Riverine Realities: Evaluating Climate Change Impacts on Habitat Dynamics of the Critically Endangered Gharial (Gavialis gangeticus) in the Indian Landscape
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Species Occurrence Records
2.2. Selection of Covariates
2.3. Ensemble Model Utilization
2.4. Assessment of Habitat Quality and Shape Geometry
3. Results
3.1. Model Validation and Variable Impact
3.2. Spatiotemporal Habitat Suitability Dynamics and Centroid Shift
3.3. Spatial Configuration and Geometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Lancet. Biodiversity Loss: A Health Crisis. Lancet 2024, 404, 1615. [Google Scholar] [CrossRef] [PubMed]
- Jaureguiberry, P.; Titeux, N.; Wiemers, M.; Bowler, D.E.; Coscieme, L.; Golden, A.S.; Guerra, C.A.; Jacob, U.; Takahashi, Y.; Settele, J.; et al. The Direct Drivers of Recent Global Anthropogenic Biodiversity Loss. Sci. Adv. 2022, 8, 9982. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Kumar, P.S.; Kabir, M.; Zuhara, F.T.; Mehjabin, A.; Tasannum, N.; Hoang, A.T.; Kabir, Z.; Mofijur, M. Threats, Challenges and Sustainable Conservation Strategies for Freshwater Biodiversity. Environ. Res. 2022, 214, 113808. [Google Scholar] [CrossRef] [PubMed]
- Harrison, I.; Abell, R.; Darwall, W.; Thieme, M.L.; Tickner, D.; Timboe, I. The Freshwater Biodiversity Crisis. Science 2018, 362, 1369. [Google Scholar] [CrossRef]
- Balian, E.V.; Segers, H.; Martens, K.; Lévéque, C. The Freshwater Animal Diversity Assessment: An Overview of the Results; Springer: Dordrecht, The Netherlands, 2008; pp. 627–637. [Google Scholar]
- Rodríguez-Caro, R.C.; Graciá, E.; Blomberg, S.P.; Santamaría, T.; Anadón, J.D.; Giménez, A. Anthropogenic Impacts on Threatened Species Erode Functional Diversity in Chelonians and Crocodilians. Nat. Commun. 2023, 14, 1542. [Google Scholar]
- Cox, N.; Young, B.E.; Bowles, P.; Aguilar, C.; Foster, C.R.; Gippet, J.M.W.; Roll, U.; Watson, J.E.M.; Böhm, M.; Tingley, R.; et al. A Global Reptile Assessment Highlights Shared Conservation Needs of Tetrapods. Nature 2022, 605, 285–290. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Papastamatiou, Y.P.; Barnett, A. Apex Predatory Sharks and Crocodiles Simultaneously Scavenge a Whale Carcass. J. Ethol. 2018, 36, 205–209. [Google Scholar] [CrossRef]
- Adame, M.F.; Jardine, T.D.; Fry, B.; Valdez, D.; Lindner, G.; Bunn, S.E. Estuarine Crocodiles in a Tropical Coastal Floodplain Obtain Nutrition from Terrestrial Prey. PLoS ONE 2018, 13, e0197159. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging Threats and Persistent Conservation Challenges for Freshwater Biodiversity. Biol. Rev. Camb. Philos. Soc. 2019, 94, 849–873. [Google Scholar]
- He, F.; Zarfl, C.; Bremerich, V.; Henshaw, A.; Darwall, W.; Tockner, K.; Jähnig, S.C. Disappearing Giants: A Review of Threats to Freshwater Megafauna. Wiley Interdiscip. Rev. Water 2017, 4, e1208. [Google Scholar] [CrossRef]
- He, F.; Zarfl, C.; Bremerich, V.; David, J.N.W.; Hogan, Z.; Kalinkat, G.; Tockner, K.; Jähnig, S.C. The Global Decline of Freshwater Megafauna. Glob. Change Biol. 2019, 25, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, V.; Bosmans, J.; Wanders, N.; King, H.; Bierkens, M.F.P.; Huijbregts, M.A.J.; Schipper, A.M. Threats of Global Warming to the World’s Freshwater Fishes. Nat. Commun. 2021, 12, 1701. [Google Scholar] [CrossRef]
- Uereyen, S.; Bachofer, F.; Klein, I.; Kuenzer, C. Multi-Faceted Analyses of Seasonal Trends and Drivers of Land Surface Variables in Indo-Gangetic River Basins. Sci. Total Environ. 2022, 847, 157515. [Google Scholar] [CrossRef] [PubMed]
- Biemans, H.; Siderius, C.; Lutz, A.F.; Nepal, S.; Ahmad, B.; Hassan, T.; von Bloh, W.; Wijngaard, R.R.; Wester, P.; Shrestha, A.B.; et al. Importance of Snow and Glacier Meltwater for Agriculture on the Indo-Gangetic Plain. Nat. Sustain. 2019, 2, 594–601. [Google Scholar] [CrossRef]
- Wijngaard, R.R.; Biemans, H.; Lutz, A.F.; Shrestha, A.B.; Wester, P.; Immerzeel, W.W. Climate Change vs. Socio-economic Development: Understanding the Future South Asian Water Gap. Hydrol. Earth Syst. Sci. 2018, 22, 6297–6321. [Google Scholar]
- Hussain, S.A. Reproductive Success, Hatchling Survival, and Rate of Increase of Gharial Gavialis gangeticus in National Chambal Sanctuary, India. Biol. Conserv. 1999, 87, 261–268. [Google Scholar]
- Maskey, T.M. Movement and Survival of Captive-Reared Gharial, Gavialis gangeticus, in the Narayani River, Nepal. Ph.D. Dissertation, University of Florida, Gainesville, FL, USA, 1989. [Google Scholar]
- Adhikari, S.; Poudel, D.; Bolakhe, S. Effect of Temperature Fluctuation on Thermoregulatory Behavior of Gharial in Winter Season: A Case Study of Gharial Conservation Breeding Centre, Chitwan National Park. Res. Zool. 2019, 9, 1–6. [Google Scholar]
- Behera, S.K.; Singh, H.; Sagar, V. Indicator Species (Gharial and Dolphin) of Riverine Ecosystem: An Exploratory of River Ganga. In Our National River Ganga; Sanghi, R., Ed.; Springer: Cham, Switzerland, 2014; pp. 39–50. [Google Scholar]
- Maskey, T.M.; Cadi, A.; Ballouard, J.M.; Fougeirol, L. Gharial Conservation in Nepal: Result of a Population Reinforcement Program. In Proceedings of the 18th Working Meeting of the Crocodile Specialist Group, Montélimar, France, 19–23 June 2006; pp. 19–23. [Google Scholar]
- Whitaker, R. The Gharial: Going Extinct Again. Iguana 2007, 14, 25–32. [Google Scholar]
- Neupane, B.; Singh, B.K.; Poudel, P.; Panthi, S.; Khatri, N.D. Habitat Occupancy and Threat Assessment of Gharial (Gavialis gangeticus) in the Rapti River, Nepal. Global Ecol. Conserv. 2020, 24, e01270. [Google Scholar]
- Sharma, S.P.; Ghazi, M.G.; Katdare, S.; Dasgupta, N.; Mondol, S.; Gupta, S.K.; Hussain, S.A. Microsatellite Analysis Reveals Low Genetic Diversity in Managed Populations of the Critically Endangered Gharial (Gavialis gangeticus) in India. Sci. Rep. 2021, 11, 5627. [Google Scholar] [CrossRef]
- Lang, J.W.; Kumar, P. Chambal Gharial Ecology Project—2016 Update. In Crocodiles, Proceedings of the 24th Working Meeting of the IUCN-SSC Crocodile Specialist Group, Skukuza, South Africa, 23–26 May 2016; IUCN: Gland, Switzerland, 2016; pp. 136–149. [Google Scholar]
- Whitaker, R.; Rajamani, V.; Basu, D.; Balakrishnan, V. Preliminary Survey of the Gharial, Gavialis gangeticus. Madras Snake Park Trust. Rep. 1974, 1–16. [Google Scholar]
- Bustard, H.R. A Future for the Gharial. Cheetal 1975, 17, 3–8. [Google Scholar]
- Lang, J.; Chowfin, S.; Ross, J.P. Gavialis gangeticus (Errata Version Published in 2019). The IUCN Red List of Threatened Species 2019. e.T8966A149227430. Available online: https://dx.doi.org/10.2305/IUCN.UK.2019-1.RLTS.T8966A149227430.en (accessed on 2 November 2024).
- Panda, A.K.; Katdare, S.; Gawan, S.; Sharma, S.P.; Badola, R.; Hussain, S.A. Population Status and Factors Influencing the Distribution of Critically Endangered Gharial (Gavialis gangeticus) in a Regulated Unprotected River System in India. Global Ecol. Conserv. 2023, 46, e02547. [Google Scholar] [CrossRef]
- Vashistha, G.; Mungi, N.A.; Lang, J.W.; Ranjan, V.; Dhakate, P.M.; Khudsar, F.A.; Kothamasi, D. Gharial Nesting in a Reservoir Is Limited by Reduced River Flow and by Increased Bank Vegetation. Sci. Rep. 2021, 11, 4805. [Google Scholar] [CrossRef]
- Singh, V.B. The Status of the Gharial (Gavialis gangeticus) in UP and Its Rehabilitation. J. Bombay Nat. Hist. Soc. 1978, 75, 668–683. [Google Scholar]
- Wildlife Institute of India. Gharial. Available online: https://wii.gov.in/nmcg/priority-species/reptiles/gharial (accessed on 18 January 2025).
- Bustard, H.R. The Government of India Crocodile Project. Cheetal 1980, 22, 11–16. [Google Scholar]
- Hussain, S.A. Basking Site and Water Depth Selection by Gharial Gavialis gangeticus Gmelin 1789 (Crocodylia, Reptilia) in National Chambal Sanctuary, India and Its Implication for River Conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2009, 19, 127–133. [Google Scholar] [CrossRef]
- Dhami, B.; Maraseni, T.; Thapa, K.; Nishan, K.C.; Subedi, S.; Gautam, S.; Ayer, S.; Bayne, E. Gharial (Gavialis gangeticus) Conservation in Bardia National Park, Nepal: Assessing Population Structure and Habitat Characteristics Along the River Channel Amidst Infrastructure Development. Ecol. Evol. 2023, 13, e10661. [Google Scholar] [CrossRef]
- Pathak, A.; Bashyal, A.; Oli, B.N.; Thapaliya, B.; Bhattarai, S.; Khanal, S.; Paudel, P. Understanding the Perception of Buffer Zone Communities to Gharial (Gavialis gangeticus) Conservation in Chitwan National Park, Nepal. Global Ecol. Conserv. 2023, 47, e02634. [Google Scholar] [CrossRef]
- Sharma, S.P.; Katdare, S.; Zaidi, Z.; Ghazi, M.G.; Gupta, S.K.; Hussain, S.A. Mitochondrial DNA Analysis Reveals Extremely Low Genetic Diversity in a Managed Population of the Critically Endangered Gharial (Gavialis gangeticus, Gmelin, 1789). Herpetol. J. 2020, 30, 202–206. [Google Scholar] [CrossRef]
- Vashistha, G.; Deepika, S.; Khudsar, F.A.; Dhakate, P.M.; Kothamasi, D. Anthropogenic Restocking of Gharial Individuals Prevents Genetic Isolation of Gharial Population in Girwa River, India by Geographic Barriers Imposed by a Barrage. preprint, 2021. [Google Scholar] [CrossRef]
- Vashistha, G.; Lang, J.W.; Dhakate, P.M.; Kothamasi, D. Sand Addition Promotes Gharial Nesting in a Regulated River-Reservoir Habitat. Ecol. Solut. Evid. 2021, 2, e12068. [Google Scholar]
- Böhm, M.; Cook, D.; Ma, H.; Davidson, A.D.; García, A.; Tapley, B.; Pearce-Kelly, P.; Carr, J. Hot and Bothered: Using Trait-Based Approaches to Assess Climate Change Vulnerability in Reptiles. Biol. Conserv. 2016, 204, 32–41. [Google Scholar]
- Tingley, R.; Meiri, S.; Chapple, D.G. Addressing Knowledge Gaps in Reptile Conservation. Biol. Conserv. 2016, 204, 1–5. [Google Scholar] [CrossRef]
- Abedin, I.; Mukherjee, T.; Abedin, J.; Kim, H.-W.; Kundu, S. Habitat Loss in the IUCN Extent: Climate Change-Induced Threat on the Red Goral (Naemorhedus baileyi) in the Temperate Mountains of South Asia. Biology 2024, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Abedin, I.; Mukherjee, T.; Kim, A.R.; Kim, H.W.; Kang, H.E.; Kundu, S. Distribution Model Reveals Rapid Decline in Habitat Extent for Endangered Hispid Hare: Implications for Wildlife Management and Conservation Planning in Future Climate Change Scenarios. Biology 2024, 13, 198. [Google Scholar] [CrossRef]
- Kundu, S.; Mukherjee, T.; Kamalakannan, M.; Barhadiya, G.; Ghosh, C.; Kim, H.W. Matrilineal Phylogeny and Habitat Suitability of the Endangered Spotted Pond Turtle (Geoclemys hamiltonii; Testudines: Geoemydidae): A Two-Dimensional Approach to Forecasting Future Conservation Consequences. PeerJ 2023, 9, e15975. [Google Scholar]
- Kundu, S.; Mukherjee, T.; Kim, A.R.; Lee, S.R.; Mukherjee, A.; Jung, W.K.; Kim, H.W. Mitochondrial DNA and Distribution Modelling Evidenced the Lost Genetic Diversity and Wild-Residence of Star Tortoise, Geochelone elegans (Testudines: Testudinidae) in India. Animals 2023, 13, 150. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar]
- Guisan, A.; Zimmermann, N.E. Predictive Habitat Distribution Models in Ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing Whether Ensemble Modelling Is Advantageous for Maximizing Predictive Performance of Species Distribution Models. Ecography 2020, 43, 549–558. [Google Scholar]
- Bachman, S.; Moat, J.; Hill, A.W.; de la Torre, J.; Scott, B. Supporting Red List Threat Assessments with GeoCAT: Geospatial Conservation Assessment Tool. ZooKeys 2011, 150, 117–126. [Google Scholar]
- Vashistha, G.; Ranjan, V.; Ansari, A.G.; Dhakate, P.M. Evidence of Gharial, Gavialis gangeticus (Crocodylia: Gavialidae), Nesting in the Ramganga River, Corbett Tiger Reserve, India. Herpetol. Notes 2022, 15, 179–182. [Google Scholar]
- Saikia, B.P.; Saud, B.J.; Kakati Saikia, M.; Saikia, P.K. Present Distribution Status and Conservation Threats of Indian Gharial in Assam, India. Indian J. Environ. Sci. 2010, 2, 382–387. [Google Scholar]
- Sarkar, D.; Ramesh, C.; Hussain, S.A.; Mondal, R.; Talukdar, G. A Field Observation of the Critically Endangered Indian Gharial, Gavialis gangeticus (Gmelin, 1789), in the Lower Ganga Canal, Narora, Uttar Pradesh, India. IRCF Reptil. Amphib. 2018, 25, 204–207. [Google Scholar]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The Next Generation Python-Based GIS Toolkit for Landscape Genetic, Biogeographic and Species Distribution Model Analyses. PeerJ 2017, 5, e4095. [Google Scholar]
- Peterson, A.T.; Soberón, J. Species Distribution Modeling and Ecological Niche Modeling: Getting the Concepts Right. Braz. J. Nat. Conserv. Essays Perspect. Natureza Conserv. 2012, 10, 102–107. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar]
- Karra, K.; Kontgis, C.; Statman-Weil, Z.; Mazzariello, J.C.; Mathis, M.; Brumby, S.P. Global Land Use/Land Cover with Sentinel-2 and Deep Learning. In Proceedings of the IGARSS 2021—2021 IEEE International Geoscience and Remote Sensing Symposium, Brussels, Belgium, 11–16 July 2021. [Google Scholar]
- SEDAC. Last of the Wild Project, Version 2, 2005 (LWP-2): Global Human Footprint Dataset (Geographic). Available online: https://doi.org/10.7927/H4M61H5F (accessed on 2 November 2024).
- Morisette, J.T.; Jarnevich, C.S.; Holcombe, T.R.; Talbert, C.B.; Ignizio, D.; Talbert, M.K.; Silva, C.; Koop, D.; Swanson, A.; Young, N.E. VisTrails SAHM: Visualization and Workflow Management for Species Habitat Modeling. Ecography 2013, 36, 129–135. [Google Scholar]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A Toolbox for Comparative Studies of Environmental Niche Models. Ecography 2010, 33, 607–611. [Google Scholar]
- De Marco, P.J.; Nóbrega, C.C. Evaluating Collinearity Effects on Species Distribution Models: An Approach Based on Virtual Species Simulation. PLoS ONE 2018, 13, e0202403. [Google Scholar]
- Zhao, N.; Zhang, X.; Shan, G.; Ye, X. Evaluating the Effects of Climate Change on Spatial Aggregation of Giant Pandas and Sympatric Species in a Mountainous Landscape. Animals 2021, 11, 3332. [Google Scholar] [CrossRef]
- Rossi, J.-P.; Rasplus, J.-Y. Climate Change and the Potential Distribution of the Glassy-Winged Sharpshooter (Homalodisca vitripennis), an Insect Vector of Xylella fastidiosa. Sci. Total Environ. 2023, 860, 160375. [Google Scholar] [PubMed]
- Chaitanya, R.; Naniwadekar, R.; Meiri, S. Why Did the Hornbill Not Cross the River? Upland Habitats Rather than a Physical Barrier Limit the Distribution of the Brown Hornbill. J. Biogeogr. 2024, 51, 2156–2169. [Google Scholar]
- Gautam, S.; Shany, V.J. Navigating Climate Change in Southern India: A Study on Dynamic Dry-Wet Patterns and Urgent Policy Interventions. Geosyst. Geoenviron. 2024, 3, 100263. [Google Scholar]
- Li, L.; Xie, F.; Yuan, N. On the Long-Term Memory Characteristic in Land Surface Air Temperatures: How Well Do CMIP6 Models Perform? Atmos. Oceanic Sci. Lett. 2023, 16, 100291. [Google Scholar]
- Allen, B.J.; Hill, D.J.; Burke, A.M.; Clark, M.; Marchant, R.; Stringer, L.C.; Williams, D.R.; Lyon, C. Projected Future Climatic Forcing on the Global Distribution of Vegetation Types. Philos. Trans. R. Soc. B Biol. Sci. 2024, 379, 20230011. [Google Scholar]
- Guisan, A.; Zimmermann, N.E.; Elith, J.; Graham, C.H.; Phillips, S.; Peterson, A.T. What Matters for Predicting the Occurrences of Trees: Techniques, Data, or Species’ Characteristics? Ecol. Monogr. 2007, 77, 615–630. [Google Scholar]
- Miller, J. Species Distribution Modeling. Geogr. Compass 2010, 4, 490–509. [Google Scholar]
- Talbert, C.B.; Talbert, M.K. User Manual for SAHM Package for VisTrails. 2012. Available online: https://pubs.usgs.gov/publication/70118102 (accessed on 2 November 2024).
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar]
- Cohen, J. Weighted Kappa: Nominal Scale Agreement Provision for Scaled Disagreement or Partial Credit. Psychol. Bull. 1968, 70, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Valverde, A.; Acevedo, P.; Barbosa, A.M.; Lobo, J.M.; Real, R. Discrimination Capacity in Species Distribution Models Depends on the Representativeness of the Environmental Domain. Glob. Ecol. Biogeogr. 2013, 22, 508–516. [Google Scholar] [CrossRef]
- Phillips, S.J.; Elith, J. POC Plots: Calibrating Species Distribution Models with Presence-Only Data. Ecology 2010, 91, 2476–2484. [Google Scholar] [CrossRef] [PubMed]
- McGarigal, K.; Marks, B.J. FRAGSTATS: Spatial Pattern Analysis Program for Quantifying Landscape Structure; Gen. Tech. Rep. PNW-GTR-351; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1995; pp. 122–351. [Google Scholar]
- Hesselbarth, M.H.K.; Sciaini, M.; With, K.A.; Wiegand, K.; Nowosad, J. landscapemetrics: An Open-Source R Tool to Calculate Landscape Metrics. Ecography 2019, 42, 1648–1657. [Google Scholar] [CrossRef]
- Sharma, S.P.; Ghazi, M.G.; Katdare, S.; Badola, R.; Hussain, S.A. Population Status and Genetic Assessment of Mugger (Crocodylus palustris) in a Tropical Regulated River System in North India. Sci. Rep. 2024, 14, 7438. [Google Scholar] [CrossRef]
- Weeks, A.R.; Sgro, C.M.; Young, A.G.; Frankham, R.; Mitchell, N.J.; Miller, K.A.; Byrne, M.; Coates, D.J.; Eldridge, M.D.B.; Sunnucks, P.; et al. Assessing the Benefits and Risks of Translocations in Changing Environments: A Genetic Perspective. Evol. Appl. 2011, 4, 709. [Google Scholar] [CrossRef]
- Berger-Tal, O.; Blumstein, D.T.; Swaisgood, R.R. Conservation Translocations: A Review of Common Difficulties and Promising Directions. Anim. Conserv. 2020, 23, 121–131. [Google Scholar]
- Lapin, K.; Hoffmann, J.A.; Braun, M.; Oettel, J. Identification and Prioritization of Stepping Stones for Biodiversity Conservation in Forest Ecosystems. Conserv. Sci. Pract. 2024, 6, e13161. [Google Scholar]
- Somaweera, R.; Brien, M.L.; Sonneman, T.; Didham, R.K.; Webber, B.L. Absence of Evidence Is Not Evidence of Absence: Knowledge Shortfalls Threaten the Effective Conservation of Freshwater Crocodiles. Global Ecol. Conserv. 2019, 20, e00773. [Google Scholar] [CrossRef]
- Rütera, S.; Vos, C.C.; van Eupen, M.; Rühmkorf, H. Transboundary Ecological Networks as an Adaptation Strategy to Climate Change: The Example of the Dutch-German Border. Basic Appl. Ecol. 2014, 15, 639–650. [Google Scholar]
- Simões, T.R.; Kammerer, C.F.; Caldwell, M.W.; Pierce, S.E. Successive Climate Crises in the Deep Past Drove the Early Evolution and Radiation of Reptiles. Sci. Adv. 2022, 8, eabq1898. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Feiner, Z.S.; Frater, P.; Hansen, G.J.A.; Ladwig, R.; Paukert, C.P.; Verhoeven, M.; Wszola, L.; Jensen, O.P. Asymmetric Impacts of Climate Change on Thermal Habitat Suitability for Inland Lake Fishes. Nat. Commun. 2024, 15, 1. [Google Scholar]
- Fukuda, Y.; McDonald, P.J.; Crase, B. Lost to the Sea: Predicted Climate Change Threats to Saltwater Crocodile Nesting Habitat. Front. Ecol. Evol. 2022, 10, 839423. [Google Scholar] [CrossRef]
- Biber, M.F.; Voskamp, A.; Hof, C. Potential Effects of Future Climate Change on Global Reptile Distributions and Diversity. Glob. Ecol. Biogeogr. 2023, 32, 519–534. [Google Scholar]
- Dayananda, B.; Bezeng, S.B.; Karunarathna, S.; Jeffree, R.A. Climate Change Impacts on Tropical Reptiles: Likely Effects and Future Research Needs Based on Sri Lankan Perspectives. Front. Ecol. Evol. 2021, 9, 2021. [Google Scholar]
- Fahrig, L. Why Do Several Small Patches Hold More Species Than Few Large Patches? Glob. Ecol. Biogeogr. 2020, 29, 615–628. [Google Scholar]
- Szangolies, L.; Rohwäder, M.S.; Jeltsch, F. Single Large AND Several Small Habitat Patches: A Community Perspective on Their Importance for Biodiversity. Basic Appl. Ecol. 2022, 65, 16–27. [Google Scholar] [CrossRef]
- Mi, C.; Ma, L.; Yang, M.; Li, X.; Meiri, S.; Roll, U.; Oskyrko, O.; Pincheira-Donoso, D.; Harvey, L.P.; Jablonski, D.; et al. Global Protected Areas as Refuges for Amphibians and Reptiles under Climate Change. Nat. Commun. 2023, 14, 1. [Google Scholar] [CrossRef]
- Rao, R.J.; Gurjwar, R.K. Crocodile Human Conflict in National Chambal Sanctuary, India. In Proceedings of the World Crocodile Conference and 22nd Working Meeting of the IUCN SSC Crocodile Specialist Group, Negombo, Sri Lanka, 20–23 May 2020. [Google Scholar]
- Singh, S.; Srivastava, S.; Singh, A.; Dutta, S. Illegal Trade in Gharial in Northern India. Crocodile Spec. Group Newslett. 2024, 43, 19. [Google Scholar]
- Rehák, I. Gharial Extinction Crisis. In Proceedings of the 62nd WAZA Annual Conference, Budapest, Hungary, 26–30 August 2007; pp. 202–209. [Google Scholar]

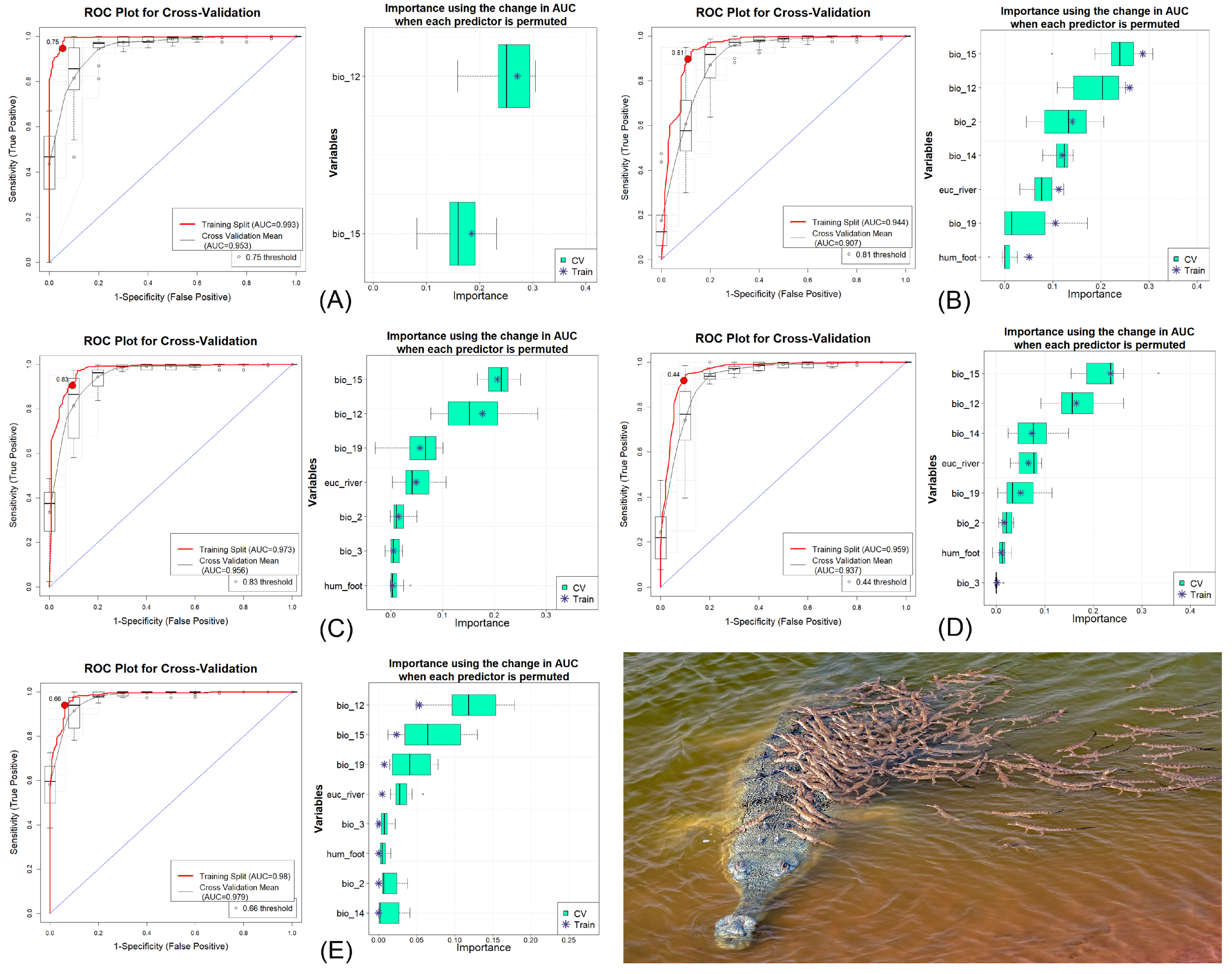
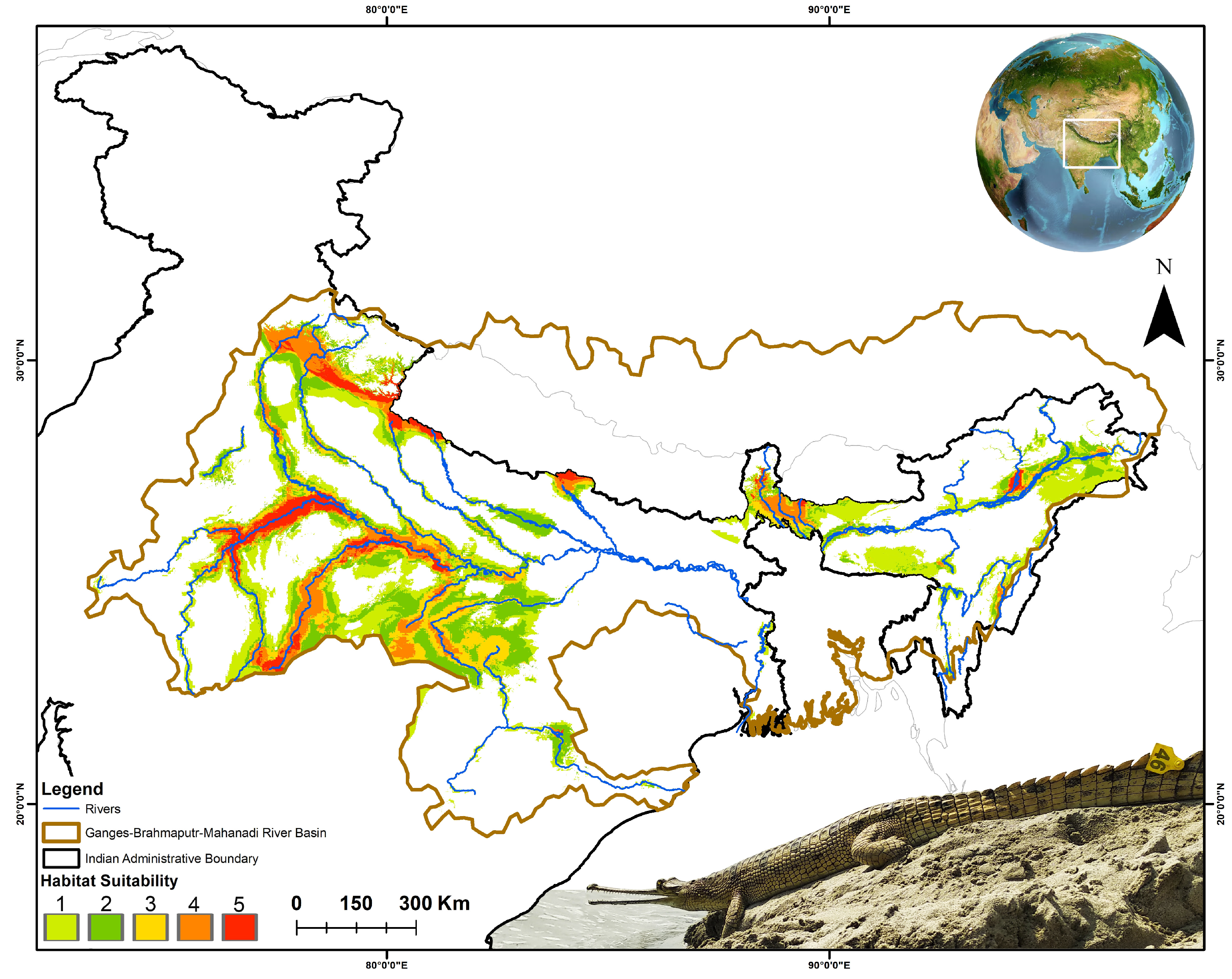
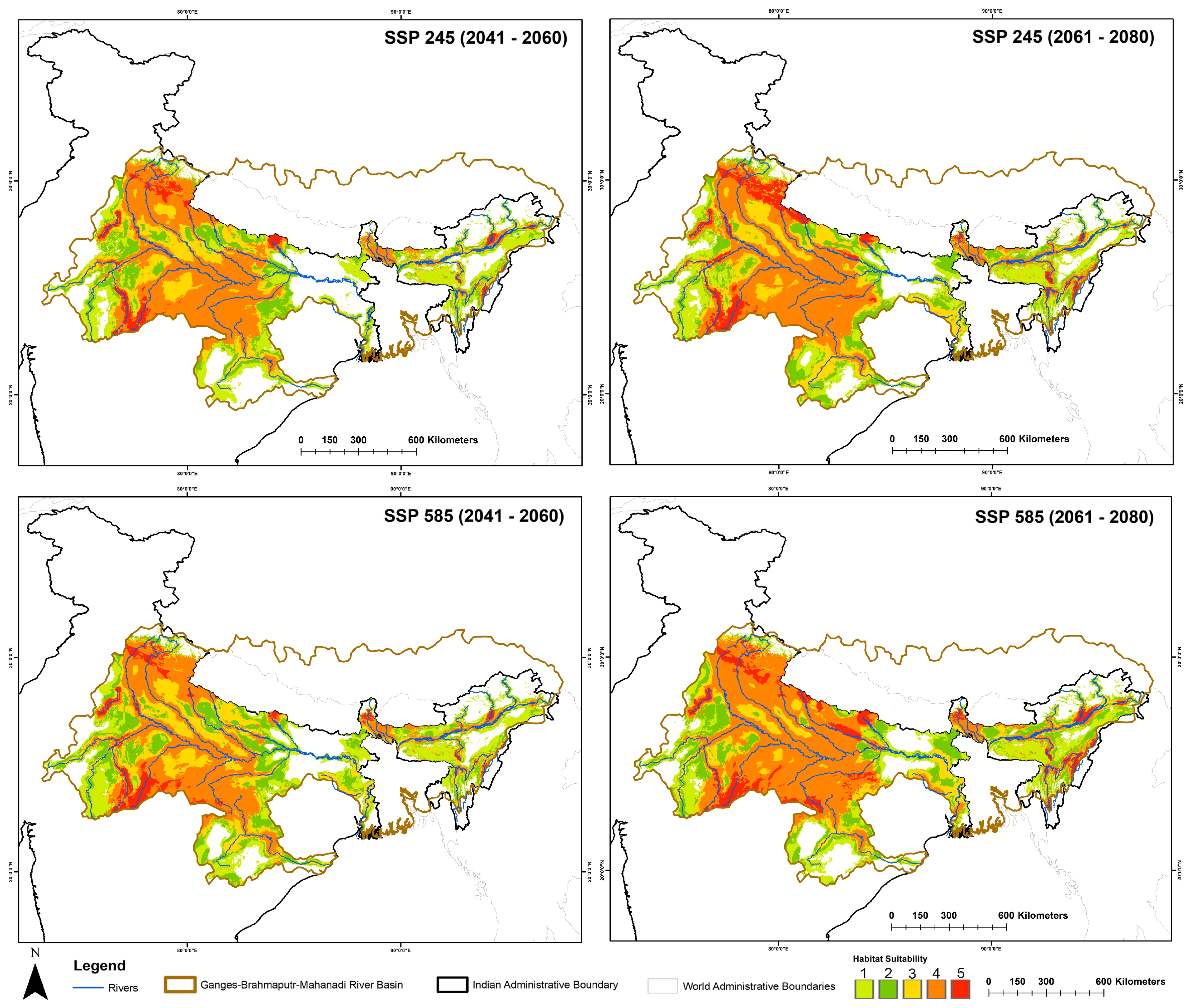

| Model | Dataset | AUC | ΔAUC | PCC | TSS | Kappa | Specificity | Sensitivity |
|---|---|---|---|---|---|---|---|---|
| BRT | Train | 0.993 | 0.04 | 94.7 | 0.893 | 0.869 | 0.946 | 0.948 |
| CV | 0.953 | 90.5 | 0.798 | 0.771 | 0.886 | 0.912 | ||
| GLM | Train | 0.944 | 0.037 | 89.6 | 0.789 | 0.75 | 0.892 | 0.898 |
| CV | 0.907 | 88.2 | 0.745 | 0.713 | 0.852 | 0.892 | ||
| MARS | Train | 0.973 | 0.017 | 90.5 | 0.81 | 0.771 | 0.905 | 0.905 |
| CV | 0.956 | 89.8 | 0.775 | 0.75 | 0.865 | 0.91 | ||
| MaxEnt | Train | 0.959 | 0.022 | 91.4 | 0.823 | 0.791 | 0.905 | 0.917 |
| CV | 0.937 | 89.6 | 0.786 | 0.749 | 0.886 | 0.9 | ||
| RF | Train | 0.98 | 0.001 | 94 | 0.879 | 0.852 | 0.939 | 0.94 |
| CV | 0.979 | 95.1 | 0.874 | 0.875 | 0.907 | 0.968 |
| Variables | Abbreviations | BRT | GLM | MARS | MAXENT | RF | μ (Mean) | μ (Mean) % |
|---|---|---|---|---|---|---|---|---|
| Annual Precipitation | bio_12 | 0.271 | 0.260 | 0.177 | 0.165 | 0.054 | 0.185 | 33.75 |
| Precipitation of Driest Month | bio_14 | 0.000 | 0.120 | 0.000 | 0.073 | 0.000 | 0.039 | 7.04 |
| Precipitation Seasonality | bio_15 | 0.185 | 0.287 | 0.206 | 0.224 | 0.023 | 0.185 | 33.70 |
| Precipitation of Coldest Quarter | bio_19 | 0.000 | 0.105 | 0.057 | 0.050 | 0.007 | 0.044 | 8.00 |
| Mean Diurnal Range | bio_2 | 0.000 | 0.141 | 0.015 | 0.016 | 0.000 | 0.035 | 6.29 |
| Isothermality | bio_3 | 0.000 | 0.000 | 0.006 | 0.001 | 0.000 | 0.002 | 0.28 |
| Euclidean Distance to Waterbodies | euc_river | 0.000 | 0.113 | 0.049 | 0.068 | 0.005 | 0.047 | 8.53 |
| Human Influence Index | hum_foot | 0.000 | 0.051 | 0.004 | 0.011 | 0.000 | 0.013 | 2.40 |
| State | Present | SSP245 (2041–2060) | SSP245 (2061–2080) | SSP585 (2041–2060) | SSP585 (2061–2080) |
|---|---|---|---|---|---|
| Uttar Pradesh | 10,954 | 5713 | 14,655 | 5682 | 19,699 |
| Madhya Pradesh | 10,797 | 18,708 | 25,027 | 32,241 | 31,150 |
| Rajasthan | 7074 | 5883 | 6320 | 6246 | 4254 |
| Uttarakhand | 5785 | 10,913 | 24,116 | 12,121 | 9501 |
| Assam | 1489 | 2576 | 3946 | 2167 | 6974 |
| Bihar | 1062 | 3008 | 3064 | 1354 | 4390 |
| Orissa | 111 | 0 | 0 | 54 | 4 |
| West Bengal | 97 | 87 | 487 | 767 | 721 |
| Haryana | 70 | 1905 | 2418 | 2699 | 3659 |
| Sikkim | 30 | 264 | 469 | 460 | 720 |
| Himachal Pradesh | 12 | 22 | 272 | 339 | 15 |
| Arunachal Pradesh | 6 | 412 | 465 | 283 | 1283 |
| Manipur | 0 | 1252 | 2878 | 667 | 2213 |
| Jharkhand | 0 | 0 | 412 | 0 | 3239 |
| Chhattisgarh | 0 | 0 | 0 | 101 | 1809 |
| Nagaland | 0 | 328 | 314 | 330 | 1378 |
| Mizoram | 0 | 1 | 27 | 23 | 802 |
| Meghalaya | 0 | 45 | 87 | 92 | 93 |
| Delhi | 0 | 25 | 158 | 72 | 0 |
| Scenario | NP | PD | LPI | TE | LSI | AI |
|---|---|---|---|---|---|---|
| Present | 388 | 3,956,579 | 1.0256 | 119.344 | 19.8265 | 90.3325 |
| SSP245 (2041–2060) | 560 | 5,716,036 | 1.102 | 202.408 | 26.8187 | 90.4336 |
| SSP245 (2061–2080) | 634 | 6,471,370 | 1.2852 | 266.928 | 29.1225 | 90.519 |
| SSP585 (2041–2060) | 555 | 5,665,000 | 1.6909 | 235.224 | 27.5614 | 90.3487 |
| SSP585 (2061–2080) | 761 | 7,809,127 | 1.3737 | 321.296 | 31.548 | 90.7826 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedin, I.; Singha, H.; Singh, S.; Mukherjee, T.; Kim, H.-W.; Kundu, S. Riverine Realities: Evaluating Climate Change Impacts on Habitat Dynamics of the Critically Endangered Gharial (Gavialis gangeticus) in the Indian Landscape. Animals 2025, 15, 896. https://doi.org/10.3390/ani15060896
Abedin I, Singha H, Singh S, Mukherjee T, Kim H-W, Kundu S. Riverine Realities: Evaluating Climate Change Impacts on Habitat Dynamics of the Critically Endangered Gharial (Gavialis gangeticus) in the Indian Landscape. Animals. 2025; 15(6):896. https://doi.org/10.3390/ani15060896
Chicago/Turabian StyleAbedin, Imon, Hilloljyoti Singha, Shailendra Singh, Tanoy Mukherjee, Hyun-Woo Kim, and Shantanu Kundu. 2025. "Riverine Realities: Evaluating Climate Change Impacts on Habitat Dynamics of the Critically Endangered Gharial (Gavialis gangeticus) in the Indian Landscape" Animals 15, no. 6: 896. https://doi.org/10.3390/ani15060896
APA StyleAbedin, I., Singha, H., Singh, S., Mukherjee, T., Kim, H.-W., & Kundu, S. (2025). Riverine Realities: Evaluating Climate Change Impacts on Habitat Dynamics of the Critically Endangered Gharial (Gavialis gangeticus) in the Indian Landscape. Animals, 15(6), 896. https://doi.org/10.3390/ani15060896











