Comparative Transcriptome Analysis Reveals the Impact of a High-Fat Diet on Hepatic Metabolic Function in Tilapia (Oreochromis niloticus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Experimental Design
2.2. Serum Parameter Measurement
2.3. Transcriptome Sequencing in Liver
2.4. Quantitative Real-Time PCR (qPCR) Assessment
2.5. Statistical Analysis
3. Results
3.1. Changes in Serum Biochemical Parameters
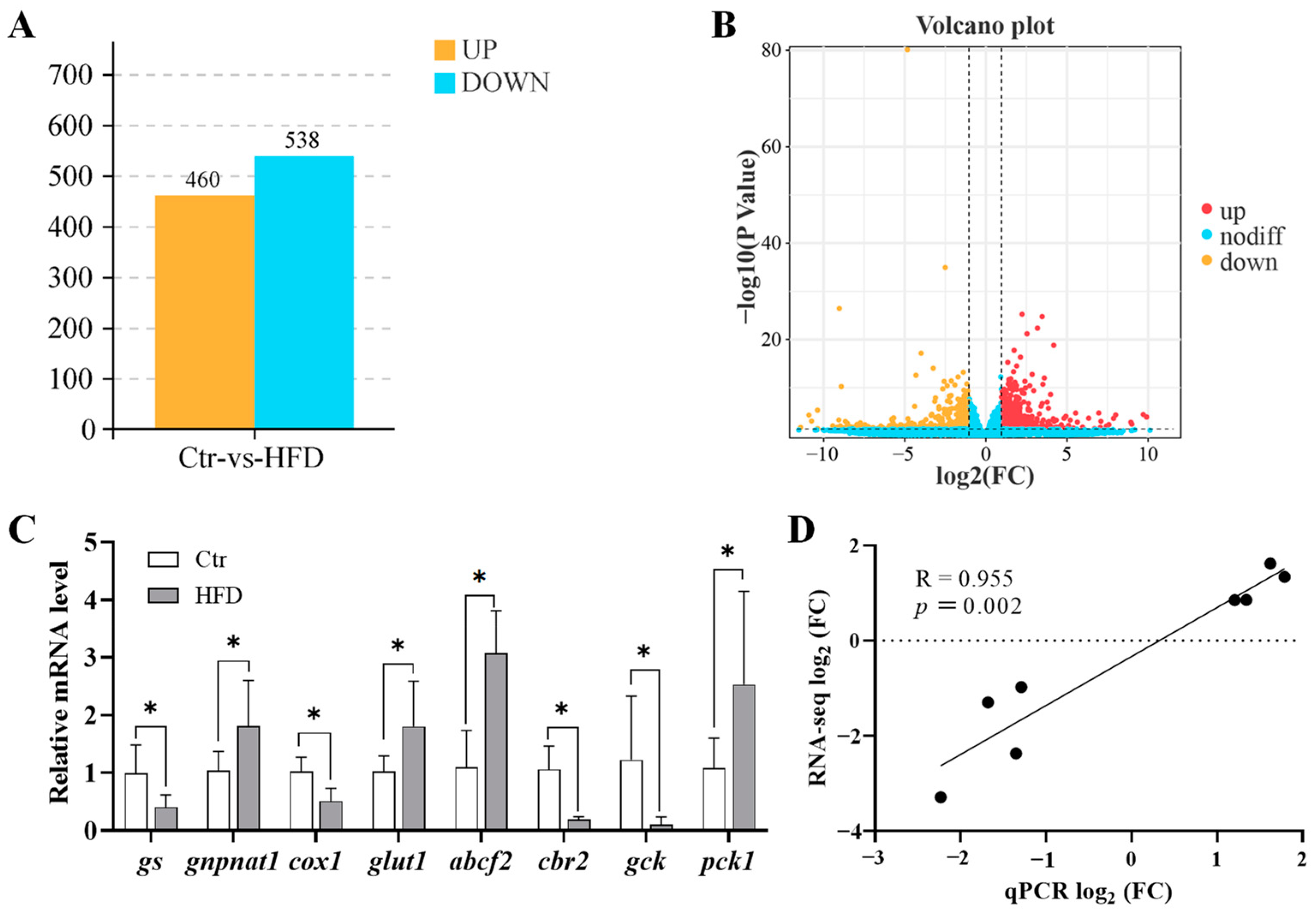
3.2. Transcriptome Sequencing and Gene Expression
3.3. GO Enrichment Analysis of DEGs
3.4. KEGG Enrichment Analysis of DEGs
3.5. Changes in the Steroid Biosynthesis Pathway
4. Discussion
4.1. The Influence of HFD Blood Paramaters
4.2. The Influence of HFD on Energy Metabolism
4.3. The Influence of HFD on Lipid Metabolism
4.4. The Influence of HFD on Protein Synthase
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Gómez-Requeni, P.; Bedolla-Cázares, F.; Montecchia, C.; Zorrilla, J.; Villian, M.; Toledo-Cuevas, E.M.; Canosa, F. Effects of increasing the dietary lipid levels on the growth performance, body composition and digestive enzyme activities of the teleost pejerrey (Odontesthes bonariensis). Aquaculture 2013, 416–417, 15–22. [Google Scholar] [CrossRef]
- Du, Z.Y.; Clouet, P.; Huang, L.M.; Degrace, P.; Zheng, W.H.; He, J.G.; Tian, L.X.; Liu, Y.J. Utilization of different dietary lipid sources at high level in herbivorous grass carp (Ctenopharyngodon idella): Mechanism related to hepatic fatty acid oxidation. Aquac. Nutr. 2008, 14, 77–92. [Google Scholar] [CrossRef]
- Naiel, M.A.; Negm, S.S.; Ghazanfar, S.; Shukry, M.; Abdelnour, S.A. The risk assessment of high-fat diet in farmed fish and its mitigation approaches: A review. J. Anim. Physiol. Anim. Nutr. 2023, 107, 948–969. [Google Scholar] [CrossRef]
- Du, Z. Causes of fatty liver in farmed fish: A review and new perspectives. J. Fish. China 2014, 38, 1628–1638. [Google Scholar]
- Sheridan, M.A. Lipid dynamics in fish: Aspects of absorption, transportation, deposition and mobilization. Comp. Biochem. Physiol. Part B Comp. Biochem. 1988, 90, 679–690. [Google Scholar] [CrossRef]
- Bruslé, J.; i Anadon, G.G. The structure and function of fish liver. In Fish Morphology; Routledge: London, UK, 2017; pp. 77–93. [Google Scholar]
- Yu, K.; Huang, K.; Jiang, S.; Tang, X.; Huang, X.; Sun, L.; Pang, L.; Mo, C. Protective function on liver and proteomic analysis of the improvement mechanism of Sedum sarmentosum Bunge extract on nonalcoholic fatty liver disease in Nile tilapia. Aquaculture 2021, 531, 735977. [Google Scholar] [CrossRef]
- Tian, J.; Tao, Q.; Li, Y.; Wang, G. Ethoxyquin attenuate oxidant stress, inflammatory response and apoptosis in liver of Channa argus fed with high-fat dietary. Aquac. Rep. 2021, 21, 100889. [Google Scholar] [CrossRef]
- Jin, M.; Shen, Y.; Pan, T.; Zhu, T.; Li, X.; Xu, F.; Betancor, M.B.; Jiao, L.; Tocher, D.R.; Zhou, Q. Dietary Betaine Mitigates Hepatic Steatosis and Inflammation Induced by a High-Fat-Diet by Modulating the Sirt1/Srebp-1/Pparalpha Pathway in Juvenile Black Seabream (Acanthopagrus schlegelii). Front. Immunol. 2021, 12, 694720. [Google Scholar] [CrossRef]
- Zhou, W.; Rahimnejad, S.; Lu, K.; Wang, L.; Liu, W. Effects of berberine on growth, liver histology, and expression of lipid-related genes in blunt snout bream (Megalobrama amblycephala) fed high-fat diets. Fish Physiol. Biochem. 2019, 45, 83–91. [Google Scholar] [CrossRef]
- Schlegel, A. Studying non-alcoholic fatty liver disease with zebrafish: A confluence of optics, genetics, and physiology. Cell. Mol. Life Sci. 2012, 69, 3953–3961. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, Y.; Terai, S.; Sakaida, I.; Nishina, H. The expanding role of fish models in understandingnon-alcoholic fatty liver disease. Dis. Models Mech. 2013, 6, 905–914. [Google Scholar]
- Chen, Q.-Q.; Liu, W.-B.; Zhou, M.; Dai, Y.-J.; Xu, C.; Tian, H.-Y.; Xu, W.-N. Effects of berberine on the growth and immune performance in response to ammonia stress and high-fat dietary in blunt snout bream Megalobrama amblycephala. Fish Shellfish Immunol. 2016, 55, 165–172. [Google Scholar] [CrossRef]
- Wu, D.; Li, J.; Fan, Z.; Sun, Z.; Zheng, X.; Zhang, H.; Xu, H.; Wang, L. Dietary Lycium barbarum Polysaccharide Modulates Growth Performance, Antioxidant Capacity, and Lipid Metabolism in Common Carp (Cyprinus carpio) Fed with High-Fat Diet. Antioxidants 2024, 13, 540. [Google Scholar] [CrossRef]
- Jia, Y.; Jing, Q.; Niu, H.; Huang, B. Ameliorative effect of vitamin E on hepatic oxidative stress and hypoimmunity induced by high-fat diet in turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2017, 67, 634–642. [Google Scholar] [CrossRef]
- Ling, S.C.; Wu, K.; Zhang, D.G.; Luo, Z. Endoplasmic Reticulum Stress-Mediated Autophagy and Apoptosis Alleviate Dietary Fat-Induced Triglyceride Accumulation in the Intestine and in Isolated Intestinal Epithelial Cells of Yellow Catfish. J. Nutr. 2019, 149, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Limbu, S.M.; Ma, Q.; Zhang, M.-L.; Du, Z.-Y. High fat diet worsens the adverse effects of antibiotic on intestinal health in juvenile Nile tilapia (Oreochromis niloticus). Sci. Total Environ. 2019, 680, 169–180. [Google Scholar] [CrossRef]
- Wang, X.; Chen, D.; Lv, Z.; Zhao, X.; Ding, C.; Liu, Y.; Xiao, T. Transcriptomics analysis provides new insights into the fish antiviral mechanism and identification of interferon-stimulated genes in grass carp (Ctenopharyngodon idella). Mol. Immunol. 2022, 148, 81–90. [Google Scholar] [CrossRef]
- Zhong, X.; Gu, J.; Zhang, S.; Chen, X.; Zhang, J.; Miao, J.; Ding, Z.; Xu, J.; Cheng, H. Dynamic transcriptome analysis of the muscles in high-fat diet-induced obese zebrafish (Danio rerio) under 5-HT treatment. Gene 2022, 819, 146265. [Google Scholar] [CrossRef]
- Inoue, Y.; Fukushima, M.; Hirasawa, G.; Furukawa, F.; Takeda, H.; Umatani, C. Maternal High-Fat Diet Affects the Contents of Eggs and Causes Abnormal Development in the Medaka Fish. Endocrinology 2024, 165, bqae006. [Google Scholar] [CrossRef]
- Xue, M.; Xu, P.; Wen, H.; Chen, J.; Wang, Q.; He, J.; He, C.; Kong, C.; Li, X.; Li, H.; et al. A High-Fat-Diet-Induced Microbiota Imbalance Correlates with Oxidative Stress and the Inflammatory Response in the Gut of Freshwater Drum (Aplodinotus grunniens). Antioxidants 2024, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Yang, H.K.; Gan, H.; Gong, Z.L.; Gan, X.; Luo, Y.J. Effects of diet-supplemental choline on fatty liver pathological changes in tilapia(Oreochromis niloticus × O. aureus). J. Fish. Sci. China 2007, 14, 257–262. [Google Scholar]
- Xu, F.; Xu, C.; Xiao, S.; Lu, M.; Limbu, S.M.; Wang, X.; Du, Z.; Qin, J.G.; Chen, L. Effects of α-lipoic acid on growth performance, body composition, antioxidant profile and lipid metabolism of the GIFT tilapia (Oreochromis niloticus) fed high-fat diets. Aquac. Nutr. 2019, 25, 585–596. [Google Scholar] [CrossRef]
- Lin, J.-J.; Liu, Y.-C.; Chang, C.-J.; Pan, M.-H.; Lee, M.-F.; Pan, B.S. Hepatoprotective mechanism of freshwater clam extract alleviates non-alcoholic fatty liver disease: Elucidated in vitro and in vivo models. Food Funct. 2018, 9, 6315–6325. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; Tao, Y.F.; Bao, J.W.; Chen, D.J.; Li, H.X.; He, J.; Xu, P. High Fat Diet-Induced miR-122 Regulates Lipid Metabolism and Fat Deposition in Genetically Improved Farmed Tilapia (GIFT, Oreochromis niloticus) Liver. Front. Physiol. 2018, 9, 1442. [Google Scholar] [CrossRef]
- Ma, Q.; Li, L.Y.; Le, J.Y.; Lu, D.L.; Qiao, F.; Zhang, M.L.; Du, Z.Y.; Li, D.L. Dietary microencapsulated oil improves immune function and intestinal health in Nile tilapia fed with high-fat diet. Aquaculture 2018, 496, 19–29. [Google Scholar] [CrossRef]
- Jia, R.; Cao, L.P.; Du, J.L.; He, Q.; Gu, Z.Y.; Jeney, G.; Xu, P.; Yin, G.J. Effects of High-Fat Diet on Steatosis, Endoplasmic Reticulum Stress and Autophagy in Liver of Tilapia (Oreochromis niloticus). Front. Mar. Sci. 2020, 7, 14. [Google Scholar] [CrossRef]
- Jia, R.; Cao, L.-P.; Du, J.-L.; He, Q.; Gu, Z.-Y.; Jeney, G.; Xu, P.; Yin, G.-J. Effects of high-fat diet on antioxidative status, apoptosis and inflammation in liver of tilapia (Oreochromis niloticus) via Nrf2, TLRs and JNK pathways. Fish Shellfish. Immunol. 2020, 104, 391–401. [Google Scholar] [CrossRef]
- Blüthgen, N.; Meili, N.; Chew, G.; Odermatt, A.; Fent, K. Accumulation and effects of the UV-filter octocrylene in adult and embryonic zebrafish (Danio rerio). Sci. Total Environ. 2014, 476–477, 207–217. [Google Scholar] [CrossRef]
- Yang, E.-J.; Amenyogbe, E.; Zhang, J.-D.; Wang, W.-Z.; Huang, J.-S.; Chen, G. Integrated transcriptomics and metabolomics analysis of the intestine of cobia (Rachycentron canadum) under hypoxia stress. Aquac. Rep. 2022, 25, 101261. [Google Scholar] [CrossRef]
- WU, Y.; Zhang, M.; Zhang, J.; Wang, H.; Wen, Y.; Katherine, C. Relationship of plasma acylation stimulating protein with blood lipid profile in women with pulycystic ovary syndrome. Chin. J. Endocrinol. Metab. 2008, 513–516. [Google Scholar]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Chan, C.-K.K. Analysis of RNA-Seq Data Using TopHat and Cufflinks. In Plant Bioinformatics: Methods and Protocols; Edwards, D., Ed.; Springer: New York, NY, USA, 2016; pp. 339–361. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.Z.; Jhaveri, D.J.; Marshall, V.M.; Bauer, D.C.; Janette, E.; Narayanan, R.K.; Robinson, G.J.; Lundberg, A.E.; Bartlett, P.F.; Wray, N.R. A Comparative Study of Techniques for Differential Expression Analysis on RNA-Seq Data. PLoS ONE 2014, 9, e103207. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chang, G.Y.; Xian, L.W.; Tian, J.; Wei, L.; Fan, W.; Ming, J.; Hua, W. Evaluation of reference genes for quantitative real-time RT-PCR analysis of gene expression in Nile tilapia (Oreochromis niloticus). Gene 2013, 527, 183–192. [Google Scholar]
- Huang, S.C.; Lin, J.J.; Lee, M.F.; Liu, Y.C.; Pan, B.S. Freshwater clam extracts alleviate dyslipidaemia of tilapia fed a high-fat diet as an animal model. J. Funct. Foods 2016, 25, 559–567. [Google Scholar] [CrossRef]
- Wei, M.K.; Song, L.; Yuan, X.T.; Li, H.D.; Ji, H.; Sun, J. Dietary supplementation with a PPARγ agonist promotes adipocyte hyperplasia and improves high-fat diet tolerance and utilization in grass carp (Ctenopharyngodon idellus). Aquaculture 2024, 578, 740081. [Google Scholar] [CrossRef]
- Zhu, H.; Qiang, J.; He, J.; Tao, Y.; Bao, J.; Xu, P. Physiological parameters and gut microbiome associated with different dietary lipid levels in hybrid yellow catfish (Tachysurus fulvidraco♀× Pseudobagrus vachellii♂). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 37, 100777. [Google Scholar] [CrossRef]
- Lu, K.L.; Xu, W.N.; Li, J.Y.; Li, X.F.; Huang, G.Q.; Liu, W.B. Alterations of liver histology and blood biochemistry in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish. Sci. 2013, 79, 661–671. [Google Scholar] [CrossRef]
- Tao, Y.F.; Qiang, J.; Bao, J.W.; Chen, D.J.; Yin, G.J.; Xu, P.; Zhu, H.J. Changes in Physiological Parameters, Lipid Metabolism, and Expression of MicroRNAs in Genetically Improved Farmed Tilapia (Oreochromis niloticus) With Fatty Liver Induced by a High-Fat Diet. Front. Physiol. 2018, 9, 1521. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Li, T.; Wan, R.; Sha, L. Cordycepin attenuates high-fat diet-induced non-alcoholic fatty liver disease via down-regulation of lipid metabolism and inflammatory responses. Int. Immunopharmacol. 2021, 91, 107173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yan, Y.; Tian, H.; Jiang, G.; Li, X.; Liu, W. Resveratrol supplementation improves lipid and glucose metabolism in high-fat diet-fed blunt snout bream. Fish Physiol. Biochem. 2018, 44, 163–173. [Google Scholar] [CrossRef]
- Yang, L.; Liu, M.; Zhao, M.; Zhi, S.; Zhang, W.; Qu, L.; Xiong, J.; Yan, X.; Qin, C.; Nie, G.; et al. Dietary Bile Acid Supplementation Could Regulate the Glucose, Lipid Metabolism, and Microbiota of Common Carp (Cyprinus carpio L.) Fed with a High-Lipid Diet. Aquac. Nutr. 2023, 2023, 9953927. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, I.; Solís-Muñoz, P.; Fernández-Moreira, D.; Grau, M.; Colina, F.; Muñoz-Yagüe, T.; Solís-Herruzo, J.A. High-fat diet decreases activity of the oxidative phosphorylation complexes and causes nonalcoholic steatohepatitis in mice. Dis. Models Mech. 2014, 7, 1287–1296. [Google Scholar] [CrossRef]
- Pérez-Carreras, M.; Del Hoyo, P.; Martín, M.A.; Rubio, J.C.; Martı́n, A.; Castellano, G.; Colina, F.; Arenas, J.n.; Solis-Herruzo, J.A. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 2003, 38, 999–1007. [Google Scholar] [CrossRef]
- García-Ruiz, I.; Rodríguez-Juan, C.; Díaz-Sanjuan, T.; del Hoyo, P.; Colina, F.; Muñoz-Yagüe, T.; Solís-Herruzo, J.A. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology 2006, 44, 581–591. [Google Scholar] [CrossRef]
- Heydemann, A.; González-Vega, M.; Berhanu, T.K.; Mull, A.J.; Sharma, R.; Holley-Cuthrell, J. Hepatic Adaptations to a High Fat Diet in the MRL Mouse Strain are Associated with an Inefficient Oxidative Phosphorylation System. Jacobs J. Diabetes Endocrinol. 2016, 2, 013. [Google Scholar]
- Li, C.X.; Chen, L.L.; Li, X.C.; Ng, K.T.-P.; Yang, X.X.; Lo, C.M.; Guan, X.Y.; Man, K. ApoA-1 accelerates regeneration of small-for-size fatty liver graft after transplantation. Life Sci. 2018, 215, 128–135. [Google Scholar] [CrossRef]
- Thoma, C.; Day, C.P.; Trenell, M.I. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: A systematic review. J. Hepatol. 2012, 56, 255–266. [Google Scholar] [CrossRef]
- Pospisilik, J.A.; Knauf, C.; Joza, N.; Benit, P.; Orthofer, M.; Cani, P.D.; Ebersberger, I.; Nakashima, T.; Sarao, R.; Neely, G. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 2007, 131, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, T.; Zheng, S.; Wu, G. Hepatic glucose metabolism and its disorders in fish. In Recent Advances in Animal Nutrition and Metabolism; Springer: Cham, Switzerland, 2022; pp. 207–236. [Google Scholar]
- Kim, C.-H.; Youn, J.H.; Park, J.-Y.; Hong, S.K.; Park, K.S.; Park, S.W.; Suh, K.I.; Lee, K.-U. Effects of high-fat diet and exercise training on intracellular glucose metabolism in rats. Am. J. Physiol.-Endocrinol. Metab. 2000, 278, E977–E984. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, R.; Koike, E.; Win-Shwe, T.-T.; Yamamoto, M.; Takano, H. Impaired lipid and glucose homeostasis in hexabromocyclododecane-exposed mice fed a high-fat diet. Environ. Health Perspect. 2014, 122, 277–283. [Google Scholar] [CrossRef]
- Wilson, J.E. Isozymes of mammalian hexokinase: Structure, subcellular localization and metabolic function. J. Exp. Biol. 2003, 206, 2049–2057. [Google Scholar] [CrossRef]
- Figueiredo-Silva, A.C.; Panserat, S.; Kaushik, S.; Geurden, I.; Polakof, S. High levels of dietary fat impair glucose homeostasis in rainbow trout. J. Exp. Biol. 2012, 215, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.A.; Owen, G.I. Glucose transporters: Expression, regulation and cancer. Biol. Res. 2002, 35, 9–26. [Google Scholar] [CrossRef]
- Seyer, P.; Vallois, D.; Poitry-Yamate, C.; Schütz, F.; Metref, S.; Tarussio, D.; Maechler, P.; Staels, B.; Lanz, B.; Grueter, R. Hepatic glucose sensing is required to preserve β cell glucose competence. J. Clin. Investig. 2013, 123, 1662–1676. [Google Scholar] [CrossRef]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Sokolovska, J.; Isajevs, S.; Rostoka, E.; Sjakste, T.; Trapiņa, I.; Ošiņa, K.; Paramonova, N.; Sjakste, N. Changes in glucose transporter expression and nitric oxide production are associated with liver injury in diabetes. Cell Biochem. Funct. 2015, 33, 366–374. [Google Scholar] [CrossRef]
- Karim, S.; Adams, D.H.; Lalor, P.F. Hepatic expression and cellular distribution of the glucose transporter family. World J. Gastroenterol. 2012, 18, 6771–6781. [Google Scholar] [CrossRef]
- Jha, D.; Mitra Mazumder, P. High fat diet administration leads to the mitochondrial dysfunction and selectively alters the expression of class 1 GLUT protein in mice. Mol. Biol. Rep. 2019, 46, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, Y.; Pan, M.; Li, X.; Huang, D.; Liu, Y.; Wu, C.; Zhang, W.; Mai, K. Functions of forkhead box O on glucose metabolism in abalone Haliotis discus hannai and its responses to high levels of dietary lipid. Genes 2021, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Q.; Huang, Z.; Jiang, Q. Adropin inhibited tilapia hepatic glucose output and triglyceride accumulation via AMPK activation. J. Endocrinol. 2020, 246, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, H.; Li, H.; Guo, J.; Wang, J.; Zhang, L. Potential role of glucosamine-phosphate N-acetyltransferase 1 in the development of lung adenocarcinoma. Aging 2021, 13, 7430. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Pérez-Felpete, N.; Fernández-Fernández, C.; Donapetry-García, C.; Pazos-García, C. Liver glucose metabolism in humans. Biosci. Rep. 2016, 36, e00416. [Google Scholar] [CrossRef]
- Yu, S.; Meng, S.; Xiang, M.; Ma, H. Phosphoenolpyruvate carboxykinase in cell metabolism: Roles and mechanisms beyond gluconeogenesis. Mol. Metab. 2021, 53, 101257. [Google Scholar] [CrossRef]
- Ye, Q.; Liu, Y.; Zhang, G.; Deng, H.; Wang, X.; Tuo, L.; Chen, C.; Pan, X.; Wu, K.; Fan, J. Deficiency of gluconeogenic enzyme PCK1 promotes metabolic-associated fatty liver disease through PI3K/AKT/PDGF axis activation in male mice. Nat. Commun. 2023, 14, 1402. [Google Scholar] [CrossRef]
- Matsumoto, T.; Terai, S.; Oishi, T.; Kuwashiro, S.; Fujisawa, K.; Yamamoto, N.; Fujita, Y.; Hamamoto, Y.; Furutani-Seiki, M.; Nishina, H.; et al. Medaka as a model for human nonalcoholic steatohepatitis. Dis. Models Mech. 2010, 3, 431–440. [Google Scholar] [CrossRef]
- Lu, K.L.; Xu, W.N.; Wang, L.N.; Zhang, D.D.; Zhang, C.N.; Liu, W.B. Hepatic beta-oxidation and regulation of carnitine palmitoyltransferase (CPT) I in blunt snout bream Megalobrama amblycephala fed a high fat diet. PLoS ONE 2014, 9, e93135. [Google Scholar] [CrossRef]
- Lu, K.L.; Wang, L.N.; Zhang, D.D.; Liu, W.B.; Xu, W.N. Berberine attenuates oxidative stress and hepatocytes apoptosis via protecting mitochondria in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish Physiol. Biochem. 2017, 43, 65–76. [Google Scholar] [CrossRef]
- Mantha, L.; Palacios, E.; Deshaies, Y. Modulation of triglyceride metabolism by glucocorticoids in diet-induced obesity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1999, 277, R455–R464. [Google Scholar] [CrossRef] [PubMed]
- Peckett, A.J.; Wright, D.C.; Riddell, M.C. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism 2011, 60, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Adar, T.; Ben Ya’acov, A.; Shabat, Y.; Mizrahi, M.; Zolotarov, L.; Lichtenstein, Y.; Ilan, Y. Steroid-mediated liver steatosis is CD1d-dependent, while steroid-induced liver necrosis, inflammation, and metabolic changes are CD1d-independent. BMC Gastroenterol. 2022, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ma, F.; Guan, M. Role of steroid hormones in the pathogenesis of nonalcoholic fatty liver disease. Metabolites 2021, 11, 320. [Google Scholar] [CrossRef]
- Merrick, W.C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol. Rev. 1992, 56, 291–315. [Google Scholar] [CrossRef]
- Deldicque, L.; Cani, P.D.; Philp, A.; Raymackers, J.-M.; Meakin, P.J.; Ashford, M.L.J.; Delzenne, N.M.; Francaux, M.; Baar, K. The unfolded protein response is activated in skeletal muscle by high-fat feeding: Potential role in the downregulation of protein synthesis. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E695–E705. [Google Scholar] [CrossRef]
- Oie, S.; Matsuzaki, K.; Yokoyama, W.; Tokunaga, S.; Waku, T.; Han, S.-I.; Iwasaki, N.; Mikogai, A.; Yasuzawa-Tanaka, K.; Kishimoto, H.; et al. Hepatic rRNA Transcription Regulates High-Fat-Diet-Induced Obesity. Cell Rep. 2014, 7, 807–820. [Google Scholar] [CrossRef]



| Gene Name | Primer Sequence (5′-3′) | GenBank Number/References | Product Length (bp) | Tm (°C) | Amplification Efficiency (%) |
|---|---|---|---|---|---|
| Glucosamine-phosphate N-acetyltransferase 1 (gnpnat1) | F: GAAGTCGTCGTCAGCGATGT | XM_003437497.4 | 118 | 60.45 | 97.3 |
| R: TGGGTGCACATTCAAGAGTGA | 59.86 | ||||
| Glucose transporter 1 (glut1) | F: AGTCTGCAATCAACTGGCCTC | FJ914657.1 | 249 | 60.61 | 98.6 |
| R: CCCATCTGGTGGAGTGACATAG | 59.9 | ||||
| ATP binding cassette subfamily F member 2 (abcf2) | F: GACCCAATGGAGCTGGGAAA | XM_005448995.3 | 131 | 59.96 | 97.9 |
| R: CAGTTGCTCAGTCAGGTGCT | 60.25 | ||||
| Glucokinase (gck) | F: CTGTGACATTGTGCGTCTGG | XM_003451020.5 | 101 | 59.49 | 99.3 |
| R: GTCTCTCCCGCATCAGGTTG | 60.46 | ||||
| Phosphoenolpyruvate carboxykinase 1 (pck1) | F: CGCATTCTGGACTGGATGTTC | XM_003448375.4 | 181 | 59.33 | 99.7 |
| R: TCCTGATCTCATCCACCTCCC | 60.41 | ||||
| Glutamine synthase a (gs) | F: AGCTACCACATTCGTGCCTAC | NM_001279668.1 | 139 | 60.13 | 101.2 |
| R: TACGAGGAATGCGAATGCTGG | 60.81 | ||||
| NADH-cytochrome b5 reductase 2 (cbr2) | F: ATCGCTGGTGGAACAGGTATC | XM_003439423.3 | 200 | 59.86 | 98.8 |
| R: TGTGGAGGTTTGTCCAGTGT | 59.08 | ||||
| Cytochrome c oxidase subunit I (cox1) | F: GGCCGGGGTGTCATCTATTT | NC_013663 | 154 | 59.82 | 101.6 |
| R: GGCAAGAACGGGTAGGGATAG | 59.93 | ||||
| Ubiquitin-conjugating enzyme (ubce) | F: CTCTCAAATCAATGCCACTTCC | [38] | 130 | 57.63 | 103.8 |
| R: CCCTGGTGGAGGTTCCTTGT | 61.43 |
| Parameters | Ctr Group | HFD Group | p-Value |
|---|---|---|---|
| GPT (U/L) | 13.17 ± 1.26 | 25.66 ± 2.07 | <0.001 |
| GOT (U/L) | 6.92 ± 1.35 | 15.02 ± 2.67 | 0.019 |
| TG (mmol/L) | 1.89 ± 0.08 | 3.60 ± 0.41 | 0.001 |
| TC (mmol/L) | 6.49 ± 0.55 | 19.72 ± 2.18 | <0.001 |
| Samples | Raw Reads | Clean Reads | Q20 (%) | Q30 (%) | GC (%) | Total Mapping Ratio (%) |
|---|---|---|---|---|---|---|
| Ctr-1 | 41,161,756 | 40,460,582 (98.3%) | 98.17 | 94.28 | 50.01 | 89.45 |
| Ctr-2 | 51,320,144 | 50,572,504 (98.54%) | 98.30 | 94.58 | 50.04 | 87.50 |
| Ctr-3 | 43,276,462 | 42,573,850 (98.38%) | 98.23 | 94.41 | 50.39 | 88.17 |
| HFD-1 | 32,736,674 | 32,146,546 (98.2%) | 98.01 | 93.85 | 50.31 | 89.30 |
| HFD-2 | 41,827,178 | 41,095,560 (98.25%) | 98.21 | 94.36 | 50.09 | 88.43 |
| HFD-3 | 42,561,088 | 41,819,868 (98.26%) | 98.06 | 94.02 | 50.69 | 87.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, R.; Hou, Y.; Zhou, L.; Zhang, L.; Li, B.; Zhu, J. Comparative Transcriptome Analysis Reveals the Impact of a High-Fat Diet on Hepatic Metabolic Function in Tilapia (Oreochromis niloticus). Animals 2024, 14, 3204. https://doi.org/10.3390/ani14223204
Jia R, Hou Y, Zhou L, Zhang L, Li B, Zhu J. Comparative Transcriptome Analysis Reveals the Impact of a High-Fat Diet on Hepatic Metabolic Function in Tilapia (Oreochromis niloticus). Animals. 2024; 14(22):3204. https://doi.org/10.3390/ani14223204
Chicago/Turabian StyleJia, Rui, Yiran Hou, Linjun Zhou, Liqiang Zhang, Bing Li, and Jian Zhu. 2024. "Comparative Transcriptome Analysis Reveals the Impact of a High-Fat Diet on Hepatic Metabolic Function in Tilapia (Oreochromis niloticus)" Animals 14, no. 22: 3204. https://doi.org/10.3390/ani14223204
APA StyleJia, R., Hou, Y., Zhou, L., Zhang, L., Li, B., & Zhu, J. (2024). Comparative Transcriptome Analysis Reveals the Impact of a High-Fat Diet on Hepatic Metabolic Function in Tilapia (Oreochromis niloticus). Animals, 14(22), 3204. https://doi.org/10.3390/ani14223204







