Simple Summary
The increasing number of dogs in towns worldwide may be increasing the risk of environmental contamination by parasites whose growth forms are present in dogs’ feces. Canine, soil-transmitted helminths (cSTHs), most of which have a proven zoonotic potential, are particularly dangerous. In this study, we investigated the presence of cSTH eggs in dogs’ feces left in city parks and dog parks. This study also showed that the presence of dogs’ feces in public areas is still a problem. We observed that dog owners did not pick up their dog’s feces, even though cleaning after one’s dog during walks may be a simple and effective way of limiting the spread of parasitic invasions within the environment.
Abstract
A constant increase in dog numbers, especially in large towns, has been observed recently. The presence of dogs in urban spaces increases the risk of pollution by dogs’ feces, which may contain growth forms of parasites including canine, soil-transmitted helminths (cSTHs), most of which have a proven zoonotic potential. This study assessed the frequency of occurrence and estimated the potential risk associated with the presence of cSTHs in dogs’ feces left uncollected in urban areas. The study material consisted of 200 fecal samples obtained from city and dog parks situated in selected Warsaw districts. Each fecal sample was processed using the flotation technique. Eggs of cSTHs, including Toxocara canis, Toxascaris leonina, Trichuris vulpis, and hookworms from the Ancylostomatidae family were found in 23 (11.5%) of the examined fecal samples. The most prevalent species were hookworms from the family Ancylostomatidae (8%). The presence of parasites was confirmed in 14 out of 20 studied locations (70%), including eight city parks (72.7%) and six dog parks (66.7%). City and dog parks did not differ significantly in the frequency of parasite occurrence. This study indicated that dogs’ feces, left uncollected, may cause environmental contamination with cSTHs. It also indicated that the presence of dogs’ feces in public areas and the associated presence of parasites is still a problem.
1. Introduction
About 85 million domestic dogs are estimated to inhabit Europe [1]. Having an animal gives people the chance to improve their physical and mental health [2,3]. This, however, also increases the risk of animal-borne diseases [4], particularly when many dog owners are not aware of the potential threats [5].
The constantly increasing number of dogs is a serious hygienic, epidemiological, and ecological problem in towns worldwide. Dogs can be a source of pathogens, including parasites [6]. An important factor in the spread of parasites and in the infection of subsequent hosts is the possibility of finding potentially new hosts by means of invasion [7]. Canine, soil-transmitted helminths (cSTHs) are a group of parasites that is present in dogs; they require appropriate external environmental conditions and time to make their growth stages invasive for the next host. Some may cause diseases in people (e.g., Toxocara canis, Ancylostoma caninum), while others do not harm people but do affect the health of other animals (e.g., Toxascaris leonina) [8,9]. The zoonotic potential of Trichuris vulpis is still controversial [10].
The growth forms of cSTH spread in the surface soil layer by way of dog feces that contaminate these areas [3,11,12]. Under favorable climatic conditions, the invasive eggs of cSTHs may survive for years, which is particularly dangerous in places frequented by people, like gardens, parks, and playgrounds [8,13]. Infection happens through the accidental consumption of invasive eggs present in the environment [11,14]. Therefore, environmental contamination by dog feces containing the growth stages of parasites is the main source of infection in people and animals [15].
In towns, dogs defecate in green areas that are also visited by people. Green areas may include various recreational, social, and sport locations used by both children and adults [16,17]. If dog owners do not remove their pets’ feces, these areas may become a source of contagious factors, including cSTHs that infect other dogs, wild animals, and people [18].
Prophylaxis for pets may improve the health status of dogs and restrict the spread of animal-borne diseases among people [19,20]. The occurrence of parasites in dogs depends on many factors, including the natural resistance of a given individual, the natural habitat of the animals, and the actions undertaken by owners. The last exerts an important effect on limiting parasites in dogs. Therefore, one of the significant aspects of prophylaxis is educating animal owners [21]. There are, however, factors restricting prophylactic actions by dog owners: for example, poor education, limited financial resources, living in poor areas, and limited access to veterinary services [22,23,24].
One of the important ways for preventing the occurrence of parasites in dogs is the removal of pets’ feces. The proper removal by owners of dog excrement in public places may decrease the environmental contamination caused by the eggs of parasites [25]. In this way, infections can be prevented in people, particularly in children, whose normal behavior (such as touching their face with their hands) make them most vulnerable to infection [7,26]. Unfortunately, despite a range of educational activities, uncollected dog feces are still a serious problem in urban areas.
An additional problem is created by dogs not being under human control. A lack of veterinary supervision and free roaming result in a significant potential for the transmission of parasitic diseases [27]. Studies on the presence of parasites in dogs usually pertain to domestic animals, but these studies do not consider the degree of infection in stray dogs [28].
The application of simple and effective prophylactic actions is important in preventing parasitic invasions. Such actions may include the use of gloves when removing dog feces, preventing animals from accessing sandpits, and observing basic hygienic rules (such as washing hands and food) [29].
Veterinarians can play a significant role in restricting the occurrence of parasites in dogs. By educating their clients about the risks associated with parasitic diseases and about effective methods for their prevention, they can contribute to increasing social awareness of the need for cleaning up dogs’ excrement, having regular parasitological tests, and de-worming pets if necessary [30,31]. A statistically significant relationship was found between dog owners who visited veterinary clinics once or twice a year and who properly removed their dog’s feces, and those owners who used veterinary services only in an emergency [32].
The main method employed for the treatment and prevention of parasites in dogs is the application of de-worming remedies [33]. The risk of infection by parasites increases in dogs that are not regularly de-wormed [34,35,36]. It is, however, the animal owners’ responsibility as to whether their pets are regularly checked and treated. Despite various de-worming agents being readily available, their application is limited due to a lack of education about the use of the drugs and the risk factors associated with the transmission of parasites [37]. In a questionnaire survey carried out among dog owners in Ireland, 52% of respondents declared that they de-wormed their animals once or twice a year or not at all. Moreover, 13% of respondents living in towns admitted that they had never treated their dogs [31]. The so-called prophylactic use of de-worming agents is also common practice. This approach increases the risk of parasites developing a resistance to drugs [38]. The prophylactic de-worming of dogs, even three to four times a year, does not guarantee total protection from parasites [39,40]; therefore, an individual approach to each case, including taking into consideration the risk and physiological status of the dog, is important [38]. Educating dog owners about the importance of de-worming may positively affect owners’ behavior and decrease the risk of infection [25,32,35]. A statistically significant, positive relationship was found between the frequency of visits to veterinary clinics and the dog owners’ adherence to the rules for de-worming dogs. This result suggests that veterinarians may affect the dog owners’ awareness of the risks of parasite infection [32].
The aim of this study was to estimate the frequency of occurrence and determine the risk associated with the presence of cSTHs in dog feces in city parks and dog parks in the northwestern districts of Warsaw.
2. Materials and Methods
2.1. Study Area
The study material was collected from public green areas. The 20 selected areas included 11 city parks and nine dog parks (green fenced areas where dogs can roam without a lead or muzzle, under the supervision of their owners) (Table 1). The study areas were localized within the administrative boundaries of four northwestern districts of Warsaw: Bielany, Bemowo, Żoliborz, and Wola.

Table 1.
The number of selected city parks and dog parks in particular districts of Warsaw.
The surface area of the district, the percentage share of green areas based on data from the Statistics Poland 2021 (https://bdl.stat.gov.pl/bdl/start, accessed on 15 October 2021), and the availability of city parks and dog parks were considered when selecting the number of green areas in each district. According to these data, Bielany had the largest surface area (32.34 km2, with 11.69% occupied by green areas, including 7 city parks), followed by Bemowo (24.95 km2, with 10.37% occupied by green areas, including 4 city parks), then Wola (19.26 km2, with 17.2% occupied by green areas, including 5 city parks), and finally Żoliborz (8.47 km2, with 29.69% occupied by green areas, including 6 city parks).
2.2. Sample Collection and Coprological Analysis
From July to October 2022, 200 fresh canine fecal samples were collected—10 samples from each park. The freshly collected samples were packed in plastic bags with labels stating the location, the number of the sample, and the date. The samples were placed in a refrigerator and later examined in the laboratory. Each sample was processed using a flotation technique (saturated NaCl solution, 1.20 specific gravity) [41,42]. Each sample was microscopically examined at 100× and 400× magnifications. Egg identification was performed using morphological references [43]. Samples were classified as positive if the presence of eggs was confirmed [44].
2.3. Data Analysis
Basic parasitological parameters were calculated and defined according to Bush [45]. The prevalence was estimated using the percentage of positive samples among all tested samples of dog feces. The software package Statistica v. 13.1 for Windows was used for the statistical analysis of the data (StatSoft, Inc., Tulsa, OK, USA, 2013). Attributes were represented via frequencies and percentages. Chi-squared (χ2) frequency and Fisher’s tests were applied to analyze the differences between attributes. The relationships between the prevalence of the examined dog feces samples, the nature and purpose of the analyzed parks (city parks, dog parks), and their location in various districts of Warsaw (Żoliborz, Bielany, Wola and Bemowo) were examined. A p-value of less than 0.05 (p < 0.05) was considered statistically significant.
3. Results
Microscopic analyses revealed the presence of eggs of Toxocara spp., Toxascaris leonina, Trichuris vulpis, and hookworms of the family Ancylostomatidae (Table 2).

Table 2.
The prevalence of canine soil-transmitted helminths (cSTHs) found in dog fecal samples.
The most prevalent species were hookworms from the family Ancylostomatidae (8%), followed by Trichuris vulpis (3%) (Table 2).
From among the 200 analyzed fecal samples, 23 (11.5%) contained the eggs of parasites. Eleven of these samples (10%) were collected from city parks and 12 (13.4%) from dog parks. No statistically significant difference was found between the frequency of the occurrence of dog parasites (infected and healthy dogs) and the types of parks analyzed (χ2 = 0.44, df = 1, p = 0.658) (Table 2); hence, no difference in parasite prevalence between city parks and dog parks was confirmed.
Hookworms from the family Ancylostomatidae had the greatest share among the noted cSTH species, for both city parks and dog parks (5.45% and 11.1%, respectively; Table 2).
The presence of parasites was noted in 14 out of the 20 studied locations (70%), including eight out of 11 (72.7%) city parks and six out of nine (66.7%) dog parks. No significant differences were noted in the presence of parasites in the dogs’ feces between the particular study districts in Warsaw (χ2 = 7.57, df = 3, p = 0.056) (Table 3). Three samples showed one type of co-infection (Table 3).

Table 3.
Prevalence of canine, soil-transmitted helminths (cSTHs) found in dog fecal samples collected in the examined districts of Warsaw.
Wola was the district where the highest percentage of infected dogs was found. In both city parks (χ2 = 10.69, df = 3, p = 0.014) and dog parks (χ2 = 66.67, df = 3, p = 0.000), the greatest prevalence values (20% and 40.0%, respectively) were noted in Wola (Table 3). Moreover, in the park areas of the district, the frequency of representatives of Ancylostomatidae gen. sp. was significantly higher compared to other parasites (Table 4).

Table 4.
Evaluation of the prevalence and the number of analyzed dog feces samples (n) in city parks and dog parks in the district of Wola.
The prevalence of Ancylostomatidae in the analyzed city parks of the district of Wola was about 15.0% (χ2 = 8.73, df = 2, p = 0.013), whereas in the dog parks, the prevalence was 35.0% (χ2 = 37.20, df = 2, p = 0.000) (Figure 1). The difference in prevalence between the two types of sites was statistically significant (χ2 = 10.67, df = 1, p = 0.002). Therefore, a higher percentage of hosts infected by Ancylostomatidae was noted for dog parks in the district of Wola. Of note, Toxocara spp. was excluded from statistical analysis since it was found in only one dog park and in one host, and was not found at all in city parks.
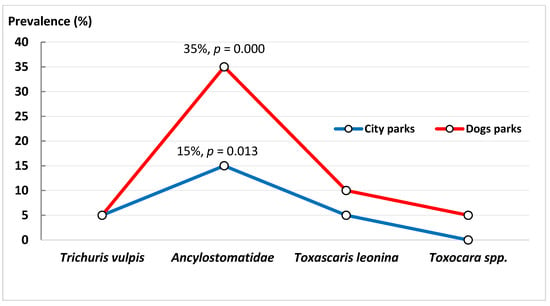
Figure 1.
The relationship between the percentage of infected hosts and the type of park in the Wola district. A significantly higher prevalence of representatives of Ancylostomatidae gen. sp. was detected compared to other identified parasites.
4. Discussion
The overall apparent prevalence of cSTH eggs that contaminated fecal samples in Warsaw was 11.5%. In Poland, similar results were obtained in earlier studies in Warsaw (18.8%) [46] and Olsztyn (19%) [47]. However, again in Poland, a higher prevalence of parasites was found in Łódź (29.5%) [48] and in Szczecin (34.8%) [49]. According to information from the available literature, different degrees of contamination by helminth eggs and larvae determined from canine feces have been established for Europe—from 8.6% in Italy [50] to 71.4% in Spain [51] and 75.7% in Albania [52]. The problem in comparing such results is that studies in a given area are performed rarely and comparisons between far-distant populations may be not reliable.
The differences in research results across the world may be attributed to various factors, such as different diagnostic techniques [53] or the socioeconomic status of the countries where the research was conducted (veterinary control and animal care, hygienic standards) [13,54]. Taking into account that the samples analyzed here were from the environment and that the status of each definitive host was unknown, associating results with the dogs’ characteristics (e.g., age, gender, breed, and underlying living conditions) and with the owners’ conduct of care, i.e., veterinary care and antiparasitic treatment, may not be possible [55,56]. Moreover, there is a chance that the analyzed feces samples were from other, similar-sized animals like foxes, whose presence has been noted in Warsaw.
Numerous studies on dog feces describe discrepancies in the diagnostic efficacy of different parasitological techniques [57,58]. According to Stefański [43], none of the techniques described in the literature can ensure the detection of all types of parasites. The procedure used in this study for detection of cSTH eggs was the common fecal flotation method in a saturated sodium chloride solution (NaCl) with a specific gravity of 1.20 g/mL [41,42]. This method is simple, relatively inexpensive, and effective, particularly for recovering light eggs (e.g., hookworms from the family Ancylostomatidae) [43]. However, it also has some limitations that influence its accuracy. Testing for the presence of heavy (e.g., ascarids: Toxocara spp. and Toxascaris leonina; trichurids: Trichuris spp.) or operculate (e.g., flukes) eggs often produces false negative results, because heavy eggs do not float well [43]. Moreover, saturated NaCl solution causes the lysis of most intestinal protozoa cysts (e.g., Giardia sp.) [59]. Therefore, it is necessary to take into account that in this study, the prevalence of cSTHs may have been underestimated.
The occurrence of cSTH eggs (Toxocara spp., Toxascaris leonina, Trichuris vulpis, and hookworms of the family Ancylostomatidae) was noted in this study. If we look at studies conducted worldwide, we can see that these taxa are globally most frequent in dogs and that they are the most common contaminants of the urban areas [11,13,14,35,55,56,60,61,62,63]. Apart from the effect on the dogs’ health, most cSTHs also have a zoonotic potential [64].
Analyzed samples most often contained the eggs of hookworms of the family Ancylostomatidae (8.5%). The prevalence of hookworms of the family Ancylostomatidae in dogs in Poland has been reported as being 3.0–7.4% in central Poland [48,65], 13.1–16.2% in northern and northwestern Poland [49,66], and 22.2% in central and southern Poland [67]. Globally, hookworms are the most-often diagnosed internal parasites in dogs [68]. Some studies have found an increased risk of hookworm infection in dogs when dog feces have not been disposed of in a timely or proper way, thus contributing to the development of infective hookworm larvae and environmental contamination [34,35,69].
While hookworms were frequently reported in this study, the zoonotic roundworm Toxocara spp. was detected with a prevalence of 0.5%. Eggs of Toxocara spp. were found in one fecal sample from a dog park in the district of Wola. A similar result was obtained in studies carried out in Australia, where T. canis. was detected with a prevalence of less than 0.5% in dogs [35]. Although its prevalence appears negligible, a significantly higher rate of egg laying occurs in puppies and dogs less than 1 year old, while confirmed infections are less common in adult dogs owing to the somatic migration of larvae [70]. Despite the relatively low prevalence in canine fecal samples, seroprevalence of toxocariasis in humans in Australia has been estimated at 7%, indicating that people’s exposure to the highly resistant infectious stage of Toxocara spp. is high [71]. Moreover, in the current study, due to the low number of city parks and dog parks studied (N = 20), one cannot estimate the degree of contamination by the eggs of Toxocara spp. in the public areas in Warsaw. Further studies on a greater number of locations across all districts of the city are needed. In previous studies conducted in Poland, higher results have been obtained: 4.1–16.8% in central Poland [48,65], 6.5–23.4% in northern and northwestern Poland [49,66], and 7.2% in central and southern Poland [67].
T. canis is a common roundworm parasite found in dogs and has a worldwide distribution [72]. There are many studies on the prevalence of Toxocara infection in dogs globally. The prevalence of T. canis in dogs has been reported as being 1.2% in Australia [35], 4.4% in the Netherlands [73], 4.6% in Belgium [74], and 6.1% in Germany [56]. In some surveys, in countries such as Portugal, Nigeria, India, and China, the prevalence was found to be as high as 51–100% in puppies and 1–45% in adult dogs [72,75,76,77].
Geographic location, outdoor access, and behavior while outdoors are also key factors influencing parasitic infection of domestic pets. The risk of transmission of Toxocara eggs was found to increase in free-roaming, unleashed dogs [78]. Dogs that roamed regularly transferred the eggs of T. canis on their paws, while their owners did the same on shoes [79]. Coprophagy and rolling around in the feces of other animals may also increase the risk of infection by T. canis [80]. Hence, the access of T. canis-infected dogs to parks, playgrounds, and recreational spaces has been identified as the main factor influencing the global prevalence of human toxocariasis [81]. However, the influence of this factor could drop significantly upon compliance with a de-worming regimen and cleaning up dog feces [82].
If ingested, the eggs of T. canis can pose a health risk to humans, as well as being of veterinary significance [83]. Toxocariasis that is caused by T. canis is the most common animal-borne parasitic disease found in humans [84]. Recent epidemiological research has estimated that approximately 1.4 billion people worldwide, particularly in subtropical and tropical regions, are infected with, or have been exposed to, Toxocara sp. [85]. The reported seroprevalence of the infection in different geographic regions varies from 2.4 to 92.8% worldwide [86,87]. Some human risk factors, especially in children, have highlighted the importance of soil and it being the main source of infection in the spread of toxocariasis [88]. Environmental contamination with Toxocara eggs is common in public places in most countries [89,90,91].
The presence of Toxascaris leonina (1.5%) was also noted in this study. In Poland, similar results were obtained in earlier studies for Warsaw (0.7%) [65], Łódź (1.1%) [48], and Szczecin (2.3%) [49]. Generally, T. leonina displays lower prevalence in dogs than T. canis [92]. However, in this study, T. leonina was found more often than T. canis, although both species were characterized by low prevalence. This very low prevalence of T. leonina is consistent with what has been estimated for dogs worldwide (2.9%), and can be attributed to the limited routes for T. leonina transmission among dogs compared to T. canis [92]. T. leonina is one of the cSTH species devoid of zoonotic potential. The accidental infection of people is, however, possible—several cases of toxocariasis have been reported globally [93,94].
Trichuris vulpis is common in Europe, being one of the most frequent alimentary tract parasites in dogs. Depending on the country and the population being studied, from 0.2% to as many as 60% of dogs are infected [95]. T. vulpis eggs survive for long periods in the environment, especially in temperate climates [10], where they become a constant source of infection and often lead to high infection rates in dogs. Reports from Belgium and Holland have found that T. vulpis is the second most common helminth [96,97]. In our study the prevalence of T. vulpis in dogs was 3%, which was similar to the prevalence found by earlier studies conducted in Warsaw (3.1%) [65]. A survey in Spain found that 1.66% of dogs were infected with T. vulpis [51]; other studies found infection rates of 10–18% in Italy [98], 10.93% in Serbia [61], and 13–48% in Hungary [99]. Older dogs tend to be more often infected with T. vulpis [100]. T. vulpis has previously been reported as a cause of visceral larva migrans (VLM) and as an intestinal parasite in humans. However, it is not commonly considered a zoonotic nematode in pets, despite a few reported cases of human infection [10,100,101].
In the current study, the presence of cSTH eggs was noted in 14 out of the 20 studied locations (70%), including eight out of 11 city parks (72.7%) and six out of nine dog parks (66.7%). Similar results were obtained in studies performed in other European countries and in Australia, where cSTHs were found in almost half of the urban green areas being studied [16,17,62]. In the current study, no statistically significant differences were found in the frequency of parasites between city parks and dog parks. However, due to the dog parks having a much smaller area compared with that of the city parks, the dogs’ risk of infection with parasites may be higher in the former.
5. Conclusions
The presented study indicates that dog feces in city parks and dog parks in selected districts of northwestern Warsaw may be the reason for environmental contamination with cSTHs, most of which have a proven zoonotic potential. Performed studies showed that the presence of dogs’ feces in public areas and the associated presence of parasites are still a problem. Dog owners were observed not cleaning up the excrement of their animals, even though cleaning during dog walks is a simple and effective way to restrict the spread of parasitic invasions in the environment. Therefore, educational activities are necessary to increase the awareness of dog owners regarding the risks associated with the presence of parasites in public areas.
It is particularly important to carry out further studies to monitor the presence of dog parasites in public areas, especially in densely populated areas and in those visited by children. The results of such studies should be presented to local authorities. This might persuade local authorities to undertake actions aimed at protecting the environment from contamination and, consequently, limiting the risk of parasitic invasions.
Author Contributions
Conceptualization, A.T. and N.M.; methodology, A.T.; collection and analysis of samples, N.M.; formal analysis, A.T., N.M. and M.S.; writing—review and editing, A.T., N.M. and M.M.K.; visualization, A.T.; supervision, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during the study are included in this published article. The datasets used and/or analyzed in the current study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carvelli, A.; Scaramozzino, P.; Iacoponi, F.; Condoleo, R.; Marta, U. Della Size, demography, ownership profiles, and identification rate of the owned dog population in central Italy. PLoS ONE 2020, 15, e0240551. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.B. Potential Benefits of Pet Ownership in Health Promotion. J. Holist. Nurs. 1997, 15, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D. Pet roundworms and hookworms: A continuing need for global worming. Parasites Vectors 2012, 5, 91. [Google Scholar] [CrossRef]
- Overgaauw, P.; Vinke, C.M.; van Hagen, M.A.E.; Lipman, L.J.A. A One Health Perspective on the Human–Companion Animal Relationship with Emphasis on Zoonotic Aspects. Int. J. Environ. Res. Public Health 2020, 17, 3789. [Google Scholar] [CrossRef]
- Do Vale, B.; Lopes, A.P.; da Conceição-Fontes, M.; Silvestre, M.; Cardoso, L.; Coelho, A.C. A Cross-sectional Study of Knowledge on Ownership, Zoonoses and Practices among Pet Owners in Northern Portugal. Animals 2021, 11, 3543. [Google Scholar] [CrossRef] [PubMed]
- Bowman, D.D. Georgis’ Parasitology for Veterinarians, 10th ed.; Elsevier: Philadelphia, PA, USA, 2014. [Google Scholar]
- Kohansal, M.H.; Fazaeli, A.; Nourian, A.; Haniloo, A.; Kamali, K. Dogs’ Gastrointestinal Parasites and their Association with Public Health in Iran. J. Vet. Res. 2017, 61, 189–195. [Google Scholar] [CrossRef]
- Traversa, D.; Frangipane di Regalbono, A.; Di Cesare, A.; La Torre, F.; Drake, J.; Pietrobelli, M. Environmental contamination by canine geohelminths. Parasites Vectors 2014, 1, 67. [Google Scholar] [CrossRef]
- Zendejas-Heredia, P.A.; Crawley, A.; Byrnes, H.; Traub, R.J.; Colella, V. Zoonotic soil-transmitted helminths in free-roaming dogs, Kiribati. Emerg. Infect. Dis. 2021, 27, 2163–2165. [Google Scholar] [CrossRef]
- Traversa, D. Are we paying too much attention to cardio-pulmonary nematodes and neglecting old-fashioned worms like Trichuris vulpis? Parasites Vectors 2011, 4, 32. [Google Scholar] [CrossRef]
- Dado, D.; Izquierdo, F.; Vera, O.; Montoya, A.; Mateo, M.; Fenoy, S.; Galván, A.L.; García, S.; García, A.; Aránguez, E.; et al. Detection of zoonotic intestinal parasites in public parks of Spain. Potential epidemiological role of microsporidia. Zoonoses Pub. Health 2012, 59, 23–28. [Google Scholar] [CrossRef]
- Núñez, C.R.; Durán, N.R.; Barrera, G.E.M.; Barrera, E.M.; Gómez, L.G.B. Dipylidium caninum, Ancylostoma spp. and Trichuris spp. contamination in public parks in Mexico. Acta Sci. Vet. 2014, 42, 1189. [Google Scholar]
- Thevenet, P.S.; Ñancufil, A.; Oyarzo, C.M.; Torrecillas, C.; Raso, S.; Mellado, I.; Flores, M.E.; Cordoba, M.G.; Minvielle, M.C.; Basualdo, J.A. An eco-epidemiological study of contamination of soil with infective forms of intestinal parasites. Eur. J. Epidemiol. 2004, 19, 481–489. [Google Scholar] [CrossRef]
- Dubná, S.; Langrová, I.; Nápravník, J.; Jankovská, I.; Vadlejch, J.; Pekár, S.; Fechtner, J. The prevalence of intestinal parasites in dogs from Prague, rural areas, and shelters of the Czech Republic. Vet. Parasitol. 2007, 145, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Bentoinsi, B.; Meradi, S.; Ayachi, A.; Cabaret, J. Cestodes of untreated large stray dog populations in Algeria: A reservoir for herbivore and human parasitic diseases. Open Vet. Sci. J. 2009, 3, 64–67. [Google Scholar] [CrossRef]
- Smith, A.F.; Semeniuk, C.A.; Kutz, S.J.; Massolo, A. Dog-walking behaviours affect gastrointestinal parasitism in park-attending dogs. Parasites Vectors 2014, 7, 429. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Alho, A.M.; Otero, D.; Gomes, L.; Nijsse, R.; Overgaauw, P.A.M.; Madeira de Carvalho, L. Urban dog parks as sources of canine parasites: Contamination rates and pet owner behaviours in Lisbon, Portugal. J. Environ. Public Health 2017, 2017, 5984086. [Google Scholar] [CrossRef] [PubMed]
- Rahim, T.; Barrios, P.R.; McKee, G.; McLaws, M.; Kosatsky, T. Public health considerations associated with the location and operation of off-leash dog parks. J. Community Health 2018, 43, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Alho, A.M.; Lima, C.; Colella, V.; Madeira de Carvalho, L.; Otranto, D.; Cardoso, L. Awareness of zoonotic diseases and parasite control practices: A survey of dog and cat owners in Qatar. Parasites Vectors 2018, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Benavides, J.A.; Valderrama, W.; Recuenco, S.; Uieda, W.; Suzan, G.; Avila-Flores, R.; Velasco-Villa, A.; Almeida, M.; Andrade, F.A.G.; de Molina-Flores, B.; et al. Defining new pathways to manage the ongoing emergence of bat rabies in Latin America. Viruses 2020, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, M.; Sokół, R. An assessment of the relationship between the infection by intestinal parasites of dogs and cats and the breeding-veterinary awareness of the owners. Wiadomości Parazytol. 2008, 54, 245–247. (In Polish) [Google Scholar]
- Brady, S.; Norris, J.M.; Kelman, M.; Ward, M.P. Canine parvovirus in Australia: The role of socio-economic factors in disease clusters. Vet. J. 2012, 193, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vizcaíno, F.; Noble, P.J.M.; Jones, P.H.; Menacere, T.; Buchan, I.; Reynolds, S.; Dawson, S.; Gaskell, R.M.; Everitt, S.; Radford, A.D. Demographics of dogs, cats, and rabbits attending veterinary practices in Great Britain as recorded in their electronic health records. BMC Vet. Res. 2017, 13, 218. [Google Scholar] [CrossRef]
- Rigas, K.; Singleton, D.A.; Radford, A.D.; Amores-Fuster, I.; Killick, D.R. Do socioeconomic factors impact management of suspected canine multicentric lymphoma in UK first opinion practice? Vet. Rec. 2022, 191, e1319. [Google Scholar] [CrossRef]
- Harriott, L.; Gentle, M.; Traub, R.J.; Soares Magalhães, R.J.; Cobbold, R. Zoonotic and economically significant pathogens of peri-urban wild dogs across North-Eastern New South Wales and South-Eastern Queensland, Australia. Wildl. Res. 2019, 46, 212–221. [Google Scholar] [CrossRef]
- Manini, M.P.; Marchioro, A.A.; Colli, C.M.; Nishi, L.; Falavigna Guilherme, A.L. Association between contamination of public squares and seropositivity for Toxocara spp. in children. Vet. Parasitol. 2012, 188, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Kaleta, T. Dogs out of control as a global problem. Życie Wet. 2015, 90, 715–720. (In Polish) [Google Scholar]
- Khan, W.; Nisa, N.N.; Ullah, S.; Ahmad, S.; Mehmood, S.A.; Khan, M.; Ahmad, S.; Ali, W.; Ullah, H.; Anwa, K. Gastrointestinal helminths in dog feces surrounding suburban areas of Lower Dir district, Pakistan: A public health threat. Braz. J. Biol. 2020, 80, 511–517. [Google Scholar] [CrossRef]
- Własienko, A.; Kuchar, E. Diagnostics, treatment and prophylaxis of most frequent parasitic diseases in children. Lekarz POZ 2017, 3, 154–160. (In Polish) [Google Scholar]
- Golden, O.; Hanlon, A.J. Towards the Development of Day One Competences in Veterinary Behaviour Medicine: Survey of Veterinary Professionals Experience in Companion Animal Practice in Ireland. Ir. Vet. J. 2018, 71, 12. [Google Scholar] [CrossRef]
- Sherlock, C.; Holland, C.V.; Keegan, J.D. Caring for Canines: A Survey of Dog Ownership and Parasite Control Practices in Ireland. Vet. Sci. 2023, 10, 90. [Google Scholar] [CrossRef]
- Nguyen, T.; Clark, N.; Jones, M.K.; Herndon, A.; Mallyon, J.; Soares Magalhaes, R.J.; Abdullah, S. Perceptions of dog owners towards canine gastrointestinal parasitism and associated human health risk in Southeast Queensland. One Health 2021, 12, 100226. [Google Scholar] [CrossRef]
- Lynn, R.C.; Duquette, R.A. 6—Antiparasitic drugs. In Georgis’ Parasitology for Veterinarians, 11th ed.; Saunders, W.B., Bowman, D.D., Eds.; Elsevier Ltd.: Oxford, UK, 2021; pp. 286–348. [Google Scholar]
- Palmer, C.S.; Traub, R.J.; Robertson, I.D.; Hobbs, R.P.; Elliot, A.; While, L.; Rees, R.; Thompson, R.C.A. The veterinary and public health significance of hookworm in dogs and cats in Australia and the status of A. ceylanicum. Vet. Parasitol. 2007, 145, 304–313. [Google Scholar] [CrossRef]
- Palmer, C.S.; Thompson, R.C.A.; Traub, R.J.; Rees, R.; Robertson, I.D. National study of the gastrointestinal parasites of dogs and cats in Australia. Vet. Parasitol. 2008, 151, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Massetti, L.; Colella, V.; Zendejas, P.A.; Nguyen, D.; Harriott, L.; Marwedel, L.; Wiethoelter, A.; Traub, R.J. High throughput multiplex qPCRs for the surveillance of zoonotic species of canine hookworms. PLoS Neglected Trop. Dis. 2020, 14, e0008392. [Google Scholar] [CrossRef]
- Matos, M.; Alho, A.M.; Owen, S.P.; Nunes, T.; Madeira de Carvalho, L. Parasite control practices and public perception of parasitic diseases: A survey of dog and cat owners. Prev. Vet. Med. 2015, 122, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Zawiślak, J.; Swiecicka, N.; Gulda, D.; Monkiewicz, M.; Drewka, M. Deworming as a basic element of prophylactic programmes in dogs and cats. Przegl. Hodowl. 2011, 79, 12. (In Polish) [Google Scholar]
- Mangan, A.; Lawlor, A.; de Waal, T.; Aungier, S. The Prevalence of Intestinal Parasites in Dogs and Cats in the Greater Dublin Area. Vet. Irel. J. 2019, 9, 538–541. [Google Scholar]
- Sager, H.; Moret, C.; Grimm, F.; Deplazes, P.; Doherr, M.G.; Gottstein, B. Coprological Study on Intestinal Helminths in Swiss Dogs: Temporal Aspects of Anthelminthic Treatment. Parasitol. Res. 2006, 98, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Euzeby, J. Diagnostic Experimental des Helminthoses Animals; Vigot: Paris, France, 1981; Tome 1. [Google Scholar]
- Dantas-Torres, F.; Ketzis, J.; Mihalca, A.D.; Baneth, G.; Otranto, D.; Tort, G.P. TroCCAP recommendations for the diagnosis, prevention and treatment of parasitic infections in dogs and cats in the tropics. Vet. Parasitol. 2020, 283, 109–167. [Google Scholar] [CrossRef]
- Stefański, W.; Żarnowski, E. Recognition of Parasitic Invasions in Animals; Państwowe Wydawnictwo Rolnicze i Leśne: Warszawa, Poland, 1971; pp. 27–34. (In Polish) [Google Scholar]
- Blagburn, B. Diagnostic Manual on Internal Parasites of Dogs and Cats; College of Veterinary Medicine, Auburn University: Auburn, AL, USA, 2014. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Turkowicz, M.; Cielecka, D. The occurrence of intestinal nematodes in dogs near Warsaw. Wiad. Parazytol. 2002, 48, 407–411. (In Polish) [Google Scholar] [PubMed]
- Szelagiewicz, M.; Sokol, R.; Gaca, K.; Michalski, M.; Bah, M. An assessment of dogs’ worming in Olsztyn. Med. Wet. 1996, 52, 409–476. (In Polish) [Google Scholar]
- Szwabe, K.; Blaszkowska, J. Stray dogs and cats as potential sources of soil contamination with zoonotic parasites. Ann. Agric. Environ. Med. 2017, 24, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Tylkowska, A.; Pilarczyk, B.; Gregorczyk, A.; Templin, E. Gastrointestinal helminths of dogs in Western Pomerania, Poland. Wiad. Parazytol. 2010, 56, 269–276. [Google Scholar] [PubMed]
- Papini, R.; Campisi, E.; Faggi, E.; Pini, G.; Mancianti, F. Prevalence of Toxocara canis eggs in dog faeces from public places of Florence, Italy. Helminthologia 2012, 49, 154–158. [Google Scholar] [CrossRef]
- Martínez-Moreno, F.J.; Hernández, S.; López-Cobos, E.; Becerra, C.; Acosta, I.; Martínez-Moreno, A. Estimation of canine intestinal parasites in Córdoba (Spain) and their risk to public health. Vet. Parasitol. 2007, 143, 7–13. [Google Scholar] [CrossRef]
- Xhaxhiu, D.; Kusi, I.; Rapti, D.; Kondi, E.; Postoli, R.; Rinaldi, L.; Dimitrova, Z.M.; Visser, M.; Knaus, M.; Rehbein, S. Principal intestinal parasites of dogs in Tirana, Albania. Parasitol. Res. 2011, 108, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Mandarino-Pereira, A.; de Souza, F.S.; Lopes, C.W.G.; Pereira, M.J.S. Prevalence of parasites in soil and dog feces according to diagnostic tests. Vet. Parasitol. 2010, 170, 176–181. [Google Scholar] [CrossRef]
- Pipíková, J.; Papajová, I.; Šoltys, J.; Schusterová, I.; Kočišová, D.; Toháthyová, A. Segregated settlements present an increased risk for the parasite infections spread in Northeastern Slovakia. Helminthologia 2017, 54, 199–210. [Google Scholar] [CrossRef]
- Katagiri, S.; Oliveira-Sequeira, T.C.G. Prevalence of dog intestinal parasites and risk perception of zoonotic infection by dog owners in São Paulo State, Brazil. Zoonoses Public Health 2008, 55, 406–413. [Google Scholar] [CrossRef]
- Barutzki, D.; Schaper, R. Results of parasitological examinations of faecal samples from cats and dogs in Germany between 2003 and 2010. Parasitol. Res. 2011, 109, 45–60. [Google Scholar] [CrossRef]
- Coelho, W.M.D.; Gomes, J.F.; Amarante, A.F.T.; Bresciani, K.D.S.; Lumina, G.; Koshino-Shimizu, S.; Leme, D.P.; Falcão, A.X. A new laboratorial method for the diagnosis of gastrointestinal parasites in dogs. Rev. Bras. Parasitol. Vet. 2013, 22, 1–5. [Google Scholar] [CrossRef]
- Táparo, C.V.; Perri, S.H.; Serrano, A.C.; Ishizaki, M.N.; da Costa, T.P.; do Amarante, A.F. Comparison between coproparasitological techniques for the diagnosis of helminth eggs or protozoa oocysts in dogs. Rev. Bras. Parasitol. Vet. 2006, 15, 1–5. [Google Scholar]
- Coelho, W.M.D.; Gomes, J.F.; Falcao, A.X.; dos Santos, B.M.; Soares, F.A.; Suzuki, C.T.N.; do Amarante, A.F.T.; Bresciani, K.D.S. Comparative study of five techniques for the diagnosis of canine gastrointestinal parasites. Braz. J. Vet. Parasitol. 2015, 24, 223–226. [Google Scholar] [CrossRef]
- De Liberato, C.; Berrilli, F.; Odorizi, L.; Scarcella, R.; Barni, M.; Amoruso, C.; Scarito, A.; Filippo, M.M.D.; Carvelli, A.; Iacoponi, F.; et al. Parasites in stray dogs from Italy: Prevalence, risk factors and management concerns. Acta Parasitol. 2018, 63, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Kurnosova, O.P.; Arisov, M.V.; Odoyevskaya, I.M. Intestinal parasites of pets and other house-kept animals in Moscow. Helminthologia 2019, 56, 108–117. [Google Scholar] [CrossRef]
- Moro, K.K.; Abah, A.E. Epizootiology of zoonotic parasites of dogs in Abua area of Rivers State, Nigeria. Vet. Anim. Sci. 2019, 7, 100045. [Google Scholar] [CrossRef]
- Raičević, I.N.; Pavlović, T.A.; Galonja, C. Canine intestinal parasites as a potential source of soil contamination in the public areas of Kruševac, Serbia. J. Infect. Dev. Ctries. 2021, 15, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Massetti, L.; Wiethoelter, A.; McDonagh, P.; Rae, L.; Marwedel, L.; Beugnet, F.; Colella, V.; Traub, R.J. Faecal prevalence, distribution and risk factors associated with canine soil-transmitted helminths contaminating urban parks across Australia. Int. J. Parasitol. 2022, 52, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Bartosik, J.; Łojek, J.; Puchała, M.; Kaczyk, J.; Górski, P.; Długosz, E.; Zygner, W. Prevalence of intestinal parasites detected in routine coproscopic methods in dogs and cats from the Masovian voivodeship in 2012–2015. Med. Weter. 2019, 75, 293–297. [Google Scholar] [CrossRef]
- Felsmann, J.Z.; Michalski, M.M.; Felsmann, M.; Sokół, R.; Szarek, J.; Strzyżewska-Worotyńska, E. Invesive forms of canine endoparasites as a potential threat to public health—A review and own studies. Ann. Agric. Environ. Med. 2017, 24, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Bartosik, J.; Dziwirek, K.; Łojek, J.; Kaczyk, J.; Górski, P. Prevalence of intestinal parasite infection in dogs from selected rural areas of central and southern Poland. Sci. Ann. Pol. Soc. Anim. Prod. 2017, 13, 61–69. [Google Scholar] [CrossRef]
- Reinemeyer, C.R. Formulations and Clinical Uses of Pyrimidine Compounds in Domestic Animals. In Pyrantel Parasiticide Therapy in Humans and Domestic Animals; Marchiondo, A.A., Ed.; Academic Press: Cambridge, CA, USA, 2016; pp. 67–107. [Google Scholar]
- Bowman, D.D. 4—Helminths. In Georgis’ Parasitology for Veterinarians, 11th ed.; Saunders, W.B., Bowman, D.D., Eds.; Elsevier Ltd.: Oxford, UK, 2021; pp. 135–260. [Google Scholar]
- Nijsse, R.; Mughini-Gras, L.; Wagenaar, J.A.; Ploeger, H.W. Recurrent patent infections with Toxocara canis in household dogs older than six months: A prospective study. Parasites Vectors 2016, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, W.L.; Stewart, A.C.; Walker, J.C. Toxocariasis: A serological survey of blood donors in the Australian Capital Territory together with observations on the risks of infection. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 217–221. [Google Scholar] [CrossRef]
- Lee, A.C.; Schantz, P.M.; Kazacos, K.R.; Montgomery, S.P.; Bowman, D.D. Epidemiologic and zoonotic aspects of ascarid infections in dogs and cats. Trends Parasitol. 2010, 26, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.M.R.; Garijo, M.M.; Alonso, F.D. Prevalence and viability of eggs Toxocara spp. and Toxascaris leonina in public parks in eastern Spain. J. Helminthol. 2001, 75, 169–173. [Google Scholar]
- Giacometti, A.; Cirioni, O.; Fortuna, M.; Osimani, P.; Antonicelli, L.; Del Prete, M.S.; Riva, A.; Derdico, M.M.; Petrelli, E.; Scalise, G. Environmental and serological evidence for the presence of toxocariasis in urban area of Ancona, Italy. Eur. J. Epidemiol. 2000, 16, 1023–1026. [Google Scholar] [CrossRef]
- Papavasilopoulos, V.; Pitiriga, V.; Birbas, K.; Elefsiniotis, J.; Bonatsos, G.; Tsakris, A. Soil contamination by Toxocara canis and human seroprevalence in the Attica region, Greece. Germs 2018, 8, 155–161. [Google Scholar] [CrossRef]
- Sowemimo, O.A. Prevalence and intensity of Toxocara canis (Werner, 1782) in dogs and its potential public health significance in life. Nigeria J. Helminthol. 2007, 81, 433–438. [Google Scholar] [CrossRef]
- Dai, R.; Li, Z.; Li, F.; Liu, D.; Liu, W.; Liu, G. Severe infection of adult dogs with helminths in Hunan Province, China poses significant public health concerns. Vet. Parasitol. 2009, 160, 348–350. [Google Scholar] [CrossRef]
- Nijsse, R.; Ploeger, H.W.; Wagenaar, J.A.; Mughini-Gras, L. Toxocara Canis in Household Dogs: Prevalence, Risk Factors and Owners? Attitude towards Deworming. Parasitol. Res. 2014, 114, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Panova, O.A.; Khrustalev, A.V. Dog Walking Brings Toxocara Eggs to People’s Homes. Vet. Parasitol. 2018, 262, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Nijsse, R.; Mughini-Gras, L.; Wagenaar, J.; Ploeger, H. Coprophagy in Dogs Interferes in the Diagnosis of Parasitic Infections by Faecal Examination. Vet. Parasitol. 2014, 204, 304–309. [Google Scholar] [CrossRef]
- Amaral, H.L.D.A.; Rassier, G.L.; Pepe, M.S. Presence of Toxocara canis eggs on the hair of dogs: A risk factor for Visceral Larva Migrans. Vet. Parasitol. 2010, 174, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Nijsse, R.; Mughini-Gras, L.; Wagenaar, J.A. Environmental contamination with Toxocara eggs: A quantitative approach to estimate the relative contributions of dogs, cats and foxes, and to assess the efficacy of advised interventions in dogs. Parasites Vectors 2015, 8, 397. [Google Scholar] [CrossRef] [PubMed]
- Overgaauw, P.A.M.; van Knapen, F. Veterinary and Public Health Aspects of Toxocara spp. Vet. Parasitol. 2013, 193, 398–403. [Google Scholar] [CrossRef]
- Waindok, P.; Raulf, M.K.; Springer, A.; Strube, C. The Zoonotic Dog Roundworm Toxocara canis, a Worldwide Burden of Public Health. In Dog Parasites Endangering Human Health, Parasitology Research Monographs; Strube, C., Mehlhorn, H., Eds.; Springer: Cham, Switzerland, 2021; pp. 5–26. [Google Scholar]
- Rostami, A.; Riahi, S.M.; Holland, C.V.; Taghipour, A.; Khalili-Fomeshi, M.; Fakhri, Y. Seroprevalence estimates for toxocariasis in people worldwide: A systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2019, 13, e0007809. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Skov, J.; Møller, L.N.; Jensen, P.M.; Kapel, C.M.; Petersen, E.; Nielsen, H.V. Seroprevalence of human toxocariasis in Denmark. Clin. Vaccine Immunol. 2009, 16, 1372–1373. [Google Scholar] [CrossRef]
- Magnaval, J.F.; Michault, A.; Calon, N.; Charlet, J.P. Epidemiology of human toxocariasis in La Reunion. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 531–533. [Google Scholar] [CrossRef]
- Ma, G.; Holland, C.V.; Wang, T.; Hofmann, A.; Fan, C.K.; Maizels, R.M.; Hotez, P.J.; Gasser, R.B. Human toxocariasis. Lancet Infect. Dis. 2018, 18, 14–24. [Google Scholar] [CrossRef]
- Däwel, D. The prevalence of Toxocara eggs in the sand in children’s playgrounds in Frankfurt. Ann. Trop. Med. Parasitol. 1984, 78, 633–636. [Google Scholar] [CrossRef]
- Rokicki, J.; Kucharska, A.; Dzido, J.; Karczewska, D. Contamination of playgrounds in Gdańsk city with parasite eggs. Wiad. Parazytol. 2007, 53, 227–230. [Google Scholar]
- Tiyo, R.; Guedes, T.; Falavigna, D.; Falavigna-Guilherme, A. Seasonal contamination of public squares and lawns by parasites with zoonotic potential in southern Brazil. J. Helminthol. 2008, 82, 1–6. [Google Scholar] [CrossRef]
- Rostami, A.; Riahi, S.M.; Fallah Omrani, V.; Wang, T.; Hofmann, A.; Mirzapour, A.; Foroutan, M.; Fakhri, Y.; Macpherson, C.N.L.; Gasser, R.B. Global Prevalence Estimates of Toxascaris leonina Infection in Dogs and Cats. Pathogens 2020, 9, 503. [Google Scholar] [CrossRef]
- Okulewicz, A.; Perec-Matysiak, A.; Buńkowska, K.; Hildebrand, J. Toxocara canis, Toxocara cati and Toxascaris leonina in wild and domestic carnivores. Helminthologia 2012, 49, 3–10. [Google Scholar] [CrossRef]
- Beaver, P.C.; Bowman, D.D. Ascaridoid larva (Nematoda) from the eye of a child in Uganda. Am. J. Trop. Med. Hyg. 1984, 33, 1272–1274. [Google Scholar] [CrossRef] [PubMed]
- Kirkova, Z.; Georgieva, D.; Raychev, E. Study on the prevalence of trichurosis in different categories of dogs and wild carnivores. Bulg. J. Vet. Med. 2006, 9, 141–147. [Google Scholar]
- Overgaauw, P.A.; Boersema, J.H. Nematode infections in dog breeding kennels in The Netherlands, with special reference to Toxocara. Vet. Q. 1998, 20, 12–15. [Google Scholar] [CrossRef]
- Vanparijs, O.; Hermans, L.; van der Flaes, L. Helminth and protozoan parasites in dogs and cats in Belgium. Vet. Parasitol. 1991, 38, 67–73. [Google Scholar] [CrossRef]
- Scaramozzino, P.; Carvelli, A.; Iacoponi, F.; De Liberato, C. Endoparasites in household and shelter dogs from Central Italy. Int. J. Vet. Sci. Med. 2018, 6, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Fok, E.; Szatmári, V.; Busák, K.; Rozgonyi, F. Epidemiology: Prevalence of intestinal parasites in dogs in some urban and rural areas of Hungary. Vet. Q. 2001, 23, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Little, S.E.; Johnson, E.M.; Lewis, D.; Jaklitsch, R.P.; Payton, M.E.; Blagburn, B.L.; Bowman, D.D.; Moroff, S.; Tams, T.; Rich, L.; et al. Prevalence of intestinal parasites in pet dogs in the United States. Vet. Parasitol. 2009, 166, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.J.; Columbus, S.T.; Aldeen, W.E.; Davis, M.; Carroll, K.C. Trichuris vulpis recovered from a patient with chronic diarrhea and five dogs. J. Clin. Microbiol. 2002, 40, 2703–2704. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).