Effects of Dietary Crude Protein Level on Growth Performance, Carcass Traits, Meat Quality, and Fatty Acid Composition of Ningxiang Finishing Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Slaughter Performance
2.3. Meat Quality
2.4. Determination of Fatty Acid Composition
2.5. Adipocyte Morphological Analysis
2.6. Untargeted Metabolomic Analysis of Liver
2.7. RNA Extraction and Real-Time Quantitative PCR
2.8. RNA Sequencing
2.9. Western Blot
2.10. Statistical Analyses
3. Results
3.1. Effects of Dietary CP Levels on Growth Performance, Carcass Traits, and Meat Quality of Ningxiang Finishing Pigs
3.2. Effect of Dietary CP Levels on Fatty Acid Composition of LM in Ningxiang Finishing Pigs
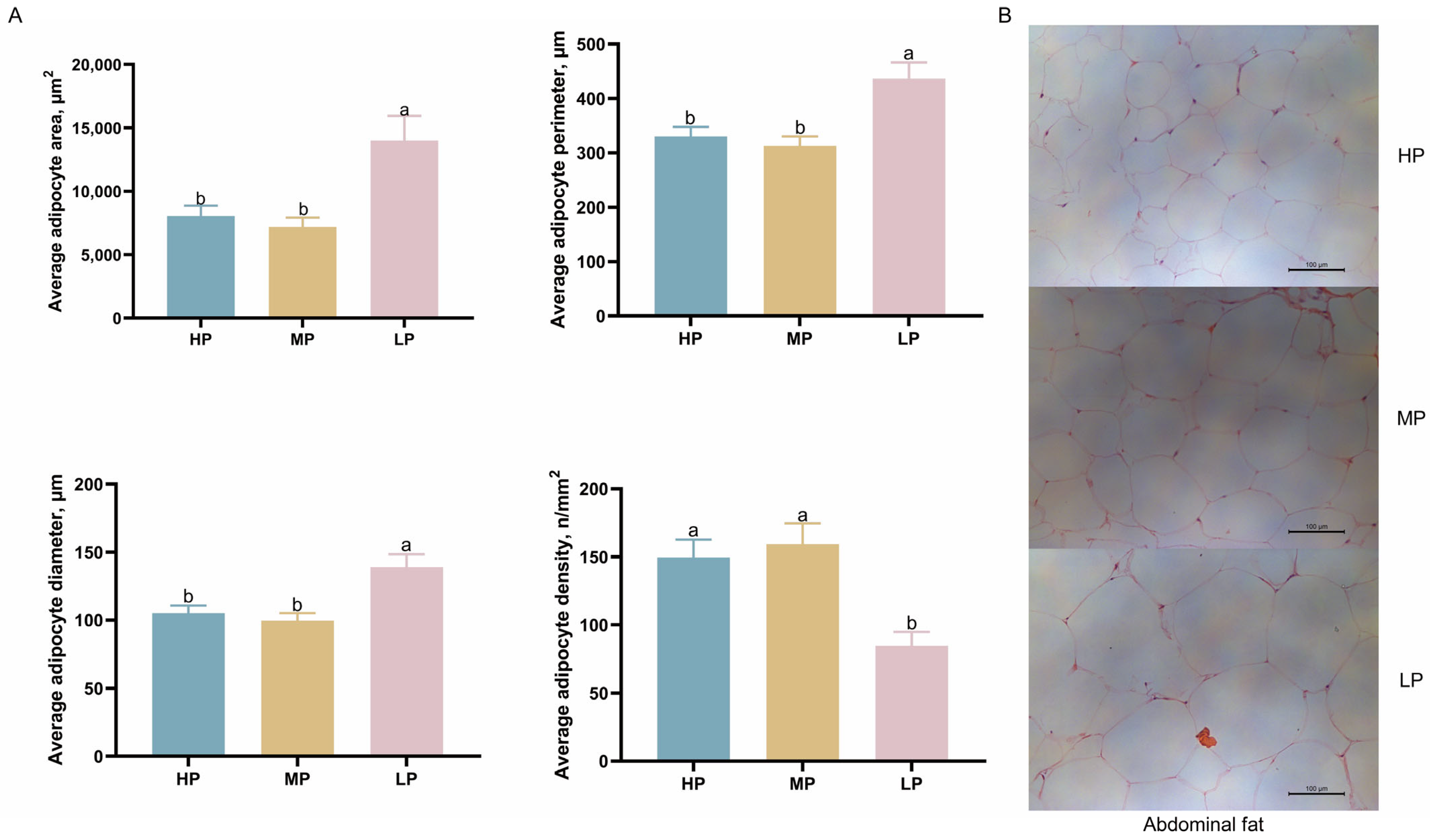
3.3. Effect of Dietary CP Levels on Adipocyte Morphology in Ningxiang Finishing Pigs
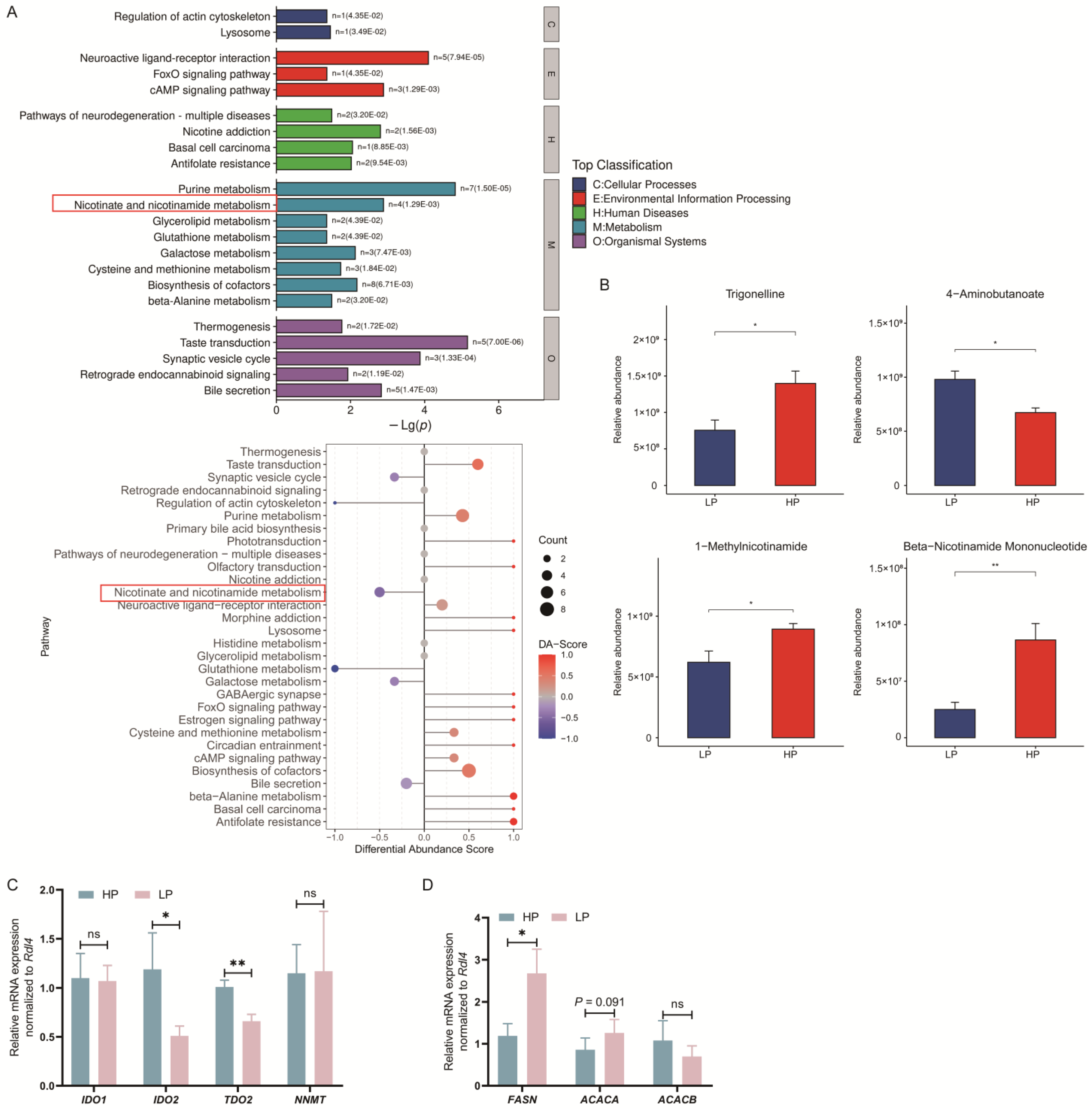
3.4. Effect of Dietary CP Levels on Liver Metabolomics in Ningxiang Finishing Pigs
3.5. Effect of Dietary CP Level on mRNA Expression of Genes Related to Tryptophan–Niacin Metabolism and Lipogenesis in Liver of Ningxiang Finishing Pigs
3.6. WGCNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Zeng, X.; Zhang, C.; Wang, Q.; Zhang, W.; Xie, J.; Chen, J.; Hu, Q.; Wang, Q.; Yang, H.; et al. Higher niacin intakes improve the lean meat rate of Ningxiang pigs by regulating lipid metabolism and gut microbiota. Front. Nutr. 2022, 9, 959039. [Google Scholar] [CrossRef]
- Lei, L.; Wang, Z.; Li, J.; Yang, H.; Yin, Y.; Tan, B.; Chen, J. Comparative microbial profiles of colonic digesta between Ningxiang pig and large white pig. Animals 2021, 11, 1862. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Ji, Y.; Lin, X.; Zhao, Y. Effect of betaine diet on growth performance, carcass quality and fat deposition in finishing Ningxiang pigs. Animals 2021, 11, 3408. [Google Scholar] [CrossRef]
- Xing, Y.; Wu, X.; Xie, C.; Xiao, D.; Zhang, B. Meat quality and fatty acid profiles of Chinese Ningxiang pigs following supplementation with n-carbamylglutamate. Animals 2020, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Li, Z.; Yang, F.; Guo, H.; Zhao, Q.; Zhang, Y.; Yin, Y.; Wu, X.; He, J. A comprehensive genomic analysis of chinese indigenous Ningxiang pigs: Genomic breed compositions, runs of homozygosity, and beyond. Int. J. Mol. Sci. 2023, 24, 14550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Gao, H.; Yang, F.; Li, Y.; Yang, Q.; Liao, Y.; Guo, H.; Xu, K.; Tang, Z.; Gao, N.; et al. Comparative characterization of volatile compounds of Ningxiang pig, duroc and their crosses (duroc x Ningxiang) by using spme-gc-ms. Foods 2023, 12, 1059. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Wang, G.; Cai, S.; Zeng, X.; Qiao, S. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 2018, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Kerr, B.J.; Southern, L.L.; Bidner, T.D.; Friesen, K.G.; Easter, R.A. Influence of dietary protein level, amino acid supplementation, and dietary energy levels on growing-finishing pig performance and carcass composition. J. Anim. Sci. 2003, 81, 3075–3087. [Google Scholar] [CrossRef]
- Fan, P.; Liu, P.; Song, P.; Chen, X.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017, 7, 43412. [Google Scholar] [CrossRef]
- Goodarzi, P.; Wileman, C.M.; Habibi, M.; Walsh, K.; Sutton, J.; Shili, C.N.; Chai, J.; Zhao, J.; Pezeshki, A. Effect of isoleucine and added valine on performance, nutrients digestibility and gut microbiota composition of pigs fed with very low protein diets. Int. J. Mol. Sci. 2022, 23, 14886. [Google Scholar] [CrossRef]
- Spring, S.; Premathilake, H.; Bradway, C.; Shili, C.; DeSilva, U.; Carter, S.; Pezeshki, A. Effect of very low-protein diets supplemented with branched-chain amino acids on energy balance, plasma metabolomics and fecal microbiome of pigs. Sci. Rep. 2020, 10, 15859. [Google Scholar] [CrossRef]
- Liu, S.; Xie, J.; Fan, Z.; Ma, X.; Yin, Y. Effects of low protein diet with a balanced amino acid pattern on growth performance, meat quality and cecal microflora of finishing pigs. J. Sci. Food Agric. 2023, 103, 957–967. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, F.N.; Duan, Y.H.; Guo, Q.P.; Wen, C.Y.; Wang, W.L.; Huang, X.G.; Yin, Y.L. Low-protein diet improves meat quality of growing and finishing pigs through changing lipid metabolism, fiber characteristics, and free amino acid profile of the muscle. J. Anim. Sci. 2018, 96, 3221–3232. [Google Scholar] [CrossRef]
- Lei, X.J.; Cheong, J.Y.; Park, J.H.; Kim, I.H. Supplementation of protease, alone and in combination with fructooligosaccharide to low protein diet for finishing pigs. Anim. Sci. J. 2017, 88, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, W.; Tang, X.; Huang, R.; Li, F.; Su, W.; Yin, Y.; Wen, C.; Liu, J. Comparison of the meat quality and fatty acid profile of muscles in finishing Xiangcun black pigs fed varied dietary energy levels. Anim. Nutr. 2022, 11, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Alonso, V.; Campo Mdel, M.; Provincial, L.; Roncales, P.; Beltran, J.A. Effect of protein level in commercial diets on pork meat quality. Meat Sci. 2010, 85, 7–14. [Google Scholar] [CrossRef]
- Tous, N.; Lizardo, R.; Vila, B.; Gispert, M.; Font, I.F.M.; Esteve-Garcia, E. Effect of reducing dietary protein and lysine on growth performance, carcass characteristics, intramuscular fat, and fatty acid profile of finishing barrows. J. Anim. Sci. 2014, 92, 129–140. [Google Scholar] [CrossRef]
- China Feed Database. Tables of Feed Composition and Nutritive Values in China 2020. Available online: https://www.chinafeeddata.org.cn/admin/Login/slcfb (accessed on 7 October 2025).
- GB/T 39235-2020; Nutrient Requirements of Swine. National Standard of the People’s Republic of China: Beijing, China, 2020; pp. 38–66.
- GB/T 45104-2024; Determination of Gross Energy of Feeds—Bomb Calorimetric Method. National Standard of the People’s Republic of China: Beijing, China, 2024.
- GB/T 6432-2018; Determination of Crude Protein in Feeds—Kjeldahl Method. National Standard of the People’s Republic of China: Beijing, China, 2018.
- Zeng, X.; Xiao, G.; Liu, W.; Yin, L.; Zhang, Y.; Geng, C.; Wang, Q.; Yang, H. Diammonium phosphate supplementation in low-protein diets enhances growth performance in growing pigs without compromising carcass traits and meat quality. J. Anim. Sci. 2025, 103, skaf088. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Xu, Z. Analysis of blueberry plant rhizosphere bacterial diversity and selection of plant growth promoting rhizobacteria. Curr. Microbiol. 2022, 79, 331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, J.; Yang, X.; Yin, L.; Wang, M.; Yin, Y.; Li, J.; Yang, H.; Yin, Y. N-acetyl-d-glucosamine improves the intestinal development and nutrient absorption of weaned piglets via regulating the activity of intestinal stem cells. Anim. Nutr. 2022, 8, 10–17. [Google Scholar] [CrossRef]
- Mansilla, W.D.; Htoo, J.K.; de Lange, C.F.M. Nitrogen from ammonia is as efficient as that from free amino acids or protein for improving growth performance of pigs fed diets deficient in nonessential amino acid nitrogen. J. Anim. Sci. 2017, 95, 3093–3102. [Google Scholar] [CrossRef]
- Guay, F.; Donovan, S.M.; Trottier, N.L. Biochemical and morphological developments are partially impaired in intestinal mucosa from growing pigs fed reduced-protein diets supplemented with crystalline amino acids. J. Anim. Sci. 2006, 84, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Tuitoek, K.; Young, L.; de Lange, C.; Kerr, B. The effect of reducing excess dietary amino acids on growing-finishing pig performance: An elevation of the ideal protein concept. J. Anim. Sci. 1997, 75, 1575–1583. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Y.; Zhang, X.; Yan, E.; He, L.; Wang, L.; Ma, C.; Zhang, P.; Yin, J. Dietary valine/isoleucine ratio impact carcass characteristics, meat edible quality and nutritional values in finishing crossbred duroc x landrace x Yorkshire pigs with different slaughter weights. Front. Nutr. 2022, 9, 899871. [Google Scholar] [CrossRef]
- Guan, X.; Santos, R.R.; Koopmans, S.J.; Molist, F. Effects of the inclusion of dietary bitter gourd (Momordica charantia) on the performance and carcass characteristics of pigs: Potential application in the feed chain. Animals 2023, 13, 2159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, G.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; et al. Effect of different dietary protein levels and amino acids supplementation patterns on growth performance, carcass characteristics and nitrogen excretion in growing-finishing pigs. J. Anim. Sci. Biotechnol. 2019, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Kerr, B.; McKeith, F.; Easter, R. Effect on performance and carcass characteristics of nursery to finisher pigs fed reduced crude protein, amino acid-supplemented diets. J. Anim. Sci. 1995, 73, 433–440. [Google Scholar] [CrossRef]
- Morales, A.; Buenabad, L.; Castillo, G.; Arce, N.; Araiza, B.; Htoo, J.; Cervantes, M. Low-protein amino acid-supplemented diets for growing pigs: Effect on expression of amino acid transporters, serum concentration, performance, and carcass composition. J. Anim. Sci. 2015, 93, 2154–2164. [Google Scholar] [CrossRef]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Naslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature. 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Gurr, M.; Kirtland, J.; Phillip, M.; Robinson, M. The consequences of early overnutrition for fat cell size and number: The pig as an experimental model for human obesity. Int. J. Obes. 1977, 1, 151–170. [Google Scholar]
- Yang, Y.; Li, F.; Guo, Q.; Wang, W.; Zhang, L.; Yin, Y.; Gong, S.; Han, M.; Yin, Y. Effects of different supplemental levels of Eucommia ulmoides leaf extract in the diet on carcass traits and lipid metabolism in growing-finishing pigs. Front. Vet. Sci. 2021, 8, 828165. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, X.; Xia, M.; Liu, Z.; Wei, H.; Deng, Z.; Wang, C.; Jiang, S.; Peng, J. Effect of oregano essential oil and benzoic acid supplementation to a low-protein diet on meat quality, fatty acid composition, and lipid stability of longissimus thoracis muscle in pigs. Lipids Health Dis. 2017, 16, 164. [Google Scholar] [CrossRef]
- Suarez-Belloch, J.; Latorre, M.A.; Guada, J.A. The effect of protein restriction during the growing period on carcass, meat and fat quality of heavy barrows and gilts. Meat Sci. 2016, 112, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, X.; Huang, Z.; Chen, D.; Li, M.; Yu, B.; He, J.; Luo, Y.; Yan, H.; Zheng, P. Dihydromyricetin improves meat quality and promotes skeletal muscle fiber type transformations via ampk signaling in growing-finishing pigs. Food Funct. 2022, 13, 3649–3659. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.C.; Kim, B.C. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005, 71, 351–357. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, B.; Liu, C.; Su, R.; Hou, Y.; Yao, D.; Zhao, L.; Su, L.; Jin, Y. Meat quality, fatty acids, volatile compounds, and antioxidant properties of lambs fed pasture versus mixed diet. Food Sci. Nutr. 2019, 7, 2796–2805. [Google Scholar] [CrossRef]
- Song, B.; Zheng, C.; Zheng, J.; Zhang, S.; Zhong, Y.; Guo, Q.; Li, F.; Long, C.; Xu, K.; Duan, Y.; et al. Comparisons of carcass traits, meat quality, and serum metabolome between Shaziling and Yorkshire pigs. Anim. Nutr. 2022, 8, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Duan, Y.; Li, F.; Li, Y.; Guo, Q.; Ji, Y.; Tan, B.; Li, T.; Yin, Y. Effects of supplementation with branched-chain amino acids to low-protein diets on expression of genes related to lipid metabolism in skeletal muscle of growing pigs. Amino Acids. 2016, 48, 2131–2144. [Google Scholar] [CrossRef]
- Teye, G.A.; Sheard, P.R.; Whittington, F.M.; Nute, G.R.; Stewart, A.; Wood, J.D. Influence of dietary oils and protein level on pork quality. 1. Effects on muscle fatty acid composition, carcass, meat and eating quality. Meat Sci. 2006, 73, 157–165. [Google Scholar] [CrossRef]
- Hao, Z.; Li, Z.; Huo, J.; Chu, Y.; Li, J.; Yu, X.; Liu, F.; Yin, P. Effects of Chinese wolfberry and astragalus extracts on growth performance, pork quality, and unsaturated fatty acid metabolism regulation in Tibetan fragrant pigs. Anim. Sci. J. 2021, 92, e13581. [Google Scholar] [CrossRef]
- Wang, D.; Chen, G.; Chai, M.; Shi, C.; Geng, Y.; Che, Y.; Li, Y.; Liu, S.; Gao, Y.; Hou, H. Effects of dietary protein levels on production performance, meat quality and flavor of fattening pigs. Front. Nutr. 2022, 9, 910519. [Google Scholar] [CrossRef] [PubMed]
- An, W.; Huang, Z.; Mao, Z.; Qiao, T.; Jia, G.; Zhao, H.; Liu, G.; Chen, X. Dietary taurine supplementation improves the meat quality, muscle fiber type, and mitochondrial function of finishing pigs. J. Agric. Food Chem. 2023, 71, 15331–15340. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Liu, Z.; Zhou, Y.; Wei, H.; Zhang, X.; Xia, M.; Deng, Z.; Zou, Y.; Jiang, S.; Peng, J. Effect of oregano essential oil supplementation to a reduced-protein, amino acid-supplemented diet on meat quality, fatty acid composition, and oxidative stability of longissimus thoracis muscle in growing-finishing pigs. Meat Sci. 2017, 133, 103–109. [Google Scholar] [CrossRef]
- Han, Y.G.; Lee, G.I.; Do, S.H.; Jang, J.C.; Kim, Y.Y. The effect of reduced crude protein on growth performance, nutrient digestibility, and meat quality in weaning to finishing pigs. Animals 2023, 13, 1938. [Google Scholar] [CrossRef]
- Wood, J.D.; Lambe, N.R.; Walling, G.A.; Whitney, H.; Jagger, S.; Fullarton, P.J.; Bayntun, J.; Hallett, K.; Bunger, L. Effects of low protein diets on pigs with a lean genotype. 1. Carcass composition measured by dissection and muscle fatty acid composition. Meat Sci. 2013, 95, 123–128. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Souza, P.R.; Peghini, B.C.; da Silva, J.S.; Cardoso, C.R. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Hu, P.; Feng, D.; Zhu, Y.Z.; Shi, Q.; Wang, J.; Zhu, W.Y. The role of liver metabolism in compensatory-growth piglets induced by protein restriction and subsequent protein realimentation. Domest. Anim. Endocrinol. 2021, 74, 106512. [Google Scholar] [CrossRef]
- Linder, K.; Willmann, C.; Kantartzis, K.; Machann, J.; Schick, F.; Graf, M.; Kummerle, S.; Haring, H.U.; Fritsche, A.; Stefan, N.; et al. Dietary niacin intake predicts the decrease of liver fat content during a lifestyle intervention. Sci. Rep. 2019, 9, 1303. [Google Scholar] [CrossRef]
- Hong, S.; Moreno-Navarrete, J.M.; Wei, X.; Kikukawa, Y.; Tzameli, I.; Prasad, D.; Lee, Y.; Asara, J.M.; Fernandez-Real, J.M.; Maratos-Flier, E.; et al. Nicotinamide n-methyltransferase regulates hepatic nutrient metabolism through sirt1 protein stabilization. Nature Med. 2015, 21, 887–894. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Yu, Q.; Bao, T.; Dai, C.; Jiang, L.; Niu, K.; Yang, J.; Wang, S.; Wu, X. Alleviation of hepatic insulin resistance and steatosis with nmn via improving endoplasmic reticulum-mitochondria miscommunication in the liver of hfd mice. Biomed. Pharmacother. 2024, 175, 116682. [Google Scholar] [CrossRef] [PubMed]
- Farias-Pereira, R.; Park, C.S.; Park, Y. Mechanisms of action of coffee bioactive components on lipid metabolism. Food Sci. Biotechnol. 2019, 28, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.; Lima, T.F.O.; Arcaro, C.A.; Inacio, M.D.; Batista-Duharte, A.; Carlos, I.Z.; Spolidorio, L.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Trigonelline and curcumin alone, but not in combination, counteract oxidative stress and inflammation and increase glycation product detoxification in the liver and kidney of mice with high-fat diet-induced obesity. J. Nutr. Biochem. 2020, 76, 108303. [Google Scholar] [CrossRef]
- Li, Y.; Hu, N.; Yang, D.; Oxenkrug, G.; Yang, Q. Regulating the balance between the kynurenine and serotonin pathways of tryptophan metabolism. FEBS J. 2017, 284, 948–966. [Google Scholar] [CrossRef]
- Dervishi, E.; Serrano, C.; Joy, M.; Serrano, M.; Rodellar, C.; Calvo, J.H. The effect of feeding system in the expression of genes related with fat metabolism in semitendinous muscle in sheep. Meat Sci. 2011, 89, 91–97. [Google Scholar] [CrossRef]
- Pang, Y.; Xu, X.; Xiang, X.; Li, Y.; Zhao, Z.; Li, J.; Gao, S.; Liu, Q.; Mai, K.; Ai, Q. High fat activates o-glcnacylation and affects ampk/acc pathway to regulate lipid metabolism. Nutrients 2021, 13, 1740. [Google Scholar] [CrossRef]
- Zang, L.; Kagotani, K.; Hayakawa, T.; Tsuji, T.; Okumura, K.; Shimada, Y.; Nishimura, N. The hexane extract of citrus sphaerocarpa ameliorates visceral adiposity by regulating the pi3k/akt/foxo1 and ampk/acc signaling pathways in high-fat-diet-induced obese mice. Molecules 2023, 28, 8026. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, X.; Qiu, L.; Zhu, W.; Huang, L.; Miao, Y. Liver x receptor alpha promotes milk fat synthesis in buffalo mammary epithelial cells by regulating the expression of fasn. J. Dairy. Sci. 2021, 104, 12980–12993. [Google Scholar] [CrossRef]
- Rosolen, D.; Kretzer, I.F.; Winter, E.; Noldin, V.F.; Rodrigues do Carmo, I.A.; Filippin-Monteiro, F.B.; Cechinel-Filho, V.; Creczynski-Pasa, T.B. N-phenylmaleimides affect adipogenesis and present antitumor activity through reduction of fasn expression. Chem. Biol. Interact. 2016, 258, 10–20. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Fu, S.; Li, W.; Ge, Y.; Cheng, J.; Liu, J. Niacin inhibits the synthesis of milk fat in bmecs through the gpr109a-mediated downstream signalling pathway. Life Sci. 2020, 260, 118415. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular mechanisms of adipogenesis: The anti-adipogenic role of amp-activated protein kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, F.; Duan, Y.; Guo, Q.; Wang, W.; Wen, C.; Huang, X.; Yin, Y. The protein and energy metabolic response of skeletal muscle to the low-protein diets in growing pigs. J. Agric. Food Chem. 2017, 65, 8544–8551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, F.; Guo, Q.; Duan, Y.; Wang, W.; Yang, Y.; Yin, Y.; Gong, S.; Han, M.; Yin, Y. Balanced branched-chain amino acids modulate meat quality by adjusting muscle fiber type conversion and intramuscular fat deposition in finishing pigs. J. Sci. Food Agric. 2022, 102, 3796–3807. [Google Scholar] [CrossRef] [PubMed]


| Ingredient | HP | MHP | MP | MLP | LP |
|---|---|---|---|---|---|
| Corn, g/kg | 590.10 | 605.80 | 624.20 | 642.00 | 657.50 |
| Soybean meal, g/kg | 164.00 | 127.00 | 90.00 | 53.00 | 15.00 |
| Rice bran meal, g/kg | 212.00 | 230.00 | 245.00 | 261.00 | 280.00 |
| L-Lysine, g/kg | 0.00 | 1.10 | 2.20 | 3.30 | 4.40 |
| DL-Methionine, g/kg | 0.00 | 0.10 | 0.30 | 0.40 | 0.60 |
| L-Threonine, g/kg | 0.00 | 0.50 | 1.00 | 1.50 | 2.10 |
| L-Tryptophan, g/kg | 0.00 | 0.10 | 0.30 | 0.40 | 0.60 |
| L-Valine, g/kg | 0.00 | 0.50 | 1.10 | 1.70 | 2.30 |
| L-Isoleucine, g/kg | 0.00 | 0.50 | 1.10 | 1.70 | 2.30 |
| Calcium carbonate, g/kg | 0.30 | 0.40 | 0.50 | 0.40 | 0.50 |
| Calcium hydrogen phosphate, g/kg | 10.60 | 11.00 | 11.30 | 11.60 | 12.00 |
| Sodium chloride, g/kg | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Premix 1, g/kg | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 |
| Nutritional level | |||||
| NE 2 MJ/kg | 9.85 | 9.85 | 9.86 | 9.86 | 9.86 |
| GE 3 MJ/kg | 16.92 | 17.34 | 17.00 | 17.22 | 17.26 |
| CP 3 % | 15.56 | 13.99 | 12.94 | 11.90 | 10.31 |
| SID Lys 2, 4, % | 0.62 | 0.62 | 0.62 | 0.62 | 0.62 |
| SID Met 2, 4, % | 0.24 | 0.24 | 0.24 | 0.24 | 0.24 |
| SID Thr 2, 4, % | 0.46 | 0.46 | 0.46 | 0.46 | 0.46 |
| SID Trp 2, 4, % | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| SID Val 2, 4, % | 0.61 | 0.61 | 0.61 | 0.61 | 0.61 |
| SID Ile 2, 4, % | 0.46 | 0.46 | 0.46 | 0.46 | 0.46 |
| Ca 2, % | 0.49 | 0.49 | 0.49 | 0.49 | 0.49 |
| STTD P 2, % | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Genes 1 | Primers | Primers Sequences (5′ to 3′) | NCBI Accession Number |
|---|---|---|---|
| IDO1 | Forward | GCCTCACCCTTACGATGCTT | NM_001246240.1 |
| Reverse | TGCTGAGCGTTGCTAACTTC | ||
| IDO2 | Forward | CCCCGGTGTGGATACTTCAG | XM_021077739.1 |
| Reverse | TCAAGAGGGGCATCAGAGGA | ||
| TDO2 | Forward | ACCGGTGGGTCTTCAGGTTA | XM_003128997.6 |
| Reverse | TCTTCGGTATCCAGTGTCGG | ||
| NNMT | Forward | TCATTGCCACCGACTACACG | NM_001123146.1 |
| Reverse | TGACTCTGTTCCCTTCGAGC | ||
| FASN | Forward | CTGCTGGACTCGCTCTTTGA | NM_001099930.1 |
| Reverse | CTTTGCCTATGTGCTTGCCC | ||
| ACACA | Forward | AGCAAGGTCGAGACCGAAAG | XM_021066238.1 |
| Reverse | TAAGACCACCGGCGGATAGA | ||
| ACACB | Forward | CCCGACCATGTTTGTCCTCA | NM_001206399.1 |
| Reverse | GGTGAGGCGGTAACTGTTGA | ||
| RPL4 | Forward | CGCTGGTCATGTCTAAAGGTCA | XM_005659862.3 |
| Reverse | ATTCGGCGACGGTTTCTCAT |
| Items 1 | HP | MHP | MP | MLP | LP | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| ANOVA | Linear | Quadratic | |||||||
| Initial BW, kg | 52.19 | 52.20 | 53.15 | 52.10 | 53.16 | 0.41 | 0.914 | 0.573 | 0.890 |
| Final BW, kg | 77.97 | 79.26 | 81.59 | 80.99 | 79.66 | 0.56 | 0.240 | 0.193 | 0.101 |
| ADG, kg/d | 0.42 c | 0.44 abc | 0.46 ab | 0.47 a | 0.43 bc | 0.01 | 0.038 | 0.119 | 0.014 |
| ADFI, kg/d | 1.95 | 1.95 | 2.00 | 2.01 | 1.96 | 0.02 | 0.839 | 0.674 | 0.470 |
| G:F | 0.22 | 0.22 | 0.23 | 0.24 | 0.22 | 0.00 | 0.378 | 0.348 | 0.114 |
| Items | HP | MP | LP | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|
| ANOVA | Linear | Quadratic | |||||
| Slaughter rate, % | 69.85 | 70.58 | 70.81 | 0.46 | 0.694 | 0.419 | 0.806 |
| Leg-to-hip ratio, % | 25.65 | 25.89 | 24.53 | 0.01 | 0.179 | 0.149 | 0.233 |
| Carcass straight length, cm | 86.64 | 86.79 | 86.43 | 0.67 | 0.979 | 0.903 | 0.870 |
| Carcass oblique length, cm | 75.50 | 76.79 | 75.93 | 0.56 | 0.659 | 0.766 | 0.394 |
| Backfat thickness, mm | 37.00 | 37.31 | 39.58 | 1.25 | 0.676 | 0.423 | 0.723 |
| Skin rate, % | 11.76 | 10.41 | 11.36 | 0.37 | 0.318 | 0.658 | 0.151 |
| Bone rate, % | 14.43 | 14.86 | 13.26 | 0.43 | 0.295 | 0.266 | 0.270 |
| Fat percentage, % | 36.69 b | 35.75 b | 40.44 a | 0.79 | 0.027 | 0.037 | 0.067 |
| Lean meat percentage, % | 37.12 ab | 38.98 a | 35.37 b | 0.55 | 0.020 | 0.147 | 0.013 |
| Items 1 | HP | MP | LP | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|
| ANOVA | Linear | Quadratic | |||||
| L* (Lightness) | 45.56 | 45.42 | 44.51 | 0.51 | 0.679 | 0.423 | 0.736 |
| a* (Redness) | 8.54 b | 9.02 b | 10.28 a | 0.26 | 0.013 | 0.004 | 0.416 |
| b* (Yellowness) | 7.06 | 7.30 | 7.77 | 0.17 | 0.232 | 0.097 | 0.734 |
| pH45min | 6.25 b | 6.25 b | 6.64 a | 0.07 | 0.026 | 0.018 | 0.157 |
| pH24h | 5.70 | 5.56 | 5.62 | 0.04 | 0.369 | 0.411 | 0.252 |
| Drip loss, % | 2.03 | 1.64 | 1.92 | 0.15 | 0.581 | 0.779 | 0.322 |
| IMF, % | 7.36 b | 6.02 b | 13.43 a | 1.05 | <0.001 | 0.001 | 0.003 |
| Items 1 | HP | MP | LP | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|
| ANOVA | Linear | Quadratic | |||||
| C16:0 | 26.60 | 26.54 | 26.95 | 0.28 | 0.829 | 0.631 | 0.716 |
| C17:0 | 0.22 | 0.08 | 0.20 | 0.03 | 0.114 | 0.736 | 0.043 |
| C18:0 | 13.69 | 13.49 | 13.10 | 0.17 | 0.373 | 0.176 | 0.791 |
| C20:0 | 0.35 | 0.31 | 0.32 | 0.03 | 0.883 | 0.757 | 0.335 |
| C23:0 | 2.13 ab | 2.65 a | 1.13 b | 0.24 | 0.020 | 0.054 | 0.026 |
| ΣSFA | 42.98 | 43.23 | 41.70 | 0.29 | 0.052 | 0.052 | 0.110 |
| C16:1 | 3.85 | 3.99 | 3.89 | 0.13 | 0.917 | 0.898 | 0.698 |
| C18:1n9t | 0.12 | 0.09 | 0.21 | 0.03 | 0.171 | 0.229 | 0.478 |
| C18:1n9c | 41.96 ab | 40.54 b | 44.69 a | 0.71 | 0.038 | 0.080 | 0.044 |
| C20:1n9 | 0.88 | 1.05 | 1.04 | 0.05 | 0.256 | 0.167 | 0.364 |
| ΣMUFA | 45.69 b | 45.90 b | 49.91 a | 0.73 | 0.011 | 0.007 | 0.113 |
| C18:2n6c | 9.13 | 9.52 | 7.39 | 0.54 | 0.251 | 0.201 | 0.281 |
| C20:3n6 | 0.40 a | 0.48 a | 0.24 b | 0.04 | 0.014 | 0.038 | 0.022 |
| Σn-6 | 9.54 | 10.00 | 7.64 | 0.58 | 0.219 | 0.184 | 0.249 |
| C18:3n3 | 0.17 | 0.32 | 0.24 | 0.06 | 0.700 | 0.688 | 0.394 |
| C20:3n3 | 0.02 | 0.15 | 0.16 | 0.04 | 0.301 | 0.177 | 0.687 |
| Σn-3 | 0.20 | 0.33 | 0.40 | 0.05 | 0.257 | 0.111 | 0.241 |
| C20:2 | 0.40 | 0.40 | 0.35 | 0.02 | 0.465 | 0.272 | 0.589 |
| ΣPUFA | 10.14 | 10.87 | 8.39 | 0.60 | 0.228 | 0.232 | 0.208 |
| ΣPUFA/ΣMUFA | 0.22 | 0.24 | 0.17 | 0.02 | 0.188 | 0.188 | 0.192 |
| ΣPUFA/ΣSFA | 0.24 | 0.25 | 0.20 | 0.01 | 0.333 | 0.311 | 0.278 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Tang, Y.; Liu, W.; Wang, Z.; Huang, P.; Wang, Q.; Yang, H. Effects of Dietary Crude Protein Level on Growth Performance, Carcass Traits, Meat Quality, and Fatty Acid Composition of Ningxiang Finishing Pigs. Animals 2025, 15, 2950. https://doi.org/10.3390/ani15202950
Zeng X, Tang Y, Liu W, Wang Z, Huang P, Wang Q, Yang H. Effects of Dietary Crude Protein Level on Growth Performance, Carcass Traits, Meat Quality, and Fatty Acid Composition of Ningxiang Finishing Pigs. Animals. 2025; 15(20):2950. https://doi.org/10.3390/ani15202950
Chicago/Turabian StyleZeng, Xianglin, Yan Tang, Wenzhi Liu, Zhaobin Wang, Pengfei Huang, Qiye Wang, and Huansheng Yang. 2025. "Effects of Dietary Crude Protein Level on Growth Performance, Carcass Traits, Meat Quality, and Fatty Acid Composition of Ningxiang Finishing Pigs" Animals 15, no. 20: 2950. https://doi.org/10.3390/ani15202950
APA StyleZeng, X., Tang, Y., Liu, W., Wang, Z., Huang, P., Wang, Q., & Yang, H. (2025). Effects of Dietary Crude Protein Level on Growth Performance, Carcass Traits, Meat Quality, and Fatty Acid Composition of Ningxiang Finishing Pigs. Animals, 15(20), 2950. https://doi.org/10.3390/ani15202950






