Distribution of Endocrine Disruptor Chemicals and Bacteria in Saline Pétrola Lake (Albacete, SE Spain) Protected Area is Strongly Linked to Land Use
Abstract
1. Introduction
2. Materials and Methods
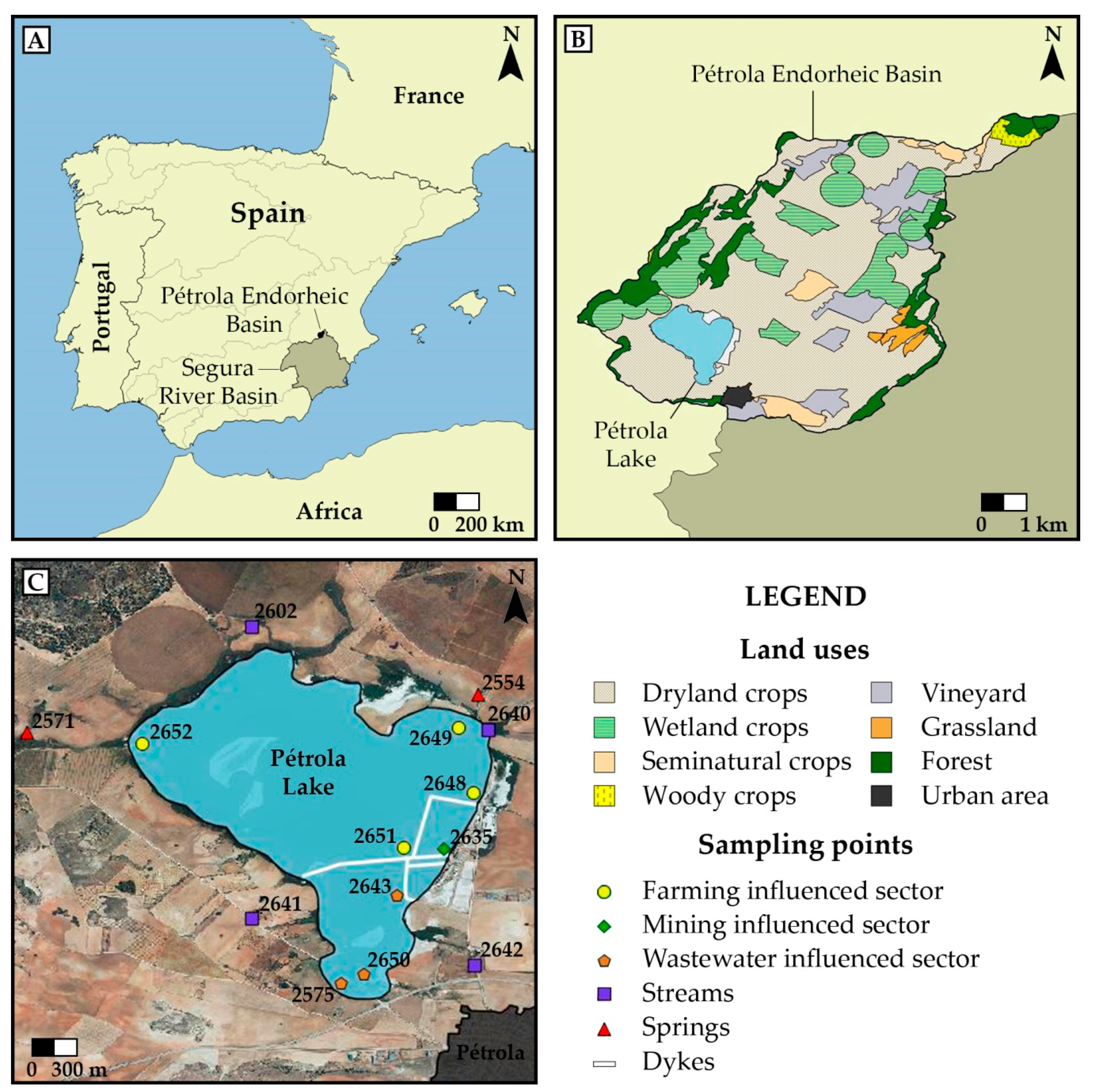
2.1. Study Area
2.2. Sample Collection
2.2.1. Water Sampling
2.2.2. Sediment Sampling
2.3. Endocrine Disrupting Chemicals Analysis
2.4. DNA Extraction and MiSeq Illumina Sequencing
2.5. Statistical Analysis
3. Results and Discussion
3.1. Endocrine Disrupting Chemicals Distribution
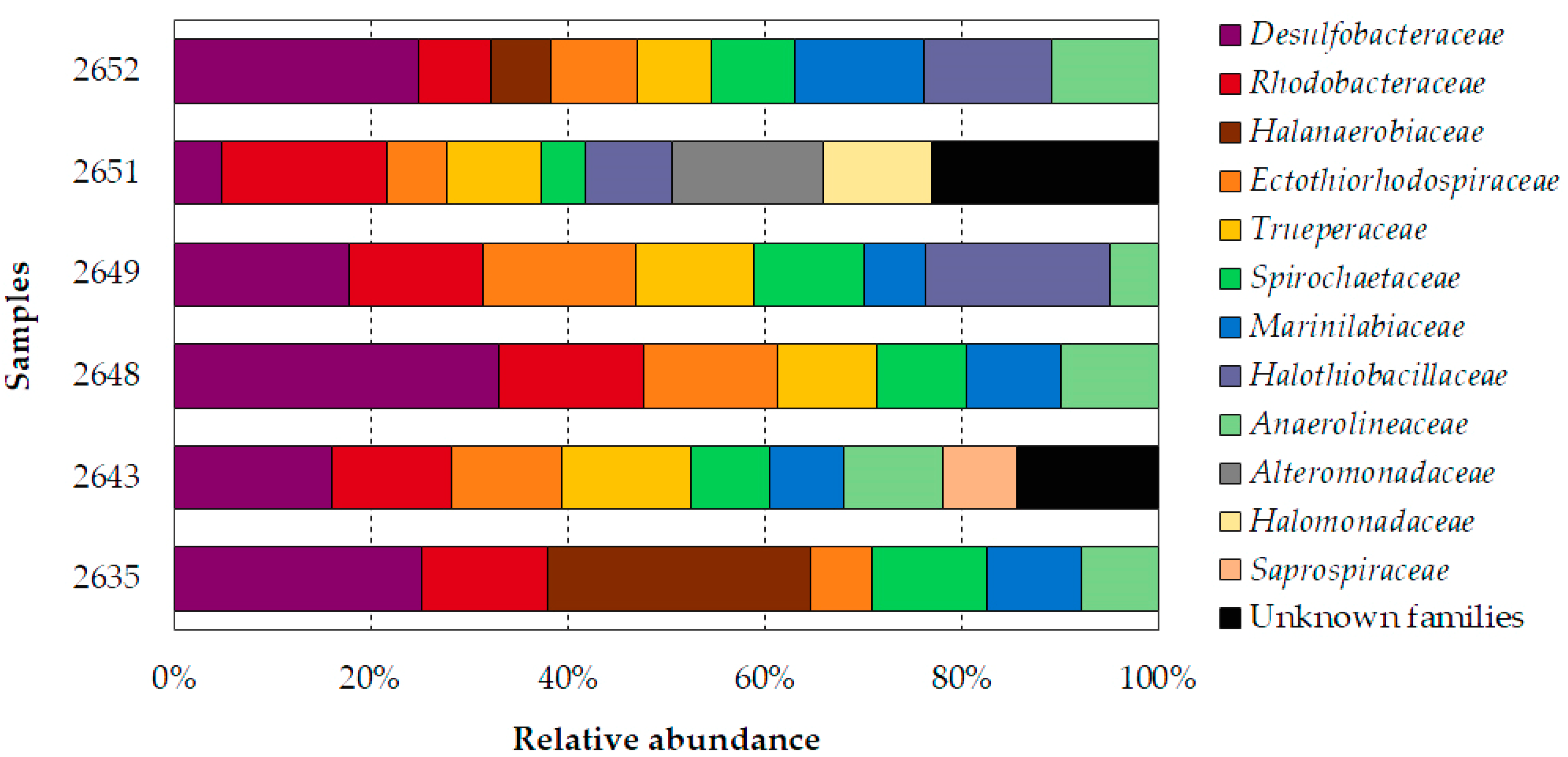
3.2. Microbial Community Structure
3.3. Land Use Influence on EDC Distribution and Bacteria Community Structure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Messerli, P.; Murniningtyas, E.; Eloundou-Enyegue, P.; Foli, E.G.; Furman, E.; Glassman, A.; Hernández-Licona, G.; Kim, E.M.; Lutz, W.; Moatti, J.-P.; et al. The Future is Now–Science for Achieving Sustainable Development. In Global Sustainable Development Report; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Colborn, T.; Vom Saal, F.S.; Soto, A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993, 101, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.M.; Hester, R.E. Endocrine Disrupting Chemicals; The Royal Society of Chemistry: London, UK, 1999; Volume 12, pp. 1–26. ISBN 978-0-85404-255-5. [Google Scholar] [CrossRef]
- Katagi, T. Behavior of pesticides in water-sediment systems. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 2006; Volume 187, pp. 133–251. ISBN 978-0-387-30237-9. [Google Scholar] [CrossRef]
- Shibata, A.; Kodaka, R.; Fujisawa, T.; Katagi, T. Degradation of flumioxazin in illuminated water–sediment systems. J. Agric. Food Chem. 2011, 59, 11186–11195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, H.; Ping, L.; Cai, X.; Wu, M.; He, H.; Zhang, C.; Zhu, Y.; Li, Z. Degradation of rizazole in water–sediment systems. J. Environ. Sci. Health B 2013, 48, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, C.; Xiong, X.; Zhang, K.; Liu, J. Partitioning and degradation of triclosan and formation of methyl-triclosan in water-sediment systems. Water Air Soil Pollut. 2014, 225, 2099. [Google Scholar] [CrossRef]
- Gómez-Alday, J.J.; Carrey, R.; Valiente, N.; Otero, N.; Soler, A.; Ayora, C.; Sanz, D.; Muñoz-Martín, A.; Castaño, S.; Recio, C.; et al. Denitrification in a hypersaline lake–aquifer system (Pétrola Basin, Central Spain): The role of recent organic matter and Cretaceous organic rich sediments. Sci. Total Environ. 2014, 497–498, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Valiente, N.; Carrey, R.; Otero, N.; Soler, A.; Sanz, D.; Muñoz-Martín, A.; Jirsa, F.; Wanek, W.; Gómez-Alday, J.J. A multi-isotopic approach to investigate the influence of land use on nitrate removal in a highly saline lake-aquifer system. Sci. Total Environ. 2018, 631–632, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Bergman, Å.; Heindel, J.J.; Kasten, T.; Kidd, K.A.; Jobling, S.; Neira, M.; Zoeller, R.T.; Becher, G.; Bjerregaard, P.; Bornman, R.; et al. The impact of endocrine disruption: A consensus statement on the state of the science. Environ. Health Perspect. 2013, 121. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Fu, Q.S.; Criddle, C.S.; Leckie, J.O. Effect of flux (transmembrane pressure) and membrane properties on fouling and rejection of reverse osmosis and nanofiltration membranes treating perfluorooctane sulfonate containing wastewater. Environ. Sci. Technol. 2007, 41, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Karigar, C.S.; Rao, S.S. Role of microbial enzymes in the bioremediation of pollutants: A review. Enzyme Res. 2011. [Google Scholar] [CrossRef] [PubMed]
- Abatenh, E.; Gizaw, B.; Tsegaye, Z.; Wassie, M. The role of microorganisms in bioremediation—A review. Open J. Environ. Biol. 2017, 2, 38–46. [Google Scholar] [CrossRef]
- Dountcheva, I.; Doña, C.; Sanz, D.; Sánchez, J.M.; Gómez-Alday, J.J. Quantitative approach to water balance in groundwater discharge hypersaline wetlands. Pétrola lake, SE Spain. Proceedings of IAH2019, the 46 Annual Congress of the Internaonal Associaon of Hydrogeologists, Málaga, Spain, 22–27 September 2019; Gómez-Hernández, J.J., Andreo-Navarro, B., Eds.; Asociación Internacional de Hidrogeólogos—Grupo Español: Barcelona, Spain, 2019. [Google Scholar]
- Valiente, N.; Carrey, R.; Otero, N.; Gutierrez-Villanueva, M.A.; Soler, A.; Sanz, D.; Castaño, S.; Gómez-Alday, J.J. Tracing sulfate recycling in the hypersaline Pétrola Lake (SE Spain): A combined isotopic and microbiological approach. Chem. Geol. 2017, 473, 74–89. [Google Scholar] [CrossRef]
- Valiente, N. A Multidisciplinary Approach for Assessing Natural Attenuation of Pollutants in a Highly Saline Lake-Aquifer System: The Case of Pétrola Lake, Spain. Ph.D. Thesis, University of Castilla-La Mancha, Albacete, Spain, August 2018. [Google Scholar]
- Wylie, P.L. Screening for 926 Pesticides and Endocrine Disruptors by GC/MS with Deconvolution Reporting Software and a New Pesticide Library; Application Note; Agilent Technologies: Wilmington, DE, USA, 2006. [Google Scholar]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, J.F.; Highlander, S.; Luna, R.A.; Gibbs, R.A.; Versalovic, J. Metagenomic Pyrosequencing and Microbial Identification. Clin. Chem. 2009, 55, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Vonberg, D.; Vanderborght, J.; Cremer, N.; Pütz, T.; Herbst, M.; Vereecken, H. 20 years of long-term atrazine monitoring in a shallow aquifer in western Germany. Water Res. 2014, 50, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Blum, K.M.; Jernstedt, H.; Renman, G.; Rodríguez-Mozaz, S.; Haglund, P.; Andersson, P.L.; Wiberg, K.; Ahrens, L. Screening and prioritization of micropollutants in wastewaters from on-site sewage treatment facilities. J. Hazard. Mater. 2017, 328, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Coelhan, M.; Bromig, K.H.; Glas, K.; Roberts, A.L. Determination and levels of the biocide ortho-phenylphenol in canned beers from different countries. J. Agric. Food Chem. 2006, 54, 5731–5735. [Google Scholar] [CrossRef] [PubMed]
- Waechter, F.; Weber, E.; Hertner, T.; May-Hertl, U. Cyprodinil: A fungicide of the anilinopyrimidine class. In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Syngenta Crop Protection AG: Basle, Switzerland, 2010; pp. 1903–1913. [Google Scholar] [CrossRef]
- Mishra, S.; Saini, M.K.; Singh, M.K.; Alam, S.; Raza, S.K.; Thakur, L.K.; Mukharjee, S. Method Development and validation for identification of 2-pheny phenol in siapton 10L by gas chromatography mass spectrometry. World J. Pharm. Pharm. Sci. 2015, 4, 783–792. [Google Scholar]
- European Union. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union 2013, 226, 1–17. [Google Scholar]
- Li, W.; Wang, S.; Li, J.; Wang, X.; Fan, B.; Gao, X.; Liu, Z. Development of aquatic life criteria for tonalide (AHTN) and the ecological risk assessment. Ecotoxicol. Environ. Saf. 2020, 189, 109960. [Google Scholar] [CrossRef] [PubMed]
- Buerge, I.J.; Poiger, T.; Müller, M.D.; Buser, H.R. Caffeine, an anthropogenic marker for wastewater contamination of surface waters. Environ. Sci. Technol. 2003, 37, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Knee, K.L.; Gossett, R.; Boehm, A.B.; Paytan, A. Caffeine and agricultural pesticide concentrations in surface water and groundwater on the north shore of Kauai (Hawaii, USA). Mar. Pollut. Bull. 2010, 60, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef]
- Gonçalves, E.S.; Rodrigues, S.V.; Silva-Filho, E.V.D. The use of caffeine as a chemical marker of domestic wastewater contamination in surface waters: Seasonal and spatial variations in Teresópolis, Brazil. Rev. Ambiente Água 2017, 12, 192–202. [Google Scholar] [CrossRef]
- Dvory, N.Z.; Livshitz, Y.; Kuznetsov, M.; Adar, E.; Gasser, G.; Pankratov, I.; Lev, O.; Yakirevich, A. Caffeine vs. carbamazepine as indicators of wastewater pollution in a karst aquifer. Hydrol. Earth Syst. Sci. 2018, 22, 6371–6381. [Google Scholar] [CrossRef]
- Campbell, B.J.; Engel, A.S.; Porter, M.L.; Takai, K. The versatile ε-proteobacteria: Key players in sulphidic habitats. Nat. Rev. Microbiol. 2006, 4, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Labrenz, M.; Hirsch, P. Roseovarius. In Bergey’s Manual of Systematics of Archaea and Bacteria; Bergey’s Manual Trust and John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Andreote, F.D.; Jiménez, D.J.; Chaves, D.; Dias, A.C.; Luvizotto, D.M.; Dini-Andreote, F.; Fasanella, C.C.; Lopez, M.V.; Baena, S.; Taketani, R.G.; et al. The microbiome of Brazilian mangrove sediments as revealed by metagenomics. PLoS ONE 2012, 7, e38600. [Google Scholar] [CrossRef] [PubMed]
- Kuever, J. The Family Desulfobacteraceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin, Germany, 2014; pp. 45–73. [Google Scholar] [CrossRef]
- Pujalte, M.J.; Lucena, T.; Ruvira, M.A.; Arahal, D.R.; Macián, M.C. The Family Rhodobacteraceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin, Germany, 2014; pp. 439–512. [Google Scholar] [CrossRef]
- Reyes, C.; Schneider, D.; Thürmer, A.; Kulkarni, A.; Lipka, M.; Sztejrenszus, S.Y.; Böttcher, M.E.; Daniel, R.; Friedrich, M.W. Potentially Active Iron, Sulfur, and Sulfate Reducing Bacteria in Skagerrak and Bothnian Bay Sediments. Geomicrobiol. J. 2017, 34, 840–850. [Google Scholar] [CrossRef]
- Imhoff, J.F. The Family Ectothiorhodospiraceae. In The Prokaryotes; Balows, A., Trüper, H.G., Dworkin, M., Harder, W., Schleifer, K.H., Eds.; Springer: New York, NY, USA, 1992; pp. 3222–3229. [Google Scholar] [CrossRef]
- Sinkko, H.; Lukkari, K.; Sihvonen, L.M.; Sivonen, K.; Leivuori, M.; Rantanen, M.; Paulin, L.; Lyra, C. Bacteria Contribute to Sediment Nutrient Release and Reflect Progressed Eutrophication-Driven Hypoxia in an Organic-Rich Continental Sea. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E. The Family Marinilabiaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin, Germany, 2014; pp. 731–732. [Google Scholar] [CrossRef]
- Albuquerque, L.; Rainey, F.A.; da Costa, M.S. Trueperaceae. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Rainey, F., Kämpfer, P., Trujillo, M., Chun, J., DeVos, P., Hedlund, B., Dedysh, S., Eds.; Bergey’s Manual Trust and John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Lipsewers, Y.A.; Hopmans, E.C.; Meysman, F.J.; Sinninghe Damsté, J.S.; Villanueva, L. Abundance and diversity of denitrifying and anammox bacteria in seasonally hypoxic and sulfidic sediments of the saline lake Grevelingen. Front. Microbiol. 2016, 7, 1661. [Google Scholar] [CrossRef] [PubMed]
- González-Blanco, G.; Pérez-Pérez, V.; Orozco-Villafuerte, J.; Aguirre-Garrido, J.; Beristain-Cardoso, R.; Buendía-González, L. Kinetics and microbial structure of nitrogen cycle bacteria contained in the rhizosphere of natural wetland polluted with chromium. Rev. Mex. Ing. Quim. 2019, 19, 543–553. [Google Scholar] [CrossRef]
- López-Pérez, M.; Rodriguez-Valera, F. The Family Alteromonadaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 69–92. [Google Scholar]
- Oren, A.; Arahal, D.R.; Ventosa, A. Emended descriptions of genera of the family Halobacteriaceae. Int. J. Syst. Evol. Microbiol. 2009, 59, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Tahrioui, A.; Schwab, M.; Quesada, E.; Llamas, I. Quorum sensing in some representative species of halomonadaceae. Life 2013, 3, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Halanaerobiaceae. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Rainey, F., Kämpfer, P., Trujillo, M., Chun, J., DeVos, P., Hedlund, B., Dedysh, S., Eds.; Bergey’s Manual Trust and John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Motelay-Massei, A.; Garban, B.; Tiphagne-Larcher, K.; Chevreuil, M.; Ollivon, D. Mass balance for polycyclic aromatic hydrocarbons in the urban watershed of Le Havre (France): Transport and fate of PAHs from the atmosphere to the outlet. Water Res. 2006, 40, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xu, Y.; Liu, J.; Liu, D.; Li, J.; Zhang, G.; Li, X.; Zou, S.; Lai, S. Atmospheric deposition of polycyclic aromatic hydrocarbons (PAHs) to a coastal site of Hong Kong, South China. Atmos. Environ. 2013, 69, 265–272. [Google Scholar] [CrossRef]
- Gavrilescu, M. Fate of Pesticides in the Environment and its Bioremediation. Eng. Life Sci. 2005, 5, 497–526. [Google Scholar] [CrossRef]
- Traisup, K. Fate of two currently-used pesticides in water-sediment systems. Ph.D. Thesis, University of York, Heslington, UK, November 2012. [Google Scholar]
- Tang, X.; Xie, G.; Shao, K.; Dai, J.; Chen, Y.; Xu, Q.; Gao, G. Bacterial Community Composition in Oligosaline Lake Bosten: Low Overlap of Betaproteobacteria and Bacteroidetes with Freshwater Ecosystems. Microbes Environ. 2015, 30, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Kumbhare, S.V.; Mhatre, S.S.; Chowdhury, S.P.; Shetty, S.A.; Marathe, N.P.; Bhute, S.; Shouche, Y.S. Exploration of microbial diversity and community structure of Lonar Lake: The only hypersaline meteorite crater lake within basalt rock. Front. Microbiol. 2016, 6, 1553. [Google Scholar] [CrossRef] [PubMed]
- Carrey, R.; Rodríguez-Escales, P.; Otero, N.; Ayora, C.; Soler, A.; Gómez-Alday, J.J. Nitrate attenuation potential of hypersaline lake sediments in central Spain: Flow-through and batch experiments. J. Contam. Hydrol. 2014, 164, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Menchén, A.; Valiente, N.; Toledo, B.; Gómez-Alday, J.J. Microscale effects of oxygen and light on bacterial sulfate reduction in organic-rich lacustrine sediments. E3S Web Conf. 2019, 98, 11004. [Google Scholar] [CrossRef]
- Vilela, C.L.S.; Bassin, J.P.; Peixoto, R.S. Water contamination by endocrine disruptors: Impacts, microbiological aspects and trends for environmental protection. Environ. Pollut. 2018, 235, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Siddique, T.; Saleem, M.; Arshad, M.; Khalid, A. Impact of pesticides on soil microbial diversity, enzymes, and biochemical reactions. Adv. Agron. 2009, 102, 159–200. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang. Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Blunt, S.M.; Sackett, J.D.; Rosen, M.R.; Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Hedlund, B.P.; Moser, D.P. Association between degradation of pharmaceuticals and endocrine-disrupting compounds and microbial communities along a treated wastewater effluent gradient in Lake Mead. Sci. Total Environ. 2018, 622, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
| Spring and Stream | WWP | Petrola Lake | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Land Use | IRL | IRL | IRL | DRL | DRL | DRL | WWP | WWP | WWP | IRL | IRL | IRL | MP | LS | |
| Control Point | 2554 | 2571 | 2602 | 2640 | 2641 | 2642 | 2575 | 2643 | 2650 | 2649 | 2652 | 2651 | 2635 | 2648 | Total |
| Atrazine | - | 1 | 1 | 1 | - | 1 | - | - | 1 | 1 | 1 | - | - | - | 7 |
| Carbofuran | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | 1 |
| Chlorfenvinphos, cis- | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | 1 |
| Chlorfenvinphos, trans- | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | 1 |
| Diazinon | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | 1 |
| Dinocap | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | 1 |
| Methoprene | 1 | - | 1 | - | - | - | - | - | - | - | - | - | - | 1 | 3 |
| Mevinphos | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| Propoxur | - | - | - | - | - | - | - | - | - | 1 | 1 | 1 | 1 | 3 | 7 |
| Tetramethrin II | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | 1 |
| Boscalid (Nicobifen) | - | 4 | - | - | - | - | - | - | - | 2 | 1 | - | - | 1 | 8 |
| Cyprodinil | 6 | 6 | 6 | 8 | 7 | 4 | 7 | 4 | 2 | 5 | 3 | 4 | 3 | 4 | 69 |
| Flutolanil | 1 | - | 1 | 1 | 1 | 1 | - | - | - | - | - | - | - | - | 5 |
| Methomyl | - | - | 1 | 1 | - | - | - | 1 | - | 1 | 1 | 2 | - | - | 7 |
| o-Phenylphenol | 6 | 3 | 3 | 5 | 4 | 5 | 7 | 1 | 2 | 1 | - | 3 | 1 | 2 | 43 |
| Oxadiazon | - | - | 1 | 1 | - | - | - | 1 | - | 1 | - | - | 1 | - | 5 |
| Propiconazole-II | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | 1 |
| Pyrimethanil | - | - | - | - | - | 1 | - | 1 | - | - | - | 1 | - | - | 3 |
| Tebuconazole | - | 2 | - | - | - | - | - | 1 | - | 1 | 1 | 2 | - | 1 | 8 |
| Terbuthylazine | - | - | - | - | - | - | 2 | - | - | - | - | - | - | - | 2 |
| 2,4,6-Tribromophenol | 3 | 3 | 2 | 2 | 3 | 3 | 2 | - | - | - | - | - | - | - | 18 |
| Acenaphthylene | 1 | - | 1 | - | 1 | - | - | 1 | - | - | 1 | - | - | 1 | 6 |
| Atrazine-desethyl | - | 5 | - | 1 | - | - | - | - | - | - | - | - | - | - | 6 |
| Butylated hydroxyanisole | - | 1 | 1 | 1 | 1 | - | 2 | - | - | 1 | - | - | 1 | - | 8 |
| Caffeine | 4 | 1 | 3 | 4 | 5 | 1 | 6 | - | 2 | - | 1 | - | 1 | 1 | 29 |
| Dibenz[a,h]anthracene | - | 2 | 1 | 1 | - | 1 | - | - | - | - | - | - | 1 | 1 | 7 |
| Fluoranthene | 3 | 2 | 3 | 3 | 2 | 1 | 1 | - | - | 1 | - | 1 | - | - | 17 |
| Fluorene | 1 | 2 | 2 | 5 | 5 | 2 | 3 | 1 | 1 | - | 1 | 1 | 1 | 2 | 27 |
| Musk Ketone | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | 1 |
| Phenanthrene | 1 | - | - | - | - | - | 2 | - | - | - | - | - | - | - | 3 |
| Piperonyl butoxide | - | - | - | - | 2 | 1 | 8 | - | - | 1 | - | 1 | - | - | 13 |
| Terbuthylazine-desethyl | - | 1 | - | - | 1 | 3 | - | - | - | - | - | - | - | - | 5 |
| Tonalide | 7 | 4 | 4 | 6 | 6 | 5 | 8 | 1 | 2 | 1 | - | - | - | - | 44 |
| Triclosan | - | - | - | - | - | - | 2 | - | - | - | - | - | - | - | 2 |
| Count 1 | 11 | 15 | 15 | 14 | 13 | 13 | 18 | 9 | 6 | 12 | 9 | 10 | 8 | 10 | |
| Sum 2 | 34 | 38 | 31 | 40 | 39 | 29 | 56 | 12 | 10 | 17 | 11 | 17 | 10 | 17 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menchén, A.; Espín, Y.; Valiente, N.; Toledo, B.; Álvarez-Ortí, M.; Gómez-Alday, J.J. Distribution of Endocrine Disruptor Chemicals and Bacteria in Saline Pétrola Lake (Albacete, SE Spain) Protected Area is Strongly Linked to Land Use. Appl. Sci. 2020, 10, 4017. https://doi.org/10.3390/app10114017
Menchén A, Espín Y, Valiente N, Toledo B, Álvarez-Ortí M, Gómez-Alday JJ. Distribution of Endocrine Disruptor Chemicals and Bacteria in Saline Pétrola Lake (Albacete, SE Spain) Protected Area is Strongly Linked to Land Use. Applied Sciences. 2020; 10(11):4017. https://doi.org/10.3390/app10114017
Chicago/Turabian StyleMenchén, Alfonso, Yolanda Espín, Nicolás Valiente, Beatriz Toledo, Manuel Álvarez-Ortí, and Juan José Gómez-Alday. 2020. "Distribution of Endocrine Disruptor Chemicals and Bacteria in Saline Pétrola Lake (Albacete, SE Spain) Protected Area is Strongly Linked to Land Use" Applied Sciences 10, no. 11: 4017. https://doi.org/10.3390/app10114017
APA StyleMenchén, A., Espín, Y., Valiente, N., Toledo, B., Álvarez-Ortí, M., & Gómez-Alday, J. J. (2020). Distribution of Endocrine Disruptor Chemicals and Bacteria in Saline Pétrola Lake (Albacete, SE Spain) Protected Area is Strongly Linked to Land Use. Applied Sciences, 10(11), 4017. https://doi.org/10.3390/app10114017









