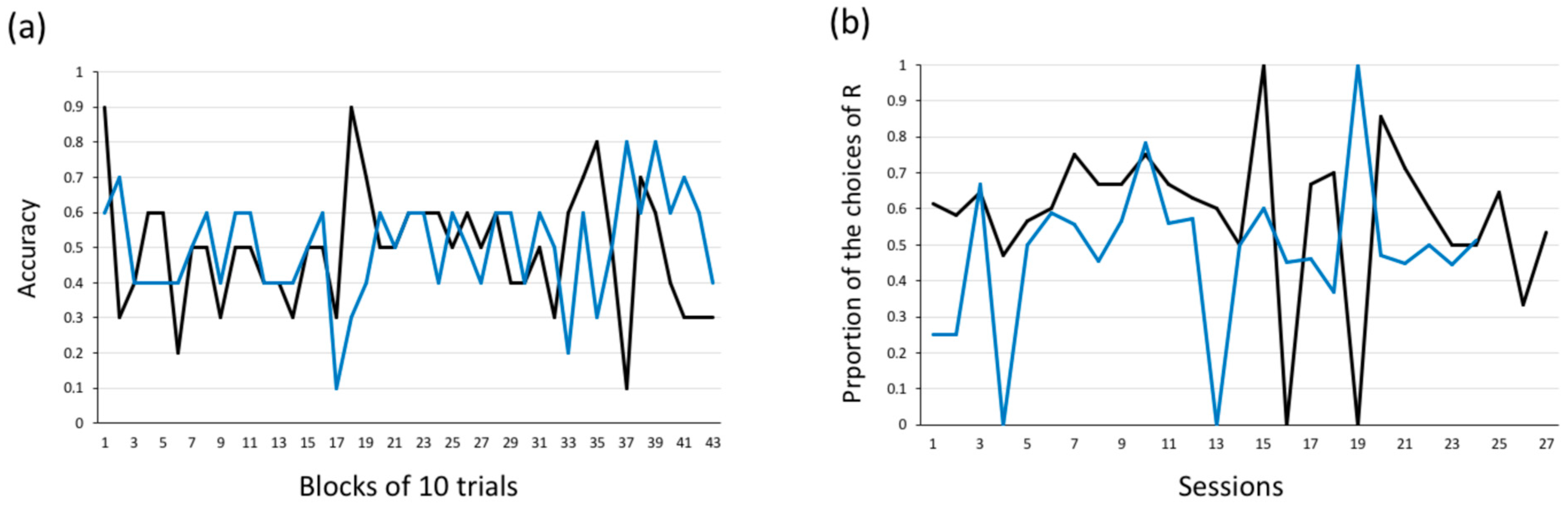
3.2.2. Experiment 2 (Yellow vs. White)
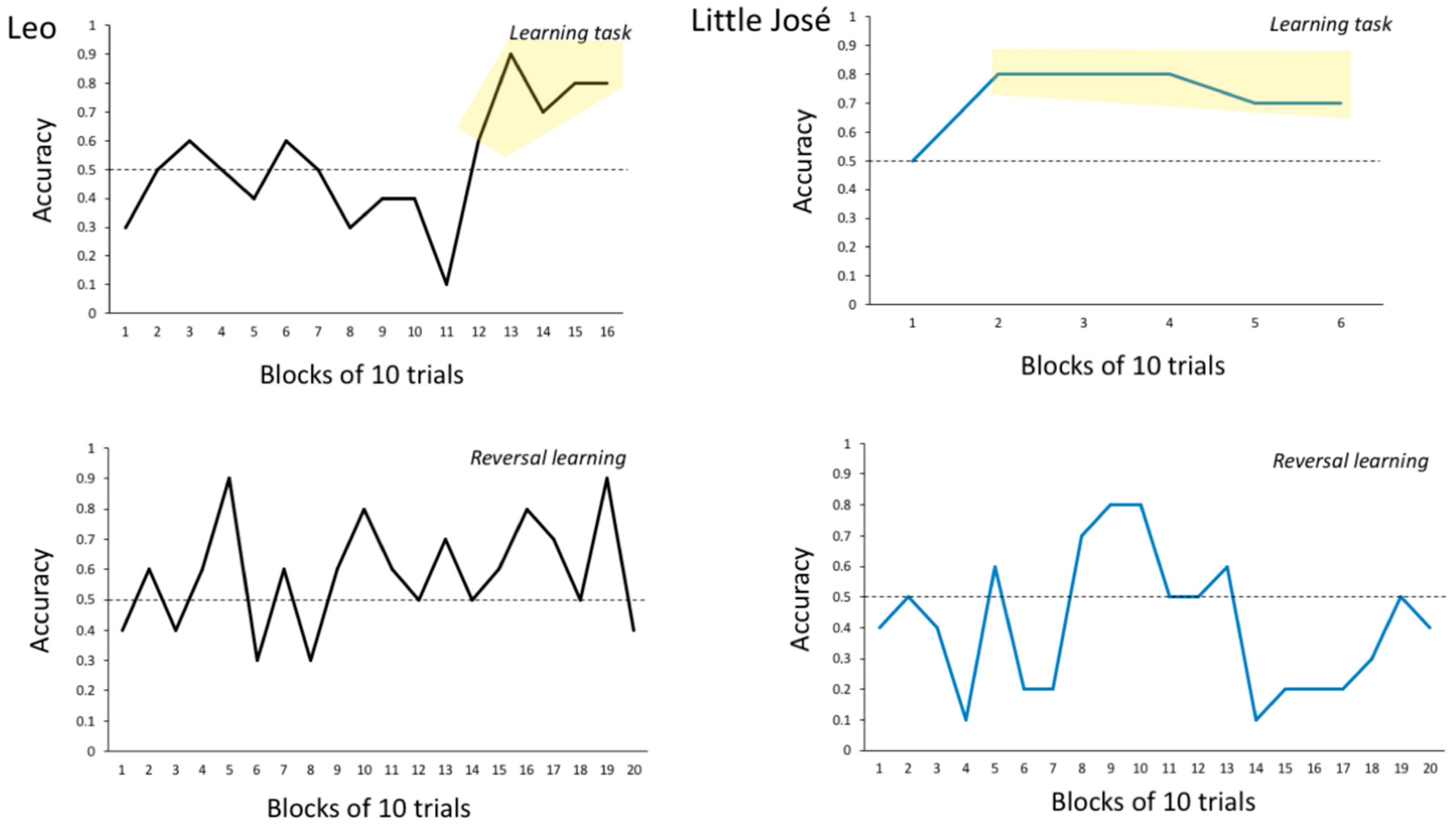
3.2.3. Experiment 3: Response Learning (Achromatic Stimuli) + Place Learning Task
4. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, C.; Laland, K.; Krause, J. Fish Cognition and Behavior; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bshary, R.; Wickler, W.; Fricke, H. Fish cognition: A primate’s eye view. Anim. Cogn. 2002, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Salena, M.G.; Turko, A.J.; Singh, A.; Pathak, A.; Hughes, E.; Brown, C.; Balshine, S. Understanding fish cognition: A review and appraisal of current practices. Anim. Cogn. 2021, 24, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Buechel, S.D.; Boussard, A.; Kotrschal, A.; van der Bijl, W.; Kolm, N. Brain size affects performance in a reversal-learning test. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172031. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.M.; Schluessel, V. Serial reversal learning in freshwater stingrays (Potamotrygon motoro). Anim. Cogn. 2020, 23, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Miletto Petrazzini, M.E.; Agrillo, C.; Izard, V.; Bisazza, A. Do humans (Homo sapiens) and fish (Pterophyllum scalare) make similar numerosity judgments? J. Comp. Psychol. 2016, 130, 380. [Google Scholar] [CrossRef]
- Miletto Petrazzini, M.E.; Pecunioso, A.; Dadda, M.; Agrillo, C. Searching for the critical p of Macphail’s null hypothesis: The contribution of numerical abilities of fish. Front. Psychol. 2020, 11, 55. [Google Scholar] [CrossRef]
- Bisazza, A.; Butterworth, B.; Piffer, L.; Bahrami, B.; Miletto Petrazzini, M.E.; Agrillo, C. Collective enhancement of numerical acuity by meritocratic leadership in fish. Sci. Rep. 2014, 4, 4560. [Google Scholar] [CrossRef]
- Gómez-Laplaza, L.M.; Gerlai, R. Spontaneous discrimination of small quantities: Shoaling preferences in angelfish (Pterophyllum scalare). Anim. Cogn. 2011, 14, 565–574. [Google Scholar] [CrossRef]
- Potrich, D.; Zanon, M.; Vallortigara, G. Archerfish number discrimination. eLife 2022, 11, e74057. [Google Scholar] [CrossRef]
- Lee, S.A.; Vallortigara, G.; Flore, M.; Spelke, E.S.; Sovrano, V.A. Navigation by environmental geometry: The use of zebrafish as a model. J. Exp. Biol. 2013, 216, 3693–3699. [Google Scholar] [CrossRef]
- Braithwaite, V.A.; De Perera, T.B. Short-range orientation in fish: How fish map space. Mar. Freshw. Behav. Physiol. 2006, 39, 37–47. [Google Scholar] [CrossRef]
- Kohda, M.; Bshary, R.; Kubo, N.; Awata, S.; Sowersby, W.; Kawasaka, K.; Kobayashi, T.; Sogawa, S. Cleaner fish recognize self in a mirror via self-face recognition like humans. Proc. Nat. Acad. Sci. USA 2023, 120, e2208420120. [Google Scholar] [CrossRef] [PubMed]
- Mair, A.; Dadda, M.; Kitaoka, A.; Agrillo, C. Illu-Shoal choice: An exploration of different means for enrichment of captive zebrafish. Animals 2023, 13, 2640. [Google Scholar] [CrossRef] [PubMed]
- Meshalkina, D.A.; Kizlyk, M.N.; Kysil, E.V.; Collier, A.D.; Echevarria, D.J.; Abreu, M.S.; Barcellos, L.J.G.; Song, C.; Kalueff, A.V. Understanding zebrafish cognition. Behav. Proc. 2017, 141, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; Laland, K.N. Social learning of a novel avoidance task in the guppy: Conformity and social release. Anim. Behav. 2002, 64, 41–47. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Bisazza, A.; Bertolucci, C. Guppies show sex and individual differences in the ability to inhibit behaviour. Anim. Cogn. 2020, 23, 535–543. [Google Scholar] [CrossRef]
- Gómez-Laplaza, L.M.; Gerlai, R. Can angelfish (Pterophyllum scalare) count? Discrimination between different shoal sizes follows Weber’s law. Anim. Cogn. 2011, 14, 1–9. [Google Scholar] [CrossRef]
- Gómez-Laplaza, L.M.; Gerlai, R. Angelfish (Pterophyllum scalare) discriminate between small quantities: A role of memory. J. Comp. Psychol. 2015, 129, 78. [Google Scholar] [CrossRef]
- Wegman, J.J.; Morrison, E.; Wilcox, K.T.; DeLong, C.M. Visual perception of photographs of rotated 3d objects in goldfish (Carassius auratus). Animals 2022, 12, 1797. [Google Scholar] [CrossRef]
- Otero Coronel, S.; Martorell, N.; Beron de Astrada, M.; Medan, V. Stimulus contrast information modulates sensorimotor decision making in goldfish. Front. Neural Circuits 2020, 14, 23. [Google Scholar] [CrossRef]
- Sibeaux, A.; Karlsson, C.; Newport, C.; Burt de Perera, T. Distance estimation in the goldfish (Carassius auratus). Proc. R. Soc. Lond. Ser. B 2022, 289, 20221220. [Google Scholar] [CrossRef] [PubMed]
- Triki, Z.; Bshary, R. Sex differences in the cognitive abilities of a sex-changing fish species Labroides dimidiatus. R. Soc. Open Sci. 2021, 8, 210239. [Google Scholar] [CrossRef] [PubMed]
- Aellen, M.; Burkart, J.M.; Bshary, R. No evidence for general intelligence in a fish. Ethology 2022, 128, 424–436. [Google Scholar] [CrossRef]
- Raick, X.; Huby, A.; Kurchevski, G.; Godinho, A.L.; Parmentier, É. Yellow-eyed piranhas produce louder sounds than red-eyed piranhas in an invasive population of Serrasalmus marginatus. J. Fish Biol. 2020, 97, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Uetanabaro, M.; Wang, T.; Abe, A.S. Breeding behaviour of the red-bellied piranha, Pygocentrus nattereri, in nature. Environ. Biol. Fishes 1993, 38, 369–371. [Google Scholar] [CrossRef]
- Velasco-Hogan, A.; Meyers, M.A. Bite force mechanics and allometry of piranha (Serrasalmidae). J. Mech. Behav. Biomed. Mater. 2021, 115, 104296. [Google Scholar] [CrossRef]
- Skinner, B.F. Selection by consequences. Science 1981, 213, 501–504. [Google Scholar] [CrossRef]
- Cantlon, J.F.; Brannon, E.M. Basic math in monkeys and college students. PLoS Biol. 2007, 5, e328. [Google Scholar] [CrossRef]
- Perdue, B.M.; Beran, M.J.; Washburn, D.A. A computerized testing system for primates: Cognition, welfare, and the Rumbaughx. Behav. Proc. 2018, 156, 37–50. [Google Scholar] [CrossRef]
- Herbranson, W.T. Pigeons, humans, and the Monty Hall dilemma. Curr. Dir. Psychol. Sci. 2012, 21, 297–301. [Google Scholar] [CrossRef]
- Nakamura, N.; Watanabe, S.; Fujita, K. Pigeons perceive the Ebbinghaus-Titchener circles as an assimilation illusion. J. Exp. Psychol. Anim. Behav. Proc. 2008, 34, 375. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Testolin, A.; Bisazza, A.; Zorzi, M.; Lucon-Xiccato, T. Poor numerical performance of guppies tested in a Skinner box. Sci. Rep. 2020, 10, 16724. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Lucon-Xiccato, T.; Bisazza, A.; Manabe, K.; Dadda, M. The devil is in the detail: Zebrafish learn to discriminate visual stimuli only if salient. Behav. Proc. 2020, 179, 104215. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.; Torgerson-White, L.; McGuire, M.; Thueme, M.; Thomas, J.; Beran, M.J. Quantity estimation and comparison in western lowland gorillas (Gorilla gorilla gorilla). Anim. Cogn. 2014, 17, 755–765. [Google Scholar] [CrossRef]
- Vonk, J.; Beran, M.J. Bears ‘count’ too: Quantity estimation and comparison in black bears, Ursus americanus. Anim. Behav. 2012, 84, 231–238. [Google Scholar] [CrossRef]
- Perdue, B.M.; Talbot, C.F.; Stone, A.M.; Beran, M.J. Putting the elephant back in the herd: Elephant relative quantity judgments match those of other species. Anim. Cogn. 2012, 15, 955–961. [Google Scholar] [CrossRef]
- Pepperberg, I.M. Grey parrot numerical competence: A review. Anim.Cogn. 2006, 9, 377–391. [Google Scholar] [CrossRef]
- Bisazza, A.; Tagliapietra, C.; Bertolucci, C.; Foà, A.; Agrillo, C. Non-visual numerical discrimination in a blind cavefish (Phreatichthys andruzzii). J. Exp. Biol. 2014, 217, 1902–1909. [Google Scholar] [CrossRef]
- Fuss, T.; Schluessel, V. The Ebbinghaus illusion in the gray bamboo shark (Chiloscyllium griseum) in comparison to the teleost damselfish (Chromis chromis). Zoology 2017, 123, 16–29. [Google Scholar] [CrossRef]
- Brown, J.L.; Shively, F.D.; LaMotte, R.H.; Sechzer, J.A. Color discrimination in the cat. J. Comp. Physiol. Psychol. 1973, 84, 534. [Google Scholar] [CrossRef]
- Miletto Petrazzini, M.E.; Pecunioso, A.; Dadda, M.; Agrillo, C. The impact of brain lateralization and anxiety-like behaviour in an extensive operant conditioning task in zebrafish (Danio rerio). Symmetry 2019, 11, 1395. [Google Scholar] [CrossRef]
- Savage, A.; Dronzek, L.A.; Snowdon, C.T. Color discrimination by the cotton-top tamarin (Saguinus oedipus oedipus) and its relation to fruit coloration. Folia Primatol. 1987, 49, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J. Place vs. response learning: History, controversy, and neurobiology. Front. Behav. Neurosci. 2021, 14, 598570. [Google Scholar] [CrossRef] [PubMed]
- Lucon-Xiccato, T.; Bisazza, A. Discrimination reversal learning reveals greater female behavioural flexibility in guppies. Biol. Lett. 2014, 10, 20140206. [Google Scholar] [CrossRef]
- Agrillo, C.; Miletto Petrazzini, M.E.; Tagliapietra, C.; Bisazza, A. Inter-specific differences in numerical abilities among teleost fish. Front. Psychol. 2012, 3, 483. [Google Scholar] [CrossRef] [PubMed]
- Agrillo, C.; Bisazza, A. Understanding the origin of number sense: A review of fish studies. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160511. [Google Scholar] [CrossRef]
- Earl, B. Humans, fish, spiders and bees inherited working memory and attention from their last common ancestor. Front. Psychol. 2023, 13, 937712. [Google Scholar] [CrossRef]
- Maximino, C.; Marques de Brito, T.; Dias, C.A.; Gouveia, A., Jr.; Morato, S. Scototaxis as anxiety-like behavior in fish. Nat. Protoc. 2010, 5, 209–216. [Google Scholar] [CrossRef]
- Miletto Petrazzini, M.E.; Gambaretto, L.; Dadda, M.; Brennan, C.; Agrillo, C. Are cerebral and behavioural lateralization related to anxiety-like traits in the animal model zebrafish (Danio rerio)? Laterality 2021, 26, 144–162. [Google Scholar] [CrossRef]
- Miletto Petrazzini, M.E.; Pecunioso, A.; Dadda, M.; Agrillo, C. Does brain lateralization affect the performance in binary choice tasks? A study in the animal model Danio rerio. Symmetry 2020, 12, 1294. [Google Scholar] [CrossRef]
- Agrillo, C.; Bisazza, A. Spontaneous versus trained numerical abilities. A comparison between the two main tools to study numerical competence in non-human animals. J. Neurosci. Methods 2014, 234, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Santacà, M.; Agrillo, C. Two halves are less than the whole: Evidence of a length bisection bias in fish (Poecilia reticulata). PLoS ONE 2020, 15, e0233157. [Google Scholar] [CrossRef] [PubMed]
- Bisazza, A.; Santacà, M. Zebrafish excel in number discrimination under an operant conditioning paradigm. Anim. Cogn. 2022, 25, 917–933. [Google Scholar] [CrossRef] [PubMed]
- Maximino, C.; Marques, T.; Dias, F.; Cortes, F.V.; Taccolini, I.B.; Pereira, P.M.; Colmanetti, R.; Gazolla, R.A.; Tavares, R.I.; Rodrigues, S.T.K.; et al. A comparative analysis of the preference for dark environments in five teleosts. Int. J. Comp. Psychol. 2007, 20, 351–367. [Google Scholar] [CrossRef]
- Jozet-Alves, C.; Viblanc, V.A.; Romagny, S.; Dacher, M.; Healy, S.D.; Dickel, L. Visual lateralization is task and age dependent in cuttlefish, Sepia officinalis. Anim. Behav. 2012, 83, 1313–1318. [Google Scholar] [CrossRef]
- O’Shea-Wheller, T.A. Honeybees show a context-dependent rightward bias. Biol. Lett. 2009, 15, 20180877. [Google Scholar] [CrossRef]
- Spiezio, C.; Pugassi, M.; Agrillo, C.; Regaiolli, B. Color preference and manual laterality in the emperor tamarin (Saguinus imperator). Am. J. Primatol. 2022, 84, e23375. [Google Scholar] [CrossRef]
- Agrillo, C.; Parrish, A.E.; Beran, M.J. Do primates see the solitaire illusion differently? A comparative assessment of humans (Homo sapiens), chimpanzees (Pan troglodytes), rhesus monkeys (Macaca mulatta), and capuchin monkeys (Cebus apella). J. Comp. Psychol. 2014, 128, 402–413. [Google Scholar] [CrossRef]
- Knudson, D.V.; Lindsey, C. Type I and Type II errors in correlations of various sample sizes. Comp. Psychol. 2014, 3, 03-CP. [Google Scholar] [CrossRef]
- Kanai, R.; Rees, G. The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 2011, 12, 231–242. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Manabe, K.; Bisazza, A. Guppies learn faster to discriminate between red and yellow than between two shapes. Ethology 2019, 125, 82–91. [Google Scholar] [CrossRef]
- Gatto, E.; Santacà, M.; Verza, I.; Dadda, M.; Bisazza, A. Automated operant conditioning devices for fish. Do they work? Animals 2021, 11, 1397. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Lucon-Xiccato, T.; Savaşçı, B.B.; Dadda, M.; Bisazza, A. Experimental setting affects the performance of guppies in a numerical discrimination task. Anim. Cogn. 2017, 20, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Camacho, D.; Carleton, K.L.; Narain, D.W.; Pierotti, M.E. Visual pigment evolution in Characiformes: The dynamic interplay of teleost whole-genome duplication, surviving opsins and spectral tuning. Mol. Ecol. 2020, 29, 2234–2253. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, B.F.; Aeschliman, B.; Pierce, P. Studies in spatial learning. VIII. Place performance and the acquisition of place dispositions. J. Comp. Phys. Psychol. 1950, 43, 73–85. [Google Scholar] [CrossRef]
- Hicks, L.H. Effects of overtraining on acquisition and reversal of place and response learning. Psychol. Rep. 1964, 15, 459–462. [Google Scholar] [CrossRef]
- Packard, M.G.; McGaugh, J.L. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: Further evidence for multiple memory systems. Behav. Neurosci. 1992, 106, 439–446. [Google Scholar] [CrossRef]
- Packard, M.G. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc. Natl. Acad. Sci. USA 1999, 96, 12881–12886. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrillo, C.; Pecunioso, A. Using an Automated Operant Conditioning Procedure to Test Colour Discrimination in Two Juvenile Piranhas, Pygocentrus nattereri: A Lesson on Failures and Pitfalls and How to Avoid Them. Animals 2024, 14, 3187. https://doi.org/10.3390/ani14223187
Agrillo C, Pecunioso A. Using an Automated Operant Conditioning Procedure to Test Colour Discrimination in Two Juvenile Piranhas, Pygocentrus nattereri: A Lesson on Failures and Pitfalls and How to Avoid Them. Animals. 2024; 14(22):3187. https://doi.org/10.3390/ani14223187
Chicago/Turabian StyleAgrillo, Christian, and Alessandra Pecunioso. 2024. "Using an Automated Operant Conditioning Procedure to Test Colour Discrimination in Two Juvenile Piranhas, Pygocentrus nattereri: A Lesson on Failures and Pitfalls and How to Avoid Them" Animals 14, no. 22: 3187. https://doi.org/10.3390/ani14223187
APA StyleAgrillo, C., & Pecunioso, A. (2024). Using an Automated Operant Conditioning Procedure to Test Colour Discrimination in Two Juvenile Piranhas, Pygocentrus nattereri: A Lesson on Failures and Pitfalls and How to Avoid Them. Animals, 14(22), 3187. https://doi.org/10.3390/ani14223187





