Dissipation Kinetics and Dietary Risk Assessment of Boscalid Residues in Two Table-Grape Varieties Under Field Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Experimental Set-Up
2.3. Sample Preparation
2.4. HPLC Analysis
2.5. Method Validation
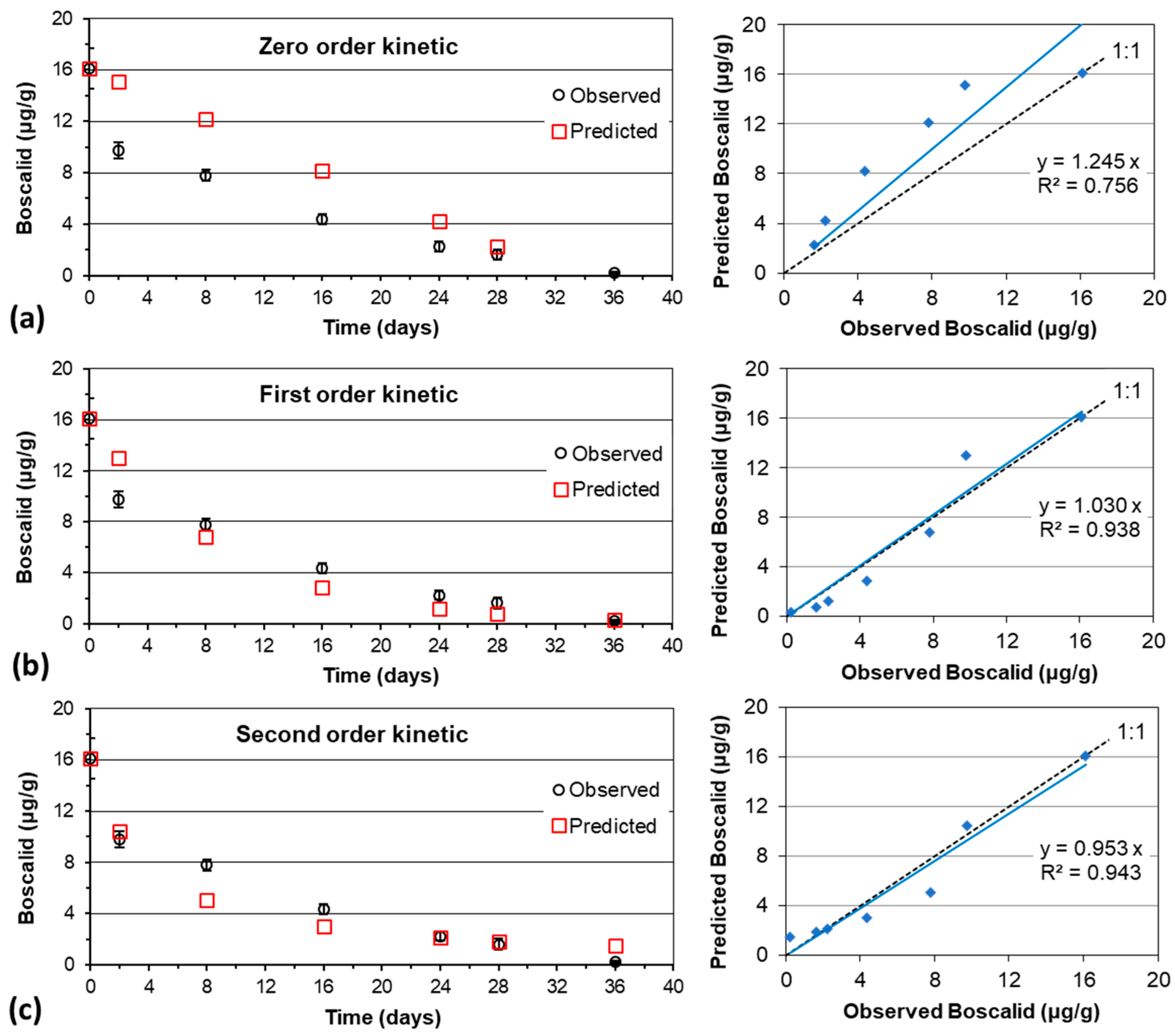
2.6. Kinetic Models for Modeling of Dissipation
2.7. Evaluation of Risk Assessment
3. Results and Discussion
3.1. Residues and Dissipation Kinetics of Boscalid in Table Grapes
3.2. Dietary Risk Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roselli, L.; Casieri, A.; de Gennaro, B.C.; Sardaro, R.; Russo, G. Environmental and Economic Sustainability of Table Grape Production in Italy. Sustainability 2020, 12, 3670. [Google Scholar] [CrossRef]
- Tegopoulos, K.; Tsirka, T.; Stekas, C.; Gerasimidi, E.; Skavdis, G.; Kolovos, P.; Grigoriou, M.E. Spatiotemporal Dynamics of Assyrtiko Grape Microbiota. Microorganisms 2024, 12, 577. [Google Scholar] [CrossRef]
- Syrgabek, Y.; Alimzhanova, M. Modern Analytical Methods for the Analysis of Pesticides in Grapes: A Review. Foods 2022, 11, 1623. [Google Scholar] [CrossRef] [PubMed]
- Lo’ay, A.A.; Rabie, M.M.; Alhaithloul, H.A.S.; Alghanem, S.M.S.; Ibrahim, A.M.; Abdein, M.A.; Abdelgawad, Z.A. On the Biochemical and Physiological Responses of ‘Crimson Seedless’ Grapes Coated with an Edible Composite of Pectin, Polyphenylene Alcohol, and Salicylic Acid. Horticulturae 2021, 7, 498. [Google Scholar] [CrossRef]
- Wang, W.-N.; Qian, Y.-H.; Liu, R.-H.; Liang, T.; Ding, Y.-T.; Xu, X.-L.; Huang, S.; Fang, Y.-L.; Ju, Y.-L. Effects of Table Grape Cultivars on Fruit Quality and Aroma Components. Foods 2023, 12, 3371. [Google Scholar] [CrossRef]
- Tsalidis, G.A. Human Health and Ecosystem Quality Benefits with Life Cycle Assessment Due to Fungicides Elimination in Agriculture. Sustainability 2022, 14, 846. [Google Scholar] [CrossRef]
- Schusterova, D.; Hajslova, J.; Kocourek, V.; Pulkrabova, J. Pesticide Residues and Their Metabolites in Grapes and Wines from Conventional and Organic Farming System. Foods 2021, 10, 307. [Google Scholar] [CrossRef]
- Gikas, G.D.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of Fungicides and Factors Affecting Their Fate and Removal Efficacy: A Review. Sustainability 2022, 14, 4056. [Google Scholar] [CrossRef]
- Golge, O.; Kabak, B. Pesticide residues in table grapes and exposure assessment. J. Agric. Food Chem. 2018, 66, 1701–1713. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Moschopoulou, G.; Tsekouras, V.; Mercader, J.V.; Abad-Fuentes, A.; Kintzios, S. Development of a Portable Cell-Based Biosensor for the Ultra-Rapid Screening for Boscalid Residues in Lettuce. Biosensors 2024, 14, 311. [Google Scholar] [CrossRef] [PubMed]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Zinc nanoparticles: Mode of action and efficacy against boscalid-resistant Alternaria alternata isolates. Sci. Total Environ. 2022, 829, 154638. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.; Srivastava, A.; Srivastava, P.C. An insight into the sorption kinetics of boscalid onto soils: Effect of general soil properties. Chemosphere 2023, 325, 138274. [Google Scholar] [CrossRef]
- d’Hose, D.; Isenborghs, P.; Brusa, D.; Jordan, B.F.; Gallez, B. The Short-Term Exposure to SDHI Fungicides Boscalid and Bixafen Induces a Mitochondrial Dysfunction in Selective Human Cell Lines. Molecules 2021, 26, 5842. [Google Scholar] [CrossRef]
- Qian, L.; Cui, F.; Yang, Y.; Liu, Y.; Qi, S.; Wang, C. Mechanisms of developmental toxicity in zebrafish embryos (Danio rerio) induced by boscalid. Sci. Total Environ. 2018, 634, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Qi, S.; Cao, F.; Zhang, J.; Zhao, F.; Li, C.; Wang, C. Toxic effects of boscalid on the growth, photosynthesis, antioxidant system and metabolism of Chlorella vulgaris. Environ. Pollut. 2018, 242, 171–181. [Google Scholar] [CrossRef]
- Papaevangelou, V.A.; Gikas, G.D.; Vryzas, Z.; Tsihrintzis, V.A. Treatment of agricultural equipment rinsing water containing a fungicide in pilot-scale horizontal subsurface flow constructed wetlands. Ecol. Eng. 2017, 101, 193–200. [Google Scholar] [CrossRef]
- Selim, M.T.; Almutari, M.M.; Shehab, H.I.; EL-Saeid, M.H. Risk Assessment of Pesticide Residues by GC-MSMS and UPLC-MSMS in Edible Vegetables. Molecules 2023, 28, 1343. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Zhang, Y.; Si, W.; Chen, L.; Shen, G.; Bai, B.; Zhou, C. Determination and dietary risk assessment of 284 pesticide residues in local fruit cultivars in Shanghai, China. Sci. Rep. 2021, 11, 9681. [Google Scholar] [CrossRef]
- Zikos, C.; Evangelou, A.; Karachaliou, C.-E.; Gourma, G.; Blouchos, P.; Moschopoulou, G.; Yialouris, C.; Griffiths, J.; Johnson, G.; Petrou, P.; et al. Commercially available chemicals as immunizing haptens for the development of a polyclonal antibody recognizing carbendazim and other benzimidazole-type fungicides. Chemosphere 2015, 119, S16–S20. [Google Scholar] [CrossRef] [PubMed]
- Angioni, A.; Dedola, F.; Garau, V.L.; Schirra, M.; Caboni, P. Fate of iprovalicarb, indoxacarb, and boscalid residues in grapes and wine by GC-ITMS analysis. J. Agric. Food Chem. 2011, 59, 6806–6812. [Google Scholar] [CrossRef]
- Kuchheuser, P.; Birringer, M. Pesticide residues in food in the European Union: Analysis of notifications in the European Rapid Alert System for Food and Feed from 2002 to 2020. Food Control 2022, 133, 108575. [Google Scholar] [CrossRef]
- Dong, M.; Wen, G.; Tang, H.; Wang, T.; Zhao, Z.; Song, W.; Wang, W.; Zhao, L. Dissipation and safety evaluation of novaluron, pyriproxyfen, thiacloprid and tolfenpyrad residues in the citrus-field ecosystem. Food Chem. 2018, 269, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Parlakidis, P.; Gounari, I.; Georgiou, A.; Adamidis, G.; Vryzas, Z.; Gikas, G.D. Removal of Two Triazole Fungicides from Agricultural Wastewater in Pilot-Scale Horizontal Subsurface Flow Constructed Wetlands. Agronomy 2023, 13, 265. [Google Scholar] [CrossRef]
- Parlakidis, P.; Adamidis, G.; Alexoudis, C.; Pythoglou, P.; Papadopoulos, S.; Vryzas, Z. Adjuvant Effects on Pyraclostrobin and Boscalid Residues, Systemic Movement, and Dietary Risk in Garlic under Field Conditions. Agriculture 2023, 13, 1636. [Google Scholar] [CrossRef]
- Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. Document No. SANTE/11945/2015. EC Directorate-General for Health and Food Safety. 2015. Available online: https://www.eurl-pesticides.eu/library/docs/allcrl/AqcGuidance_SANTE_2015_11945.pdff (accessed on 15 January 2025).
- FOCUS. Guidance Document on Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration. In Report of the FOCUS Work Group on Degradation Kinetics; EC Document Reference Sanco/10058/2005 version 2.0; European Soil Data Centre (ESDAC): Ispra, Italy; 434p, Available online: https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/dk/docs/finalreportFOCDegKinetics.pdf (accessed on 15 January 2025).
- Torabi, E.; Talebi Jahromi, K.; Homayoonzadeh, M.; Torshiz, A.O.; Tavakoli, E. Residue kinetics of neonicotinoids and abamectin in pistachio nuts under field conditions: Model selection, effects of multiple sprayings, and risk assessment. Environ. Sci. Pollut. Res. 2022, 29, 2598–2612. [Google Scholar] [CrossRef]
- Boskidis, I.; Gikas, G.D.; Pisinaras, V.; Tsihrintzis, V.A. Spatial and temporal changes of water quality, and SWAT modeling of Vosvozis river basin, North Greece. J. Environ. Sci. Health A 2010, 45, 1421–1440. [Google Scholar] [CrossRef]
- Gikas, G.D. Water quality of drainage canals and assessment of nutrient loads using QUAL2Kw. Environ. Process. 2014, 1, 369–385. [Google Scholar] [CrossRef]
- IPCS (The International Programme on Chemical Safety). Principleand Methods for the Risk Assessment of Chemicals in Food; Environmental Health Criteria 240; World Health Organization: Stuttgart, Germany, 2009. [Google Scholar]
- EFSA Scientific Committee (EFSA). Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA J. 2012, 10, 32. [Google Scholar] [CrossRef]
- He, Y.; Meng, M.; Yohannes, W.K.; Khan, M.; Wang, M.; Abd El-Aty, A.M.; Hacımüftüoğlu, F.; He, Y.; Gao, L.; She, Y. Dissipation pattern and residual levels of boscalid in cucumber and soil using liquid chromatography-tandem mass spectrometry. J. Environ. Sci. Health B 2020, 55, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Su, Y.; Dong, B.; Lu, W.; Hu, J.; Liu, X. Dissipation Residue Behaviors and Dietary Risk Assessment of Boscalid and Pyraclostrobin in Watermelon by HPLC-MS/MS. Molecules 2022, 27, 4410. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, S.; Gao, Y.; Ma, Y.; Hua, J.; Liu, X. Dissipation behavior, residue distribution and dietary risk assessment of field-incurred boscalid and pyraclostrobin in grape and grape field soil via MWCNTs-based QuEChERS using an RRLC-QqQ-MS/MS technique. Food Chem. 2019, 274, 291–297. [Google Scholar] [CrossRef]
- Alister, C.; Araya, M.; Becerra, K.; Saavedra, J.; Kogan, M. Preharvest interval periods and their relation to fruit growth stages and pesticide formulations. Food Chem. 2017, 221, 548–554. [Google Scholar] [CrossRef]
- Wołejko, E.; Oozowicka, B.; Kaczynski, P.; Jankowska, M.; Piekut, J. The Influence of Effective Microorganisms (EM) and Yeast on the Degradation of Strobilurins and Carboxamides in Leafy Vegetables Monitored by LC-MS/MS and Health Risk Assessment. Environ. Monit. Assess. 2016, 188, 64. [Google Scholar] [CrossRef]
- Bagi, F.F.; Budakov, D.B.; Bursić, V.P.; Stojšin, V.B.; Lazić, S.D.; Vuković, S.M. Efficacy of azoxystrobin for the control of cucumber downy mildew (Pseudoperonospora cubensis) and fungicide residue analysis. Crop Protet. 2014, 61, 74–78. [Google Scholar] [CrossRef]
- Munitz, M.S.; Resnik, S.L.; Montti, M.I. Method development and validation for boscalid in blueberries by solid-phase microextraction gas chromatography, and their degradation kinetics. Food Chem. 2013, 136, 1399–1404. [Google Scholar] [CrossRef]
- Jankowska, M.; Kaczynski, P.; Hrynko, I.; Lozowicka, B. Dissipation of Six Fungicides in Greenhouse-Grown Tomatoes with Processing and Health Risk. Environ. Sci. Pollut. Res. Int. 2016, 23, 11885–11900. [Google Scholar] [CrossRef]
- Sadło, S.; Szpyrka, E.; Stawarczyk, M.; Piechowicz, B. Behavior of pyrimethanil, pyraclostrobin, boscalid, cypermethrin and chlorpyrifos residues on raspberry fruit and leaves of Laszka variety. J. Environ. Sci. Health B 2014, 49, 159–168. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S. Dissipation and Residues of Boscalid in Strawberries and soils. Bull. Environ. Contam. Toxicol. 2010, 84, 301–304. [Google Scholar] [CrossRef]
- Podbielska, M.; Szpyrka, E.; Piechowicz, B.; Sadło, S.; Sudoł, M. Assessment of Boscalid and Pyraclostrobin Disappearance and Behavior following Application of Effective Microorganisms on Apples. J. Environ. Sci. Health B 2018, 53, 652–660. [Google Scholar] [CrossRef]
- Tripathi, V.; Abhilash, P.C.; Singh, H.B.; Singh, N.; Patra, D.D. Effect of temperature variation on lindane dissipation and microbial activity in soil. Ecol. Eng. 2015, 79, 54–59. [Google Scholar] [CrossRef]
- Parlakidis, P.; Rodriguez, M.S.; Gikas, G.D.; Alexoudis, C.; Perez-Rojas, G.; Perez-Villanueva, M.; Carrera, A.P.; Fernández-Cirelli, A.; Vryzas, Z. Occurrence of Banned and Currently Used Herbicides, in Groundwater of Northern Greece: A Human Health Risk Assessment Approach. Int. J. Environ. Res. Public Health 2022, 19, 8877. [Google Scholar] [CrossRef]
- Parlakidis, P.; Adamidis, G.S.; Gikas, G.D.; Vasiliou, S.; Kissa, M.; Doitsinis, K.; Alexoudis, C.; Vryzas, Z. Dissipation Kinetics, Leaching, and Ecological Risk Assessment of S-Metolachlor and Benfluralin Residues in Soil. Environments 2024, 11, 18. [Google Scholar] [CrossRef]
- Paramasivam, M. Dissipation kinetics, dietary and ecological risk assessment of chlorantraniliprole residue in/on tomato and soil using GC-MS. J. Food Sci. Technol. 2021, 58, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Torabi, E.; Talebi, K. Diazinon residues and degradation kinetics for grapes under field conditions. J. Environ. Sci. Health. B 2013, 48, 260–265. [Google Scholar] [CrossRef]
- Commission Regulation (EU). 2022/1324 of 28 July 2022 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for benzovindiflupyr, boscalid, fenazaquin, fluazifop-P, flupyradifurone, fluxapyroxad, fosetyl-Al, isofetamid, metaflumizone, pyraclostrobin, spirotetramat, thiabendazole and tolclofos-methyl in or on certain products (Text with EEA relevance). Off. J. Eur. Union 2022, 68–108. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022R1324 (accessed on 23 January 2025).
- Munir, S.; Azeem, A.; Sikandar Zaman, M.; Zia Ul Haq, M. From field to table: Ensuring food safety by reducing pesticide residues in food. Sci. Total Environ. 2024, 922, 171382. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, R.; Yuan, L. Dissipation, Residue and Human Dietary Risk Assessment of Pyraclostrobin and Cyazofamid in Grapes Using an HPLC-UV Detector. Foods 2024, 13, 314. [Google Scholar] [CrossRef]

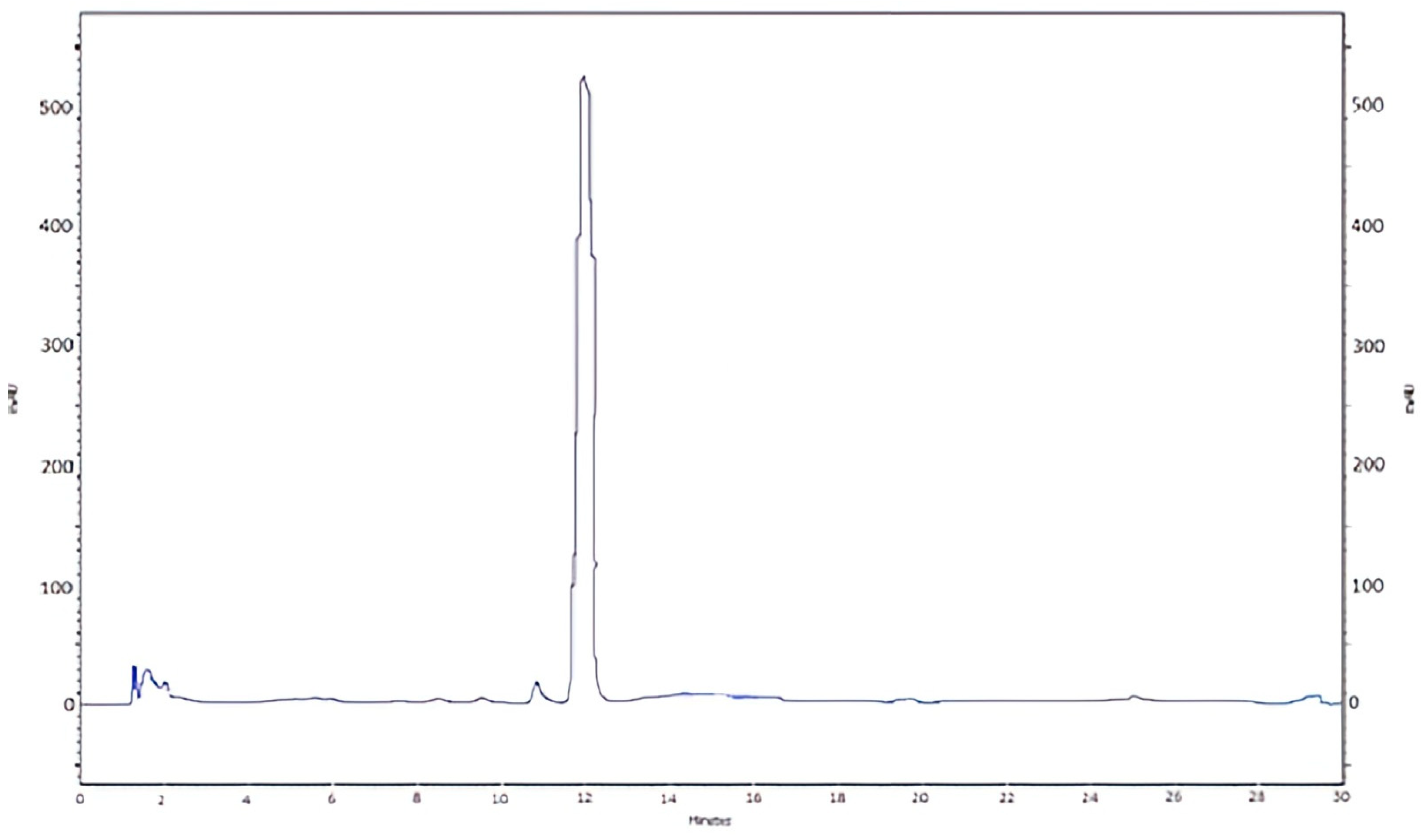

| Days After Application | Soultanina | Crimson | ||
|---|---|---|---|---|
| Residue (µg/g) 1 | Residue Decline (%) | Residue (µg/g) 1 | Residue Decline (%) | |
| 0 (2 h) | 17.70 (±2.79) | - | 16.11 (±1.58) | - |
| 2 | 10.85 (±1.58) | 38.70 | 9.77 (±0.63) | 39.35 |
| 8 | 5.75 (±1.09) | 67.51 | 7.79 (±0.42) | 51.64 |
| 16 | 3.61 (±1.06) | 79.60 | 4.37 (±0.38) | 72.87 |
| 24 | 2.20 (±0.80) | 87.57 | 2.25 (±0.39) | 86.03 |
| 28 | 1.24 (±0.39) | 92.99 | 1.62 (±0.40) | 89.94 |
| 36 | 0.11 (±0.02) | 99.38 | 0.23 (±0.10) | 98.57 |
| Model Kinetic | Performance Criteria | Table-Grape Variety | |
|---|---|---|---|
| Soultanina | Crimson | ||
| Zero order | 1 γ | 1.26 | 1.25 |
| 2 R2 | 0.66 | 0.76 | |
| 3 NOF | 0.67 | 0.51 | |
| 4 NSE | 0.54 | 0.65 | |
| 5 MAE | 2.8 | 2.34 | |
| First order | γ | 1.04 | 1.03 |
| R2 | 0.96 | 0.94 | |
| NOF | 0.23 | 0.25 | |
| NSE | 0.95 | 0.92 | |
| MAE | 0.983 | 1.100 | |
| Second order | γ | 0.99 | 0.95 |
| R2 | 0.99 | 0.94 | |
| NOF | 0.11 | 0.21 | |
| NSE | 0.99 | 0.940 | |
| MAE | 0.49 | 0.91 | |
| Parameters | Table-Grape Variety | |
|---|---|---|
| Soultanina | Crimson | |
| Zero-order model (ZO): C = C0 − kt | ||
| Kinetic equation | C = 17.72–0.585t | C = 16.11–0.495t |
| k (1/day) | 0.59 | 0.50 |
| DT50 (days) | 15.14 | 16.30 |
| DT90 (days) | 27.26 | 29.30 |
| First-order model (FO): lnC = lnC0 − kt | ||
| Kinetic equation | lnC = ln17.72–0.135t | lnC = ln16.11–0.108t |
| k (1/day) | 0.14 | 0.11 |
| DT50 (days) | 5.14 | 6.42 |
| DT90 (days) | 17.05 | 21.32 |
| PHI (days) | 9.37 | 10.90 |
| Second-order model (SO): C =C0/(1 + C0kt) | ||
| Kinetic equation | C = 17.72/(1 + 0.301t) | C = 16.11/(1 + 0.274t) |
| k (1/day) | 0.02 | 0.02 |
| DT50 (days) | 3.32 | 3.65 |
| DT90 (days) | 29.87 | 32.86 |
| PHI (days) | 8.45 | 8.11 |
| Table Grape | Days After Application | Children | Adults | ||
|---|---|---|---|---|---|
| Exposure (μg/gbw 1 day) | HQ 2 | Exposure (μg/gbw/day) | HQ | ||
| Soultanina | 0 (2 h) | 0.12 | 3.10 | 0.04 | 1.02 |
| 2 | 0.09 | 1.90 | 0.03 | 0.62 | |
| 8 | 0.04 | 1.01 | 0.01 | 0.33 | |
| 16 | 0.03 | 0.63 | 0.01 | 0.21 | |
| 24 | 0.02 | 0.39 | 0.01 | 0.13 | |
| 28 | 0.01 | 0.22 | 3.0 × 10−3 | 0.07 | |
| 36 | 1.0 × 10−3 | 0.02 | 0.00 | 0.01 | |
| Crimson | 0 (2 h) | 0.11 | 2.82 | 0.04 | 0.93 |
| 2 | 0.07 | 1.71 | 0.02 | 0.56 | |
| 8 | 0.06 | 1.36 | 0.02 | 0.45 | |
| 16 | 0.03 | 0.76 | 0.01 | 0.25 | |
| 24 | 0.02 | 0.39 | 0.01 | 0.13 | |
| 28 | 0.01 | 0.28 | 4.0 × 10−3 | 0.09 | |
| 36 | 2.0 × 10−3 | 0.04 | 1.0 × 10−3 | 0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parlakidis, P.; Adamidis, G.S.; Doulaveris, T.; Makaridis, D.; Alexoudis, C.; Vryzas, Z.; Gikas, G.D. Dissipation Kinetics and Dietary Risk Assessment of Boscalid Residues in Two Table-Grape Varieties Under Field Conditions. Environments 2025, 12, 133. https://doi.org/10.3390/environments12050133
Parlakidis P, Adamidis GS, Doulaveris T, Makaridis D, Alexoudis C, Vryzas Z, Gikas GD. Dissipation Kinetics and Dietary Risk Assessment of Boscalid Residues in Two Table-Grape Varieties Under Field Conditions. Environments. 2025; 12(5):133. https://doi.org/10.3390/environments12050133
Chicago/Turabian StyleParlakidis, Paraskevas, George S. Adamidis, Theodoros Doulaveris, Dimitrios Makaridis, Christos Alexoudis, Zisis Vryzas, and Georgios D. Gikas. 2025. "Dissipation Kinetics and Dietary Risk Assessment of Boscalid Residues in Two Table-Grape Varieties Under Field Conditions" Environments 12, no. 5: 133. https://doi.org/10.3390/environments12050133
APA StyleParlakidis, P., Adamidis, G. S., Doulaveris, T., Makaridis, D., Alexoudis, C., Vryzas, Z., & Gikas, G. D. (2025). Dissipation Kinetics and Dietary Risk Assessment of Boscalid Residues in Two Table-Grape Varieties Under Field Conditions. Environments, 12(5), 133. https://doi.org/10.3390/environments12050133






