Growth and Photosynthetic Response of Capsicum annuum L. in Biochar Amended Soil
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Physical and Chemical Parameters, and Collection Site Information
2.2. Biochar Used in the Experiment
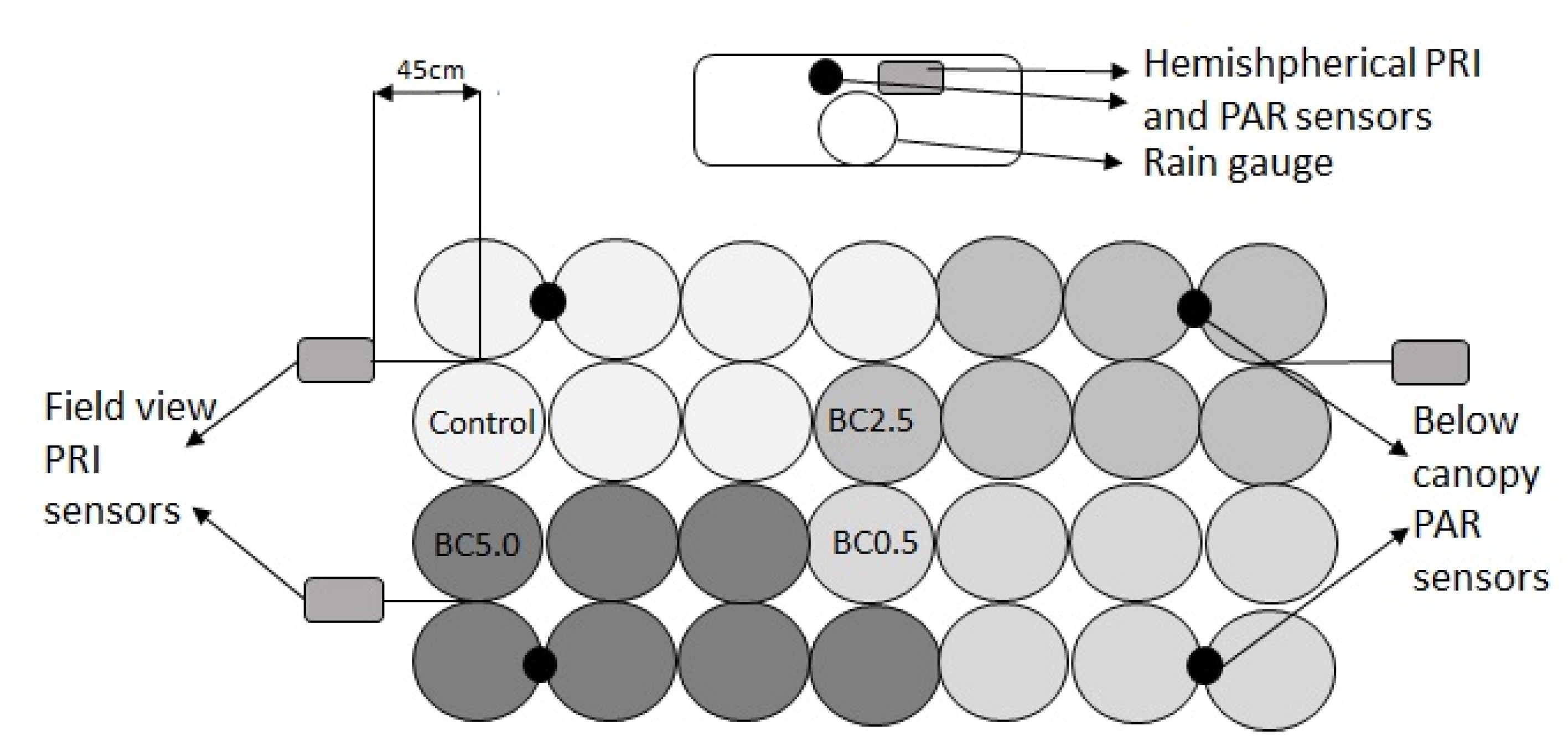
2.3. Experimental Design
2.4. Plant Root Mass, Growth Stages, and Leaf Area Determination
2.5. Statistical Analyses
3. Results
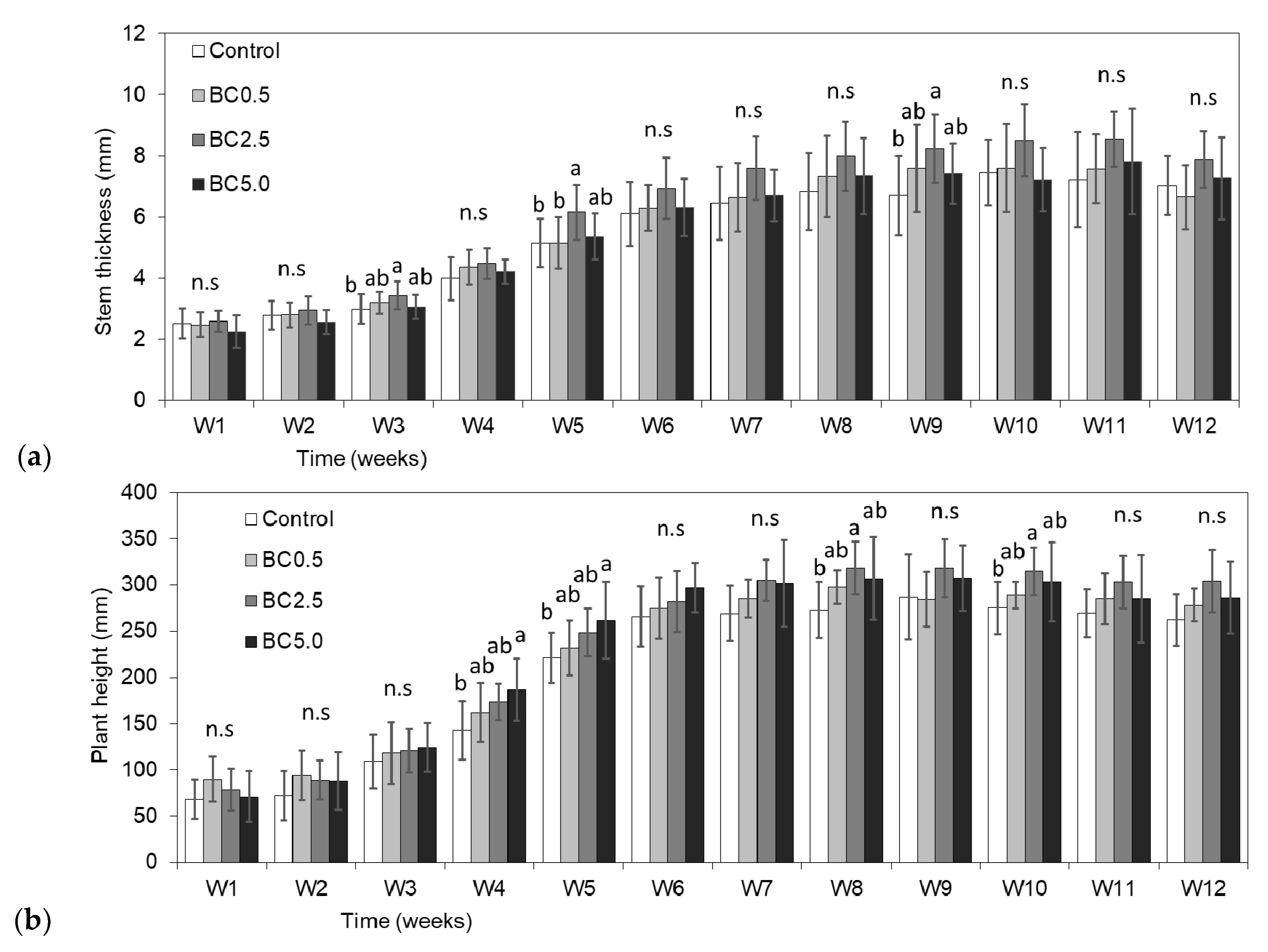
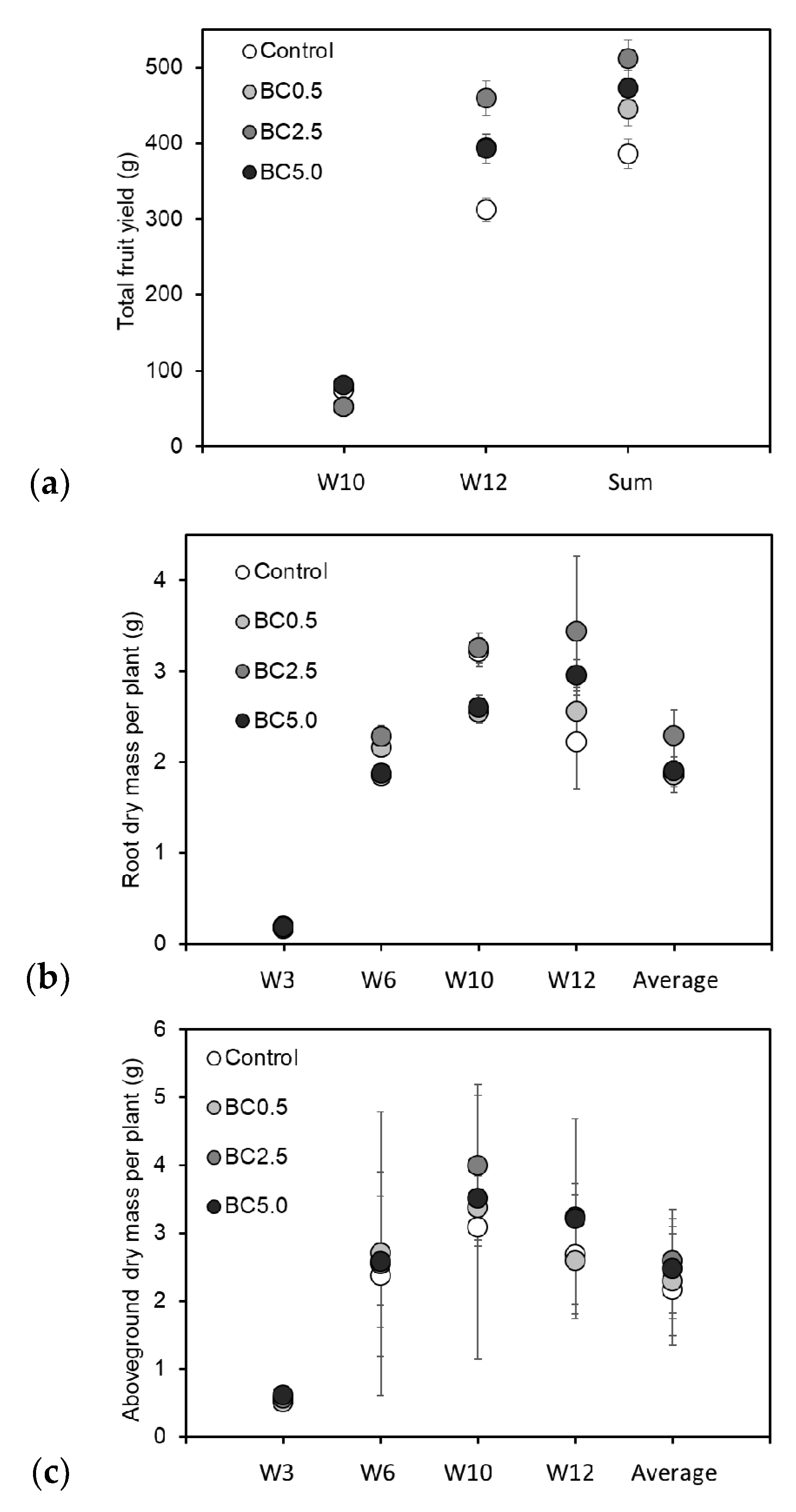
3.1. Vegetative and Reproductive Plant Growth
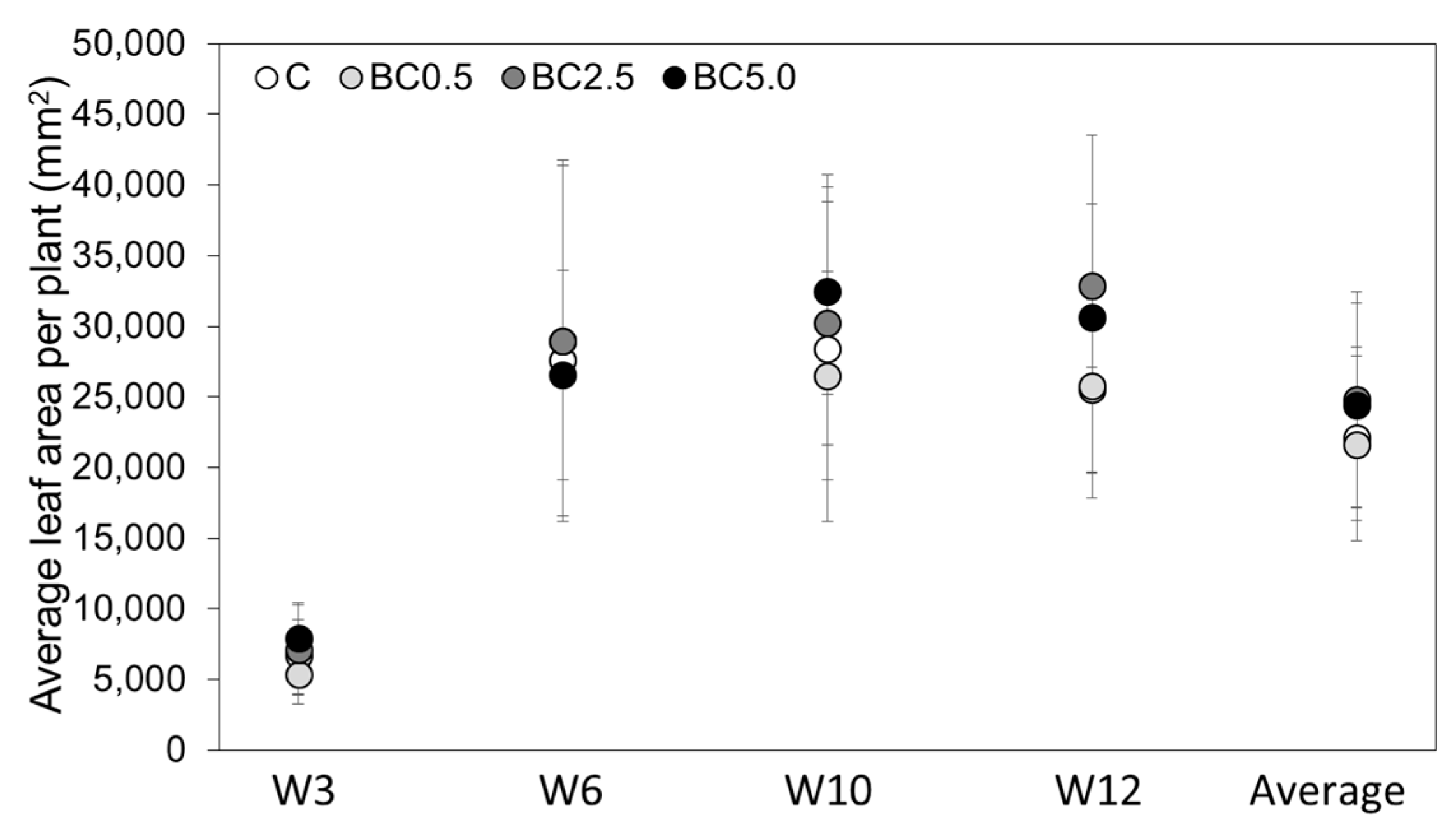
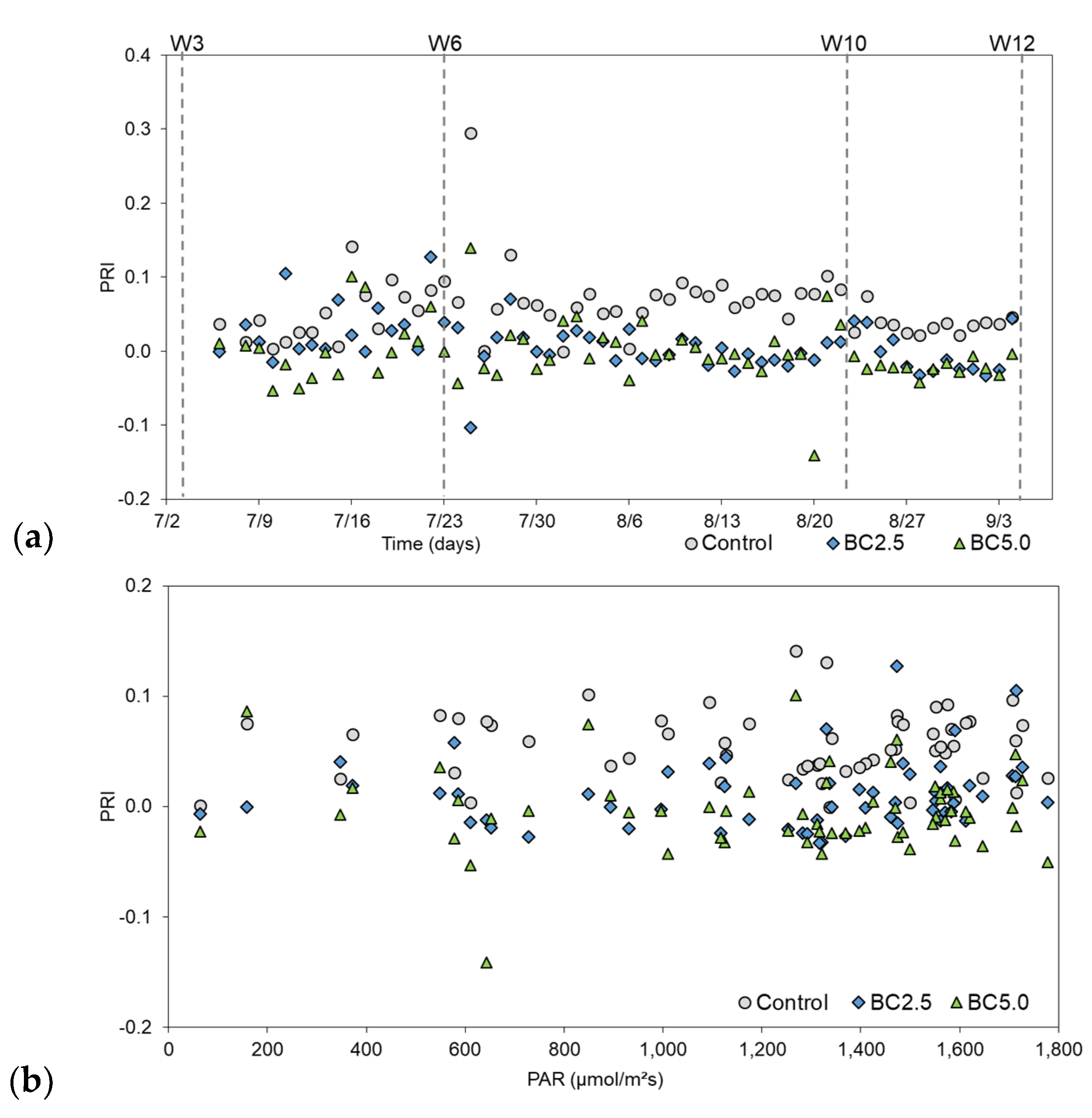
3.2. LAI and PRI Changes over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.; Pan, G.; Paz-Ferreiro, J. Biochar’s effect on crop productivity and the dependence on experimental conditions—A meta-analysis of literature data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Li, G.; Neumann Andersen, M.; Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- Githinji, L. Effect of biochar application rate on soil physical and hydraulic properties of a sandy loam. Arch. Agron. Soil Sci. 2014, 60, 457–470. [Google Scholar] [CrossRef]
- Bruun, E.W.; Petersen, C.T.; Hansen, E.; Holm, J.K.; Hauggaard-Nielsen, H. Biochar amendment to coarse sandy subsoil improves root growth and increases water retention. Soil Use Manag. 2014, 30, 109–118. [Google Scholar] [CrossRef]
- Ulyett, J.; Sakrabani, R.; Kibblewhite, M.; Hann, M. Impact of biochar addition on water retention, nitrification and carbon dioxide evolution from two sandy loam soils. Eur. J. Soil Sci. 2014, 65, 96–104. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Nelson, N.O.; Agudelo, S.C.; Yuan, W.; Gan, J. Nitrogen and phosphorus availability in biochar-amended soils. Soil Sci. Soc. Am. J. 2011, 176, 218–226. [Google Scholar] [CrossRef]
- Gunarathne, V.; Mayakaduwa, S.; Vithanage, M. Biochar’s influence as a soil amendment for essential plant nutrient uptake. In Essential Plant Nutrients: Uptake, Use Efficiency, and Management; Naeem, M., Ansari, A.A., Gill, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 47–67. [Google Scholar]
- Lehmann, J.; Kern, D.; German, L.; Mccann, J.; Martins, G.C.; Moreira, A. Soil fertility and production potential. In Amazonian Dark Earths: Origin Properties Management; Lehmann, J., Kern, D.C., Glaser, B., Wodos, W.I., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 105–124. [Google Scholar]
- Ding, Y.; Liu, Y.; Liu, S.; Huang, X.; Li, Z.; Tan, X.; Zeng, G.; Zhou, L. Potential benefits of biochar in agricultural soils: A review. Pedosphere 2017, 27, 645–661. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Akça, M.; Namli, A. Effects of poultry litter biochar on soil enzyme activities and tomato, pepper and lettuce plants growth. Eurasian J. Soil Sci. 2015, 4, 161–168. [Google Scholar] [CrossRef][Green Version]
- Gravel, V.; Dorais, M.; Menard, C. Organic potted plants amended with biochar: Its effect on growth and Pythium colonization. Can. J. Plant Sci. 2013, 93, 1217–1227. [Google Scholar] [CrossRef]
- Harel, Y.M.; Kolton, M.; Elad, Y.; Rav-David, D.; Cytryn, E.; Borenshtein, M.; Shulchani, R.; Graber, E.R. Biochar impact on plant development and disease resistance in pot trials. IOBC/WPRS Bull. 2012, 78, 141–147. [Google Scholar]
- Dao, T.T.; Canh, N.T.; Trach, N.X.; Preston, T.R. Effect of different sources of biochar on growth of maize in sandy and feralite soils. Livest. Res. Rural Dev. 2013, 25, #59. Available online: http://www.lrrd.org/lrrd25/4/dao25059.htm (accessed on 3 October 2019).
- Viger, M.; Hancock, R.D.; Miglietta, F.; Taylor, G. More plant growth but less plant defence? First global gene expression data for plants grown in soil amended with biochar. GCB Bioenergy 2015, 7, 658–672. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Calero, J.M.; Barrón, V.; Torrent, J.; del Campillo, M.C.; Gallardo, A.; Villar, R. Effects of biochars produced from different feedstocks on soil properties and sunflower growth. J. Plant Nutr. Soil Sci. 2014, 177, 16–25. [Google Scholar] [CrossRef]
- Carvalho, M.T.M.; Madari, B.E.; Bastiaans, L.; van Oort, P.A.J.; Heinemann, A.B.; da Silva, M.A.S.; Maia, A.H.N.; Meinke, H. Biochar improves fertility of a clay soil in the Brazilian Savannah: Short term effects and impact on rice yield. J. Agric. Rural Dev. Trop. Subtrop. 2013, 114, 101–107. [Google Scholar]
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; van Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017, 12, 053001. [Google Scholar] [CrossRef]
- Jeffery, S.; Abalos, D.; Spokas, K.A.; Verheijen, F.G.A. Biochar effects on crop yield. In Biochar for Environmental Management: Science and Technology, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2015; pp. 301–326. [Google Scholar]
- He, Y.; Guo, X.; Wilmshurst, J.F. Comparison of different methods for measuring leaf area index in a mixed grassland. Can. J. Plant Sci. 2007, 87, 803–813. [Google Scholar] [CrossRef]
- Aparicio, N.; Villegas, D.; Casadesus, J.; Araus, J.L.; Royo, C. Spectral vegetation indices as nondestructive tools for determining durum wheat yield. Agron. J. 2000, 92, 83–91. [Google Scholar] [CrossRef]
- Mõttus, M.; Sulev, M.; Baret, F.; Lopez-Lozano, R.; Reinart, A. Photosynthetically Active Radiation: Measurement and Modeling In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2012; pp. 7902–7932. [Google Scholar]
- Zhou, X.; Zhu, Q.; Tang, S.; Chen, X.; Wu, M. Interception of PAR and relationship between FPAR and LAI in summer maize canopy. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium, Toronto, ON, Canada, 24–28 June 2002; Volume 3256, pp. 3252–3254. [Google Scholar]
- Mougin, E.; Demarez, V.; Diawara, M.; Hiernaux, P.; Soumaguel, N.; Berg, A. Estimation of LAI, fAPAR and fCover of Sahel rangelands (Gourma, Mali). Agric. For. Meteorol. 2014, 198, 155–167. [Google Scholar] [CrossRef]
- Haider, G.; Koyro, H.-W.; Azam, F.; Steffens, D.; Müller, C.; Kammann, C. Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant Soil 2015, 395, 141–157. [Google Scholar] [CrossRef]
- Thenot, F.; Méthy, M.; Winkel, T. The photochemical reflectance index (PRI) as a water-stress index. Int. J. Remote Sens. 2002, 23, 5135–5139. [Google Scholar] [CrossRef]
- Barton, C.V.M.; North, P.R.J. Remote sensing of canopy light use efficiency using the photochemical reflectance index: Model and sensitivity analysis. Remote Sens. Environ. 2001, 78, 264–273. [Google Scholar] [CrossRef]
- Horel, Á.; Barna, G.; Makó, A. Soil physical properties affected by biochar addition at different plant phaenological phases. Part I. Int. Agrophys. 2019, 33, 255–262. [Google Scholar] [CrossRef]
- Horel, Á.; Tóth, E.; Gelybó, G.; Dencső, M.; Farkas, C. Biochar amendment affects soil water and CO2 regime during Capsicum annuum plant growth. Agronomy 2019, 9, 58. [Google Scholar] [CrossRef]
- Dövényi, Z. Magyarország kistájainak katasztere (in Hungarian); MTA Földrajztudományi Kutatóintézet: Budapest, Hungary, 2010; p. 876. [Google Scholar]
- Lim, T.J.; Spokas, K.A.; Feyereisen, G.; Novak, J.M. Predicting the impact of biochar additions on soil hydraulic properties. Chemosphere 2016, 142, 136–144. [Google Scholar] [CrossRef]
- Schon, M.K.; Compton, M.P.; Bell, E.; Burns, I. Nitrogen concentrations affect pepper yield and leachate nitrate-nitrogen from Rockwool culture. HortScience 1994, 48, 1241–1249. [Google Scholar] [CrossRef]
- Monteith, J.L. Climatic variation and the growth of crops. Q. J. R. Meteorol. Soc. 1981, 107, 749–774. [Google Scholar] [CrossRef]
- Gamon, J.A.; Kovalchuck, O.; Wong, C.Y.S.; Harris, A.; Garrity, S.R. Monitoring seasonal and diurnal changes in photosynthetic pigments with automated PRI and NDVI sensors. Biogeosciences 2015, 12, 4149–4159. [Google Scholar] [CrossRef]
- Pokovai, K.; Fodor, N. Adjusting ceptometer data to improve leaf area index measurements. Agronomy 2019, 9, 866. [Google Scholar] [CrossRef]
- Zhang, C.; Filella, I.; Garbulsky, M.F.; Peñuelas, J. Affecting factors and recent improvements of the photochemical reflectance index (PRI) for remotely sensing foliar, canopy and ecosystemic radiation-use efficiencies. Remote Sens. 2016, 8, 677. [Google Scholar] [CrossRef]
- Makó, A.; Barna, G.; Horel, A. Soil physical properties affected by biochar addition at different plant phenological phases II. Int. Agrophys. 2020, 1, 1–7. [Google Scholar] [CrossRef]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Tammeorg, P.; Simojoki, A.; Mäkelä, P.; Stoddard, F.L.; Alakukku, L.; Helenius, J. Short-term effects of biochar on soil properties and wheat yield formation with meat bone meal and inorganic fertiliser on a boreal loamy sand. Agric. Ecosyst. Environ. 2014, 191, 108–116. [Google Scholar] [CrossRef]
- Njoku, C.; Mbah, C.N.; Igboji, P.O.; Nwite, J.N.; Chibuike, C.C.; Uguru, B.N. Effect of biochar on selected soil physical properties and maize yield in an ultisol in Abakaliki southeastern Nigeria. Glob. Adv. Res. J. Agric. Sci. 2015, 4, 864–870. [Google Scholar]
- Peñuelas, J.; Garbulsky, M.F.; Filella, I. Photochemical reflectance index (PRI) and remote sensing of plant CO2 uptake. New Phytol. 2011, 191, 596–599. [Google Scholar] [CrossRef]
- Rahimzadeh-Bajgiran, P.; Munehiro, M.; Omasa, K. Relationships between the photochemical reflectance index (PRI) and chlorophyll fluorescence parameters and plant pigment indices at different leaf growth stages. Photosynth. Res. 2012, 113, 261–271. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Z.; Zhao, L.; Zhao, H.; Ren, S. Diurnal response of sun-induced fluorescence and PRI to water stress in maize using a near-surface remote sensing platform. Remote Sens. 2018, 10, 1510. [Google Scholar] [CrossRef]
- Merlier, E.; Hmimina, G.; Dufrêne, E.; Soudani, K. Explaining the variability of the photochemical reflectance index (PRI) at the canopy-scale: Disentangling the effects of phenological and physiological changes. J. Photochem. Photobiol. B Biol. 2015, 151, 161–171. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessolek, G. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 2013, 202, 183–191. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—Results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Horel, Á.; Tóth, E.; Gelybó, G.; Dencső, M.; Potyó, I. Soil CO2 and N2O emission drivers in a vineyard (Vitis vinifera) under different soil management systems and amendments. Sustainability 2018, 10, 1811. [Google Scholar] [CrossRef]
- Hardie, M.; Clothier, B.; Bound, S.; Oliver, G.; Close, D. Does biochar influence soil physical properties and soil water availability? Plant Soil 2014, 376, 347–361. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Amonette, J.E.; Ippolito, J.A.; Lima, I.M.; Gaskin, J.; Das, K.C.; Steiner, C.; Ahmedna, M.; et al. Biochars impact on soil-moisture storage in an Ultisol and two Aridisols. Soil Sci. 2012, 177, 310–320. [Google Scholar] [CrossRef]
- Ronga, D.; Caradonia, F.; Parisi, M.; Bezzi, G.; Parisi, B.; Allesina, G.; Pedrazzi, S.; Francia, E. Using digestate and biochar as fertilizers to improve processing tomato production sustainability. Agronomy 2020, 10, 138. [Google Scholar] [CrossRef]


| Chemical and Physical Parameters and Grain Size Distribution at t = 0 | Soil | SD | Biochar | SD |
|---|---|---|---|---|
| <0.002 mm (%) | 23.85 | ±0.64 | 84.52 | ±0.08 |
| 0.05–0.002 mm (%) | 55.97 | ±0.76 | 13.90 | ±0.70 |
| 2–0.5 mm (%) | 20.18 | ±0.15 | 1.57 | ±4.23 |
| pH(H2O) | 7.97 | ±0.04 | 10.33 | ±0 |
| Total N (%) | 0.14 | ±0.02 | 1.0 | ±0.1 |
| NH4+-N (mg kg−1) | 5.84 | ±1.01 | 1.9 | ±0.1 |
| CaCO3 (%) | 10.41 | ±0.34 | n.d. | - |
| P as P2O5 (mg kg−1) | 977.9 | ±158.1 | 5031.1 | ±32.6 |
| K as K2O (mg kg−1) | 443.1 | ±96.2 | 13,570.3 | ±59.0 |
| Organic C (%) | 0.93 | ±0.07 | 27.89 | ±1.73 |
| EC 2.5/EC 5.0 (mS cm−1) | 0.2 | ±0.01 | 3.03 | - |
| VWCinitial (%) | 26.51 | ±3.7 | - | - |
| Treatment | Plant Height 1 | Stem Thickness 1 | Leaf Area | Aboveground Dry Mass | Root Dry Biomass | Fruit Yield | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | |
| mm | mm | mm2 | g | g | g | |||||||
| Control | 262.38a | 27.71 | 7.02a | 0.95 | 25,547a | 5895 | 2.67a | 0.88 | 2.22a | 0.51 | 312.24a | 15.61 |
| BC0.5 | 278.25b | 17.84 | 6.66a | 1.05 | 25,735a | 6102 | 2.59a | 0.64 | 2.56ab | 0.27 | 393.60a | 19.68 |
| BC2.5 | 304.13c | 33.76 | 7.88b | 0.92 | 32,861b | 5804 | 3.23a | 0.50 | 3.44b | 0.82 | 459.52a | 22.98 |
| BC5.0 | 286.25c | 39.02 | 7.27a | 1.35 | 30,656ab | 12,840 | 3.21a | 1.47 | 2.95ab | 0.17 | 392.72a | 19.64 |
| r | Plant Height | Stem Thickness | Leaf Area | Above-Ground Mass | Root Dry Mass | Fruit Yield | |
|---|---|---|---|---|---|---|---|
| p | |||||||
| Plant height | - | 0.089 | 0.455 | 0.528 | 0.255 | 0.405 | |
| Stem thickness | 0.743 | - | 0.803 | 0.715 | 0.568 | 0.168 | |
| Leaf area | 0.077 | <0.0002 *** | - | 0.887 | 0.776 | 0.437 | |
| Aboveground mass | 0.035 * | 0.002 ** | <0.0001 *** | - | 0.507 | 0.200 | |
| Root dry mass | 0.341 | 0.022 * | <0.0005 *** | 0.045 * | - | 0.561 | |
| Fruit yield | 0.120 | 0.533 | 0.091 | 0.459 | 0.024 * | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokovai, K.; Tóth, E.; Horel, Á. Growth and Photosynthetic Response of Capsicum annuum L. in Biochar Amended Soil. Appl. Sci. 2020, 10, 4111. https://doi.org/10.3390/app10124111
Pokovai K, Tóth E, Horel Á. Growth and Photosynthetic Response of Capsicum annuum L. in Biochar Amended Soil. Applied Sciences. 2020; 10(12):4111. https://doi.org/10.3390/app10124111
Chicago/Turabian StylePokovai, Klára, Eszter Tóth, and Ágota Horel. 2020. "Growth and Photosynthetic Response of Capsicum annuum L. in Biochar Amended Soil" Applied Sciences 10, no. 12: 4111. https://doi.org/10.3390/app10124111
APA StylePokovai, K., Tóth, E., & Horel, Á. (2020). Growth and Photosynthetic Response of Capsicum annuum L. in Biochar Amended Soil. Applied Sciences, 10(12), 4111. https://doi.org/10.3390/app10124111






