Diversity and Distribution Patterns of Amphibians in the Huangshan Mountain Region: The Roles of Climate and Human Activities
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
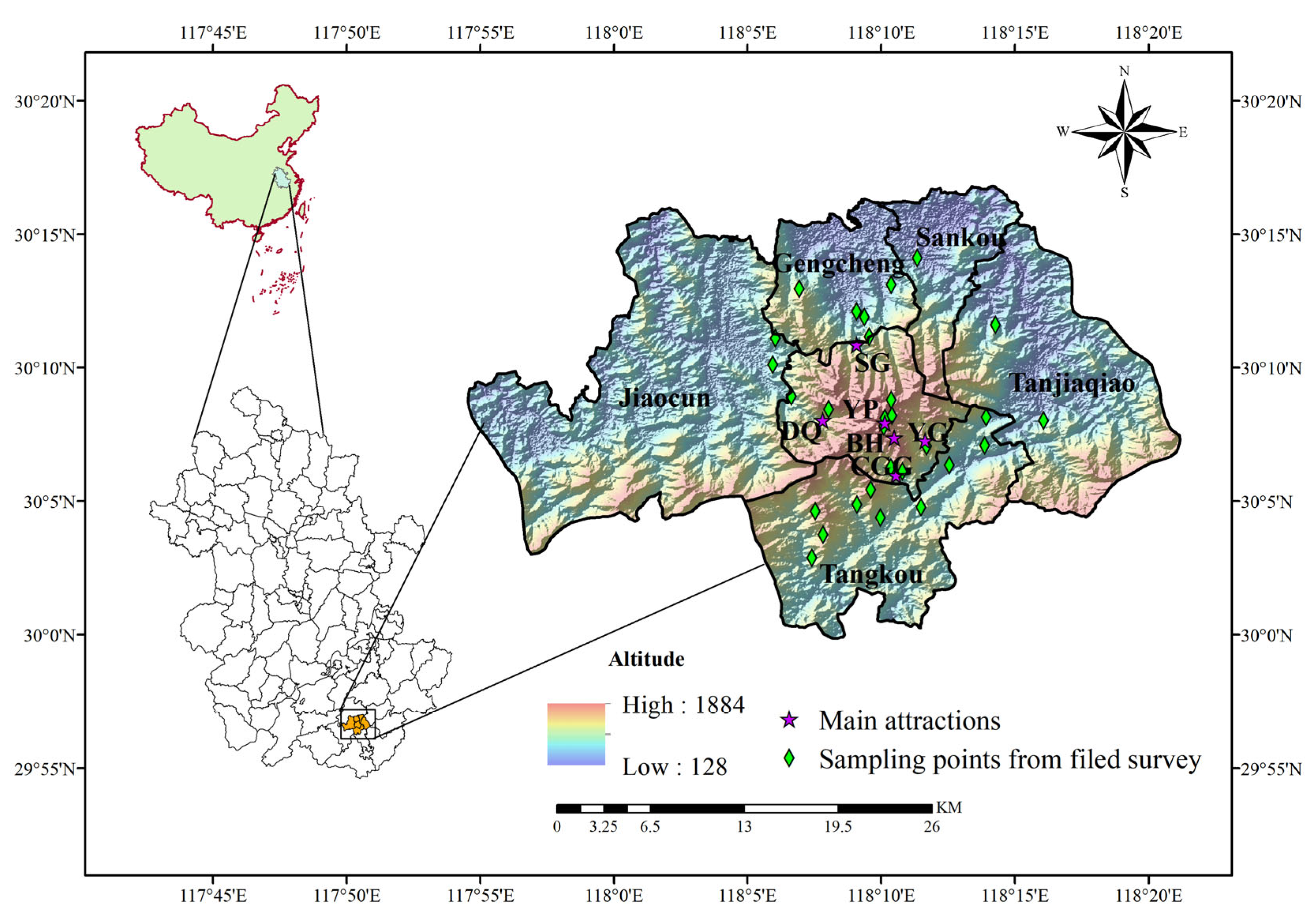
2.1. Study Area
2.2. Prediction of Amphibian Richness Based on the MaxEnt Model
- (a)
- Occurrence data
- (b)
- Environmental predictors
2.3. Survey Methods
2.4. Data Analysis
2.4.1. Model Approach
2.4.2. Diversity Analysis
- (a)
- Dominance Index
- (b)
- Shannon–Wiener Index
- (c)
- Simpson Index
- (d)
- Evenness Index [46]
- (e)
- Chao1 Index
2.4.3. Correlation Analysis
3. Results
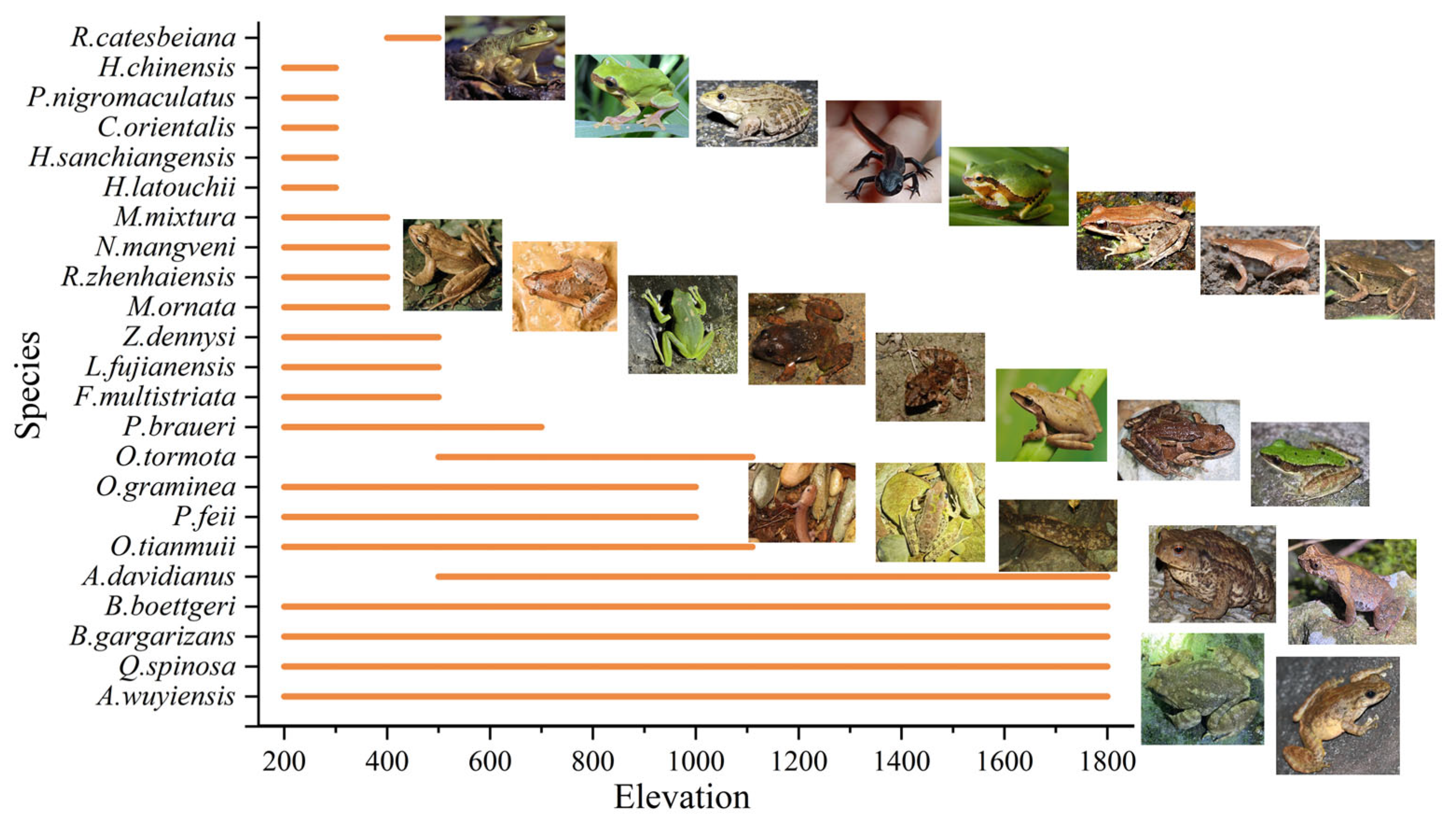
3.1. Species Richness
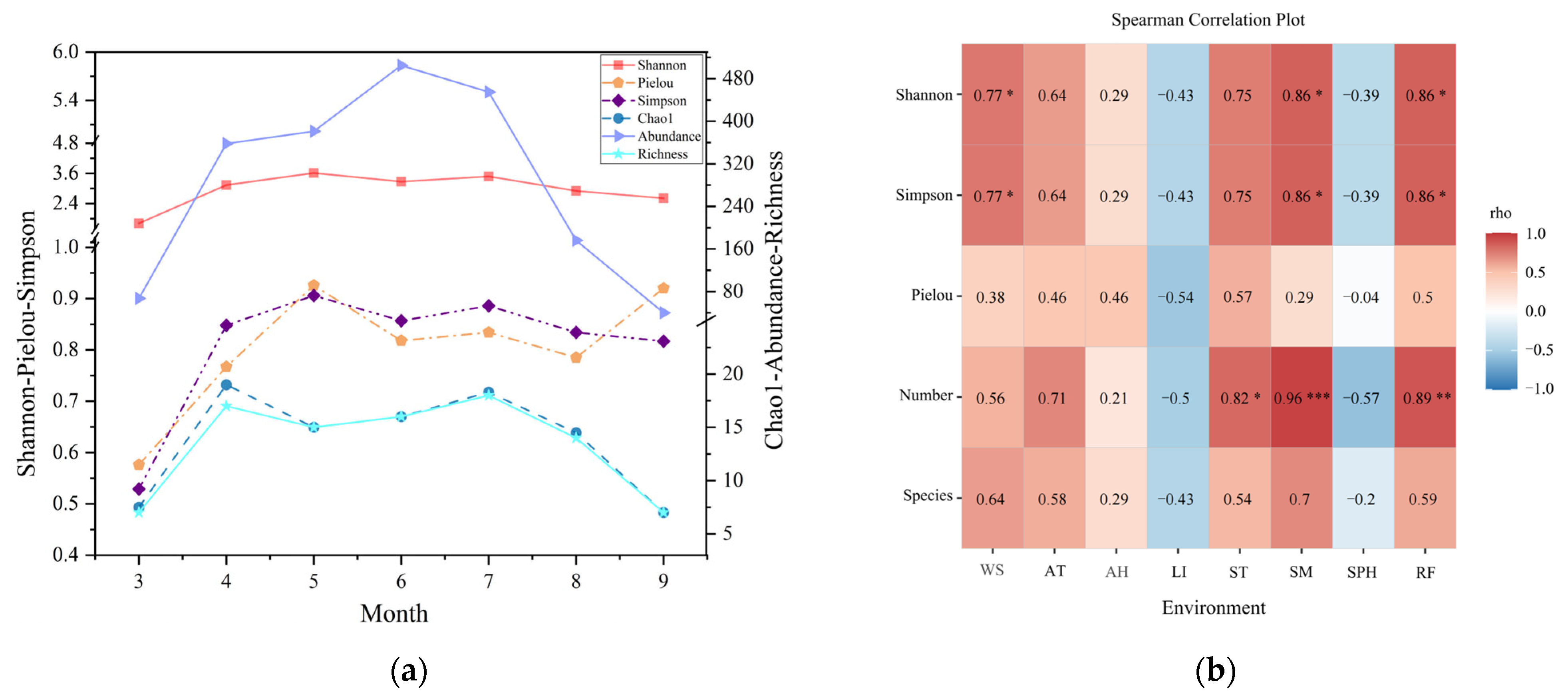
3.2. Species Diversity and Altitude Pattern
3.3. The Seasonal Variation in Amphibians’ Response to Climatic Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.; Baste, I.; Larigauderie, A.; Leadley, P.; Pascual, U.; Baptiste, B.; Demissew, S.; Dziba, L.; Erpul, G.; Fazel, A. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019; pp. 22–47. [Google Scholar]
- Mi, C.; Ma, L.; Yang, M.; Li, X.; Meiri, S.; Roll, U.; Oskyrko, O.; Pincheira-Donoso, D.; Harvey, L.P.; Jablonski, D. Global protected areas as refuges for amphibians and reptiles under climate change. Nat. Commun. 2023, 14, 1389. [Google Scholar] [PubMed]
- IUCN State of the World’s Amphibians: The Second Global Amphibian Assessment. Available online: https://www.iucnredlist.org/resources/sotwa (accessed on 28 May 2024).
- Xu, W.; Wu, Y.-H.; Zhou, W.-W.; Chen, H.-M.; Zhang, B.-L.; Chen, J.-M.; Xu, W.; Rao, D.-Q.; Zhao, H.; Yan, F. Hidden hotspots of amphibian biodiversity in China. Proc. Natl. Acad. Sci. USA 2024, 121, e2320674121. [Google Scholar] [PubMed]
- Chen, Y.; Zhang, J.; Jiang, J.; Nielsen, S.E.; He, F. Assessing the effectiveness of China’s protected areas to conserve current and future amphibian diversity. Divers. Distrib. 2017, 23, 146–157. [Google Scholar]
- Duan, R.-Y.; Kong, X.-Q.; Huang, M.-Y.; Varela, S.; Ji, X. The potential effects of climate change on amphibian distribution, range fragmentation and turnover in China. PeerJ 2016, 4, e2185. [Google Scholar]
- Ma, Q.; Wan, L.; Shi, S.; Wang, Z. Impact of climate change on the distribution of three rare salamanders (Liua shihi, Pseudohynobius jinfo, and Tylototriton wenxianensis) in Chongqing, China, and their conservation implications. Animals 2024, 14, 672. [Google Scholar] [CrossRef]
- Rahman, M.M.; Nneji, L.M.; Hossain, M.M.; Nishikawa, K.; Habib, K.A. Diversity and distribution of amphibians in central and northwest Bangladesh, with an updated checklist for the country. J. Asia-Pac. Biodivers. 2022, 15, 147–156. [Google Scholar] [CrossRef]
- Mi, C.; Huettmann, F.; Li, X.; Jiang, Z.; Du, W.; Sun, B. Effects of climate and human activity on the current distribution of amphibians in China. Conserv. Biol. 2022, 36, e13964. [Google Scholar] [CrossRef]
- Rahman, M.M.; Nneji, L.M.; Adeniyi, A.C.; Chen, J.; Eniang, E.A.; Oladipo, S.O.; Olatunde, O.; Onadeko, A.B.; Kilunda, F.K.; Ayoola, A.O. Amphibian assemblages and diversity patterns in two forest ecosystems of South-Eastern Nigeria. Afr. J. Ecol. 2020, 58, 815–827. [Google Scholar]
- Niknaddaf, Z.; Hemami, M.-R.; Pourmanafi, S.; Ahmadi, M. An integrative climate and land cover change detection unveils extensive range contraction in mountain newts. Glob. Ecol. Conserv. 2023, 48, e02739. [Google Scholar] [CrossRef]
- Jiang, D.; Zhao, X.; López-Pujol, J.; Wang, Z.; Qu, Y.; Zhang, Y.; Zhang, T.; Li, D.; Jiang, K.; Wang, B. Effects of climate change and anthropogenic activity on ranges of vertebrate species endemic to the Qinghai–Tibet Plateau over 40 years. Conserv. Biol. 2023, 37, e14069. [Google Scholar]
- Santer, B.D.; Po-Chedley, S.; Zelinka, M.D.; Cvijanovic, I.; Bonfils, C.; Durack, P.J.; Fu, Q.; Kiehl, J.; Mears, C.; Painter, J. Human influence on the seasonal cycle of tropospheric temperature. Science 2018, 361, eaas8806. [Google Scholar] [PubMed]
- Doherty-Bone, T.M.; Tamesse, J.L.; Gonwouo, L.N. What is driving declines of montane endemic amphibians? New insights from Mount Bamboutos, Cameroon. Oryx 2021, 55, 23–33. [Google Scholar]
- Ward, S.E.; Schulze, M.; Roy, B. A long-term perspective on microclimate and spring plant phenology in the Western Cascades. Ecosphere 2018, 9, e02451. [Google Scholar]
- Schmeller, D.S.; Urbach, D.; Bates, K.; Catalan, J.; Cogălniceanu, D.; Fisher, M.C.; Friesen, J.; Füreder, L.; Gaube, V.; Haver, M. Scientists’ warning of threats to mountains. Sci. Total Environ. 2022, 853, 158611. [Google Scholar]
- Lv, T.; Wang, N.; Xie, L.; Chen, S.; Zhao, R.; Feng, Y.; Li, Y.; Ding, H.; Fang, Y. Environmental heterogeneity affecting community assembly patterns and phylogenetic diversity of three forest communities at Mt. Huangshan, China. Forests 2022, 13, 133. [Google Scholar] [CrossRef]
- Díaz, S.; Malhi, Y. Biodiversity: Concepts, patterns, trends, and perspectives. Annu. Rev. Environ. Resour. 2022, 47, 31–63. [Google Scholar]
- Moore, J.W. The Capitalocene, Part I: On the nature and origins of our ecological crisis. J. Peasant. Stud. 2017, 44, 594–630. [Google Scholar]
- Jørgensen, P.S.; Folke, C.; Carroll, S.P. Evolution in the Anthropocene: Informing governance and policy. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 527–546. [Google Scholar]
- Hendry, A.P.; Gotanda, K.M.; Svensson, E.I. Human Influences on Evolution, and the Ecological and Societal Consequences; The Royal Society: London, UK, 2017; Volume 372. [Google Scholar]
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar]
- Hocking, D.J.; Babbitt, K.J. Amphibian contributions to ecosystem services. Herpetol. Conserv. Biol. 2014, 9(1), 1–17. [Google Scholar]
- Peterman, W.E.; Crawford, J.A.; Semlitsch, R.D. Productivity and significance of headwater streams: Population structure and biomass of the black-bellied salamander (Desmognathus quadramaculatus). Freshw. Biol. 2008, 53, 347–357. [Google Scholar] [CrossRef]
- Kafash, A.; Ashrafi, S.; Ohler, A.; Yousefi, M.; Malakoutikhah, S.; Koehler, G.; Schmidt, B.R. Climate change produces winners and losers: Differential responses of amphibians in mountain forests of the Near East. Glob. Ecol. Conserv. 2018, 16, e00471. [Google Scholar]
- Qiao, D.; Yuan, W.T.; Ke, S.F. China’s Natural Forest Protection Program: Evolution, impact and challenges. Int. For. Rev. 2021, 23, 338–350. [Google Scholar] [CrossRef]
- Delang, C.O.; Wang, W. Chinese forest policy reforms after 1998: The case of the natural forest protection program and the slope land conversion program. Int. For. Rev. 2013, 15, 290–304. [Google Scholar]
- Bolam, F.C.; Ahumada, J.; Akçakaya, H.R.; Brooks, T.M.; Elliott, W.; Hoban, S.; Mair, L.; Mallon, D.; McGowan, P.J.; Raimondo, D. Over half of threatened species require targeted recovery actions to avert human-induced extinction. Front. Ecol. Environ. 2023, 21, 64–70. [Google Scholar]
- Isbell, F.; Balvanera, P.; Mori, A.S.; He, J.S.; Bullock, J.M.; Regmi, G.R.; Seabloom, E.W.; Ferrier, S.; Sala, O.E.; Guerrero-Ramírez, N.R.J. Expert perspectives on global biodiversity loss and its drivers and impacts on people. Front. Ecol. Environ. 2023, 21, 94–103. [Google Scholar]
- Li, W.; Yang, P.; Xia, D.; Huffman, M.A.; Li, M.; Li, J.-H. Ecotourism Disturbance on an Endemic Endangered Primate in the Huangshan Man and the Biosphere Reserve of China: A Way to Move Forward. Biology 2022, 11, 1042. [Google Scholar] [CrossRef]
- Li, W.; Yang, P.; Li, B.; Liu, C.; Sun, L.; Li, J. Habitat characteristics or protected area size: What is more important for the composition and diversity of mammals in nonprotected areas? Ecol. Evol. 2021, 11, 7250–7263. [Google Scholar]
- Chen, S.; Ma, C.; Zhu, C.; Meadows, M.; Zhang, J.; Lu, H. Climate remained a major driving factor of vegetation dynamics over the past 600 years in Huangshan Mountain, Southeast China. Quat. Sci. Rev. 2023, 321, 108389. [Google Scholar] [CrossRef]
- Tang, X.; Fang, D.; Huang, X. Biodiversity And Conservation of Amphibians Andreptiles in Linnan Nature Reserve, Anhui Province. Sichuan J. Zool. 2001, 20, 64–67. [Google Scholar]
- Tang, X.; Nie, L. Amphibian Resources and Conservation of Huangshan City. Resour. Dev. Mark. 2008, 24, 455–457. [Google Scholar]
- Nishikawa, K.; Jiang, J.-P.; Matsui, M. Two new species of Pachytriton from Anhui and Guangxi, China (Amphibia: Urodela: Salamandridae). Curr. Herpetol. 2011, 30, 15–31. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Thapa, A.; Wu, R.; Hu, Y.; Nie, Y.; Singh, P.B.; Khatiwada, J.R.; Yan, L.I.; Gu, X.; Wei, F. Predicting the potential distribution of the endangered red panda across its entire range using MaxEnt modeling. Ecol. Evol. 2018, 8, 10542–10554. [Google Scholar] [CrossRef]
- Blank, L.; Blaustein, L. Using ecological niche modeling to predict the distributions of two endangered amphibian species in aquatic breeding sites. Hydrobiologia 2012, 693, 157–167. [Google Scholar] [CrossRef]
- Pineda, E.; Lobo, J.M. Assessing the accuracy of species distribution models to predict amphibian species richness patterns. J. Anim. Ecol. 2009, 78, 182–190. [Google Scholar] [CrossRef]
- Owen, E.; Zuliani, M.; Goldgisser, M.; Lortie, C.J. The importance of native shrubs on the distribution and diversity of reptiles and amphibians in the central drylands of Southwestern USA. Biodivers. Conserv. 2024, 33, 2131–2151. [Google Scholar] [CrossRef]
- Chen, B. Amphibian and Reptile Chronicles in Anhui Province; Anhui Science and Technology Press: Hefei, China, 1991. [Google Scholar]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Hammer, O. PAST–paleontological statistics, version 2.17. In Reference Manual 1999–2012; Natural History Museum, University of Oslo: Oslo, Norway, 2012. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Magurran, A. Ecological Diversity and its Measurement; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; pp. 1–23. [Google Scholar]
- Chao, A.; Yang, M.C.K. Stopping rules and estimation for recapture debugging with unequal failure rates. Biometrika 1993, 80, 193–201. [Google Scholar]
- Revelle, W.R. psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2017. [Google Scholar]
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2′. Creat. Elegant Data Vis. Using Gramm. Graphics. Version 2016, 2, 1–189. [Google Scholar]
- AmphibiaChina The Database of Chinese Amphibians. Electronic Database. Available online: http://www.amphibiachina.org/ (accessed on 15 March 2025).
- Che, J.; Chen, H.-M.; Yang, J.-X.; Jin, J.-Q.; Jiang, K.E.; Yuan, Z.-Y.; Murphy, R.W.; Zhang, Y.-P. Universal COI primers for DNA barcoding amphibians. Mol. Ecol. Resour. 2012, 12, 247–258. [Google Scholar] [PubMed]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Newman, J.C.; Riddell, E.A.; Williams, L.A.; Sears, M.W.; Barrett, K. Integrating physiology into correlative models can alter projections of habitat suitability under climate change for a threatened amphibian. Ecography 2022, 2022, e06082. [Google Scholar] [CrossRef]
- Kusano, T.; Nakagawa, T. Factors Affecting the Seasonal Activity of Japanese Red-Bellied Newts, Cynops pyrrhogaster (Amphibia: Salamandridae). Curr. Herpetol. 2024, 43, 55–67. [Google Scholar] [CrossRef]
- Dalpasso, A.; Seglie, D.; Eusebio Bergò, P.; Ciracì, A.; Compostella, M.; Laddaga, L.; Manica, M.; Marino, G.; Pandolfo, I.; Soldato, G. Effects of temperature and precipitation changes on shifts in breeding phenology of an endangered toad. Sci. Rep. 2023, 13, 14573. [Google Scholar]
- Pottier, P.; Kearney, M.R.; Wu, N.C.; Gunderson, A.R.; Rej, J.E.; Rivera-Villanueva, A.N.; Pollo, P.; Burke, S.; Drobniak, S.M.; Nakagawa, S. Vulnerability of amphibians to global warming. Nature 2025, 1–8. [Google Scholar]
- Rezende, E.L.; Castañeda, L.E.; Santos, M. Tolerance landscapes in thermal ecology. Funct. Ecol. 2014, 28, 799–809. [Google Scholar]
- Jørgensen, L.B.; Ørsted, M.; Malte, H.; Wang, T.; Overgaard, J. Extreme escalation of heat failure rates in ectotherms with global warming. Nature 2022, 611, 93–98. [Google Scholar]
- Guirguis, J.; Goodyear, L.E.B.; Finn, C.; Johnson, J.V.; Pincheira-Donoso, D. Risk of extinction increases towards higher elevations across the world’s amphibians. Glob. Ecol. Biogeogr. 2023, 32, 1952–1963. [Google Scholar]
- Rivera-Reyes, R.; Goyenechea Mayer-Goyenechea, I.; Ochoa Ochoa, L.M. Elevational diversity gradients of amphibians in Mexican mountain ranges: Patterns, environmental factors, and spatial scale effects. Biogeogr. J. Integr. Biogeogr. 2025, 40, a046. [Google Scholar]
- Jiang, K.; Pan, Z.; Pan, F.; Teuling, A.J.; Han, G.; An, P.; Chen, X.; Wang, J.; Song, Y.; Cheng, L. Combined influence of soil moisture and atmospheric humidity on land surface temperature under different climatic background. Iscience 2023, 26, 106837. [Google Scholar] [PubMed]
- Berg, A.; Lintner, B.R.; Findell, K.L.; Malyshev, S.; Loikith, P.C.; Gentine, P. Impact of soil moisture–atmosphere interactions on surface temperature distribution. J. Clim. 2014, 27, 7976–7993. [Google Scholar]
- The Huangshan City Statistical Yearbook in 2023. Available online: https://tjj.huangshan.gov.cn/tjnj/9158212.html (accessed on 28 October 2024).

| Codes | Environmental Predictors |
|---|---|
| Bio1 | Annual Mean Temperature |
| Bio2 | Mean Diurnal Range |
| Bio3 | Isothermal |
| Bio4 | Temperature Seasonality |
| Bio5 | Max Temperature of Warmest Month |
| Bio6 | Min Temperature of Coldest Month |
| Bio7 | Temperature Annual Range |
| Bio8 | Mean Temperature of Wettest Quarter |
| Bio9 | Mean Temperature of Driest Quarter |
| Bio10 | Mean Temperature of Warmest Quarter |
| Bio11 | Mean Temperature of Coldest Quarter |
| Bio12 | Annual Precipitation |
| Bio13 | Precipitation of Wettest Month |
| Bio14 | Precipitation of Driest Month |
| Bio15 | Precipitation of Seasonality, Coefficient of Variation |
| Bio16 | Precipitation of Wettest Quarter |
| Bio17 | Precipitation of Driest Quarter |
| Bio18 | Precipitation of Warmest Quarter |
| Bio19 | Precipitation of Coldest Quarter |
| Elev | Elevation |
| Waterbody_dis | Distance to Waterbody |
| NDVI | Normalized Difference Vegetation Index |
| Shrub | Shrub Distribution |
| Forests | Forests Distribution |
| Road_dis | Distance to Road |
| Farmland_dis | Distance to Farmland |
| Variables | Percentage Contribution | Permutation Importance |
|---|---|---|
| dis_farmland | 26.2% | 29.16% |
| dis_shrub | 15.6% | 11.16% |
| dis_waterbody | 10.6% | 11.14% |
| ndvi | 10.1% | 10.61% |
| bio3 | 8.9% | 10.59% |
| bio8 | 8.6% | 8.94% |
| Elev | 8.4% | 7.96% |
| bio15 | 6.5% | 6.80% |
| dis_forests | 5.1% | 3.63% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, F.; Pang, D.; Lin, X.; Huang, W.; Fang, J.; Li, W. Diversity and Distribution Patterns of Amphibians in the Huangshan Mountain Region: The Roles of Climate and Human Activities. Animals 2025, 15, 938. https://doi.org/10.3390/ani15070938
Hong F, Pang D, Lin X, Huang W, Fang J, Li W. Diversity and Distribution Patterns of Amphibians in the Huangshan Mountain Region: The Roles of Climate and Human Activities. Animals. 2025; 15(7):938. https://doi.org/10.3390/ani15070938
Chicago/Turabian StyleHong, Fei, Dapeng Pang, Xiaojia Lin, Weixin Huang, Jie Fang, and Wenbo Li. 2025. "Diversity and Distribution Patterns of Amphibians in the Huangshan Mountain Region: The Roles of Climate and Human Activities" Animals 15, no. 7: 938. https://doi.org/10.3390/ani15070938
APA StyleHong, F., Pang, D., Lin, X., Huang, W., Fang, J., & Li, W. (2025). Diversity and Distribution Patterns of Amphibians in the Huangshan Mountain Region: The Roles of Climate and Human Activities. Animals, 15(7), 938. https://doi.org/10.3390/ani15070938





