Ozone Deposition on Free-Running Indoor Materials and the Corresponding Volatile Organic Compound Emissions: Implications for Ventilation Requirements
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Selection and Preparation
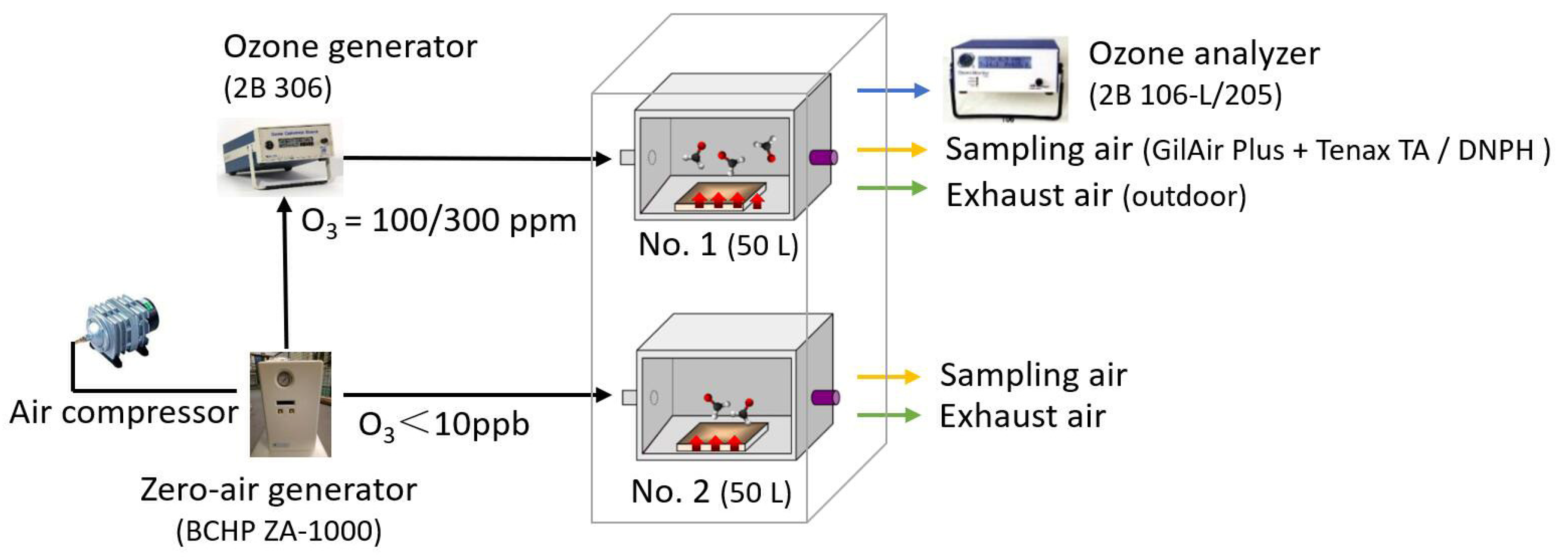
2.2. Environmental Chamber Set-Up
2.3. Testing Procedures
2.3.1. Ozone Deposition Velocities
2.3.2. Primary and Secondary VOC Emissions
2.4. The Effects of Ozone on Ventilation Requirements
- (1)
- The effects of ventilation requirements on indoor ozone concentrations after deposition.
- (2)
- The ventilation requirements for diluting the VOC emissions from the selected free-running indoor materials by considering ozone-related reactions.
2.4.1. Simplified Methods to Discuss Ventilation Requirements
- (1)
- The room air was well-mixed.
- (2)
- No indoor air cleaners or any other means were used to purify the indoor air.
- (3)
- No occupants were present, as occupants are both emission sources and sinks.
- (4)
- The VOC concentrations in the outdoor air were ignored.
- (5)
- The production of any compound from the gas-phase ozone reactions was not considered.
- (6)
- If one reaction produced by-products that could be reactants in another reaction, the effects of the by-products on the ventilation rate requirements were ignored.
2.4.2. Standard Room Configurations
3. Results and Discussion
3.1. Ozone Deposition Velocities
3.1.1. On Wood-Based Materials
3.1.2. On Synthetic Fibers
3.1.3. Comparison with Data in the Literature
3.2. Primary and Secondary VOC Emissions
3.2.1. From Wood-Based Materials
3.2.2. From Synthetic Fibers
3.2.3. Comparison with the Emission Data from the NRC Database
3.3. Ventilation Rate Determinants
3.3.1. Ventilation Rates vs. Indoor Ozone Concentrations
3.3.2. Ventilation Rates vs. VOC Emissions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; Zhou, L.; Fu, Q.; Yan, L.; Bian, Q.; Wang, N.; Xiu, G. Vertical distribution of ozone over Shanghai during late spring: A balloon-borne observation. Atmos. Environ. 2019, 208, 48–60. [Google Scholar] [CrossRef]
- Fowler, D.; Pilegaard, K.; Sutton, M.; Ambus, P.; Raivonen, M.; Duyzer, J.; Simpson, D.; Fagerli, H.; Fuzzi, S.; Schjoerring, J.K.; et al. Atmospheric composition change: Ecosystems–Atmosphere interactions. Atmos. Environ. 2009, 43, 5193–5267. [Google Scholar] [CrossRef]
- WHO. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summary of Risk Assessment; WHO Regional Office for Europe: Copenhagen, Denmark, 2006. [Google Scholar]
- Kumar, P.; Imam, B. Footprints of air pollution and changing environment on the sustainability of built infrastructure. Sci. Total. Environ. 2013, 444, 85–101. [Google Scholar] [CrossRef]
- Yin, P.; Chen, R.; Wang, L.; Meng, X.; Liu, C.; Niu, Y.; Lin, Z.; Liu, Y.; Liu, J.; Qi, J.; et al. Ambient Ozone Pollution and Daily Mortality: A Nationwide Study in 272 Chinese Cities. Environ. Health Perspect. 2017, 125, 117006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, W.; London, S.J.; Song, G.; Chen, G.; Jiang, L.; Zhao, N.; Chen, B.; Kan, H. Ozone and Daily Mortality in Shanghai, China. Environ. Health Perspect. 2006, 114, 1227–1232. [Google Scholar] [CrossRef]
- Wang, T.; Xue, L.; Brimblecombe, P.; Lam, Y.F.; Li, L.; Zhang, L. Ozone pollution in China: A review of concentrations, meteorological influences, chemical precursors, and effects. Sci. Total. Environ. 2017, 575, 1582–1596. [Google Scholar] [CrossRef]
- MEP, AQSIQ. GB 3095-2012 Ambient Air Quality Standards; China Environmental Science Press: Beijing, China, 2012. [Google Scholar]
- Maji, K.J.; Ye, W.-F.; Arora, M.; Nagendra, S.S. Ozone pollution in Chinese cities: Assessment of seasonal variation, health effects and economic burden. Environ. Pollut. 2019, 247, 792–801. [Google Scholar] [CrossRef]
- CENEMC. Report on Air Quality in Chinese Cities in April, 2019. Available online: http://www.cnemc.cn/jcbg/kqzlzkbg/201905/t20190522_703733.shtml (accessed on 9 June 2019).
- Morrison, G.C.; Shaughnessy, R.; Shu, S. Setting maximum emission rates from ozone emitting consumer appliances in the United States and Canada. Atmos. Environ. 2011, 45, 2009–2016. [Google Scholar] [CrossRef]
- Weschler, C.J. Ozone in indoor environments: Concentration and chemistry. Indoor Air 2000, 10, 269–288. [Google Scholar] [CrossRef]
- Guo, C.; Gao, Z.; Shen, J. Emission rates of indoor ozone emission devices: A literature review. Build. Environ. 2019, 158, 302–318. [Google Scholar] [CrossRef]
- Gingrich, J.; Corsi, R. Ozone decay rates in residential garages. Build. Environ. 2018, 144, 208–215. [Google Scholar] [CrossRef]
- Weschler, C.J. Ozone’s Impact on Public Health: Contributions from Indoor Exposures to Ozone and Products of Ozone-Initiated Chemistry. Environ. Health Perspect. 2006, 114, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, B.; Weschler, C.J. Assessing the Influence of Indoor Exposure to “Outdoor Ozone” on the Relationship between Ozone and Short-term Mortality in U.S. Communities. Environ. Health Perspect. 2011, 120, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Zhao, B. Surface removal rate of ozone in residences in China. Build. Environ. 2018, 142, 101–106. [Google Scholar] [CrossRef]
- Pandrangi, L.S.; Morrison, G.C. Ozone interactions with human hair: Ozone uptake rates and product formation. Atmos. Environ. 2008, 42, 5079–5089. [Google Scholar] [CrossRef]
- Weschler, C.J. Roles of the human occupant in indoor chemistry. Indoor Air 2015, 26, 6–24. [Google Scholar] [CrossRef]
- Rim, D.; Gall, E.; Ananth, S.; Won, Y. Ozone reaction with human surfaces: Influences of surface reaction probability and indoor air flow condition. Build. Environ. 2018, 130, 40–48. [Google Scholar] [CrossRef]
- Atkinson, R.; Carter, W.P.L. Kinetics and mechanisms of the gas-phase reactions of ozone with organic compounds under atmospheric conditions. Chem. Rev. 1984, 84, 437–470. [Google Scholar] [CrossRef]
- Yu, X.; Yi, B.; Wang, X.; Chen, J. Predicting reaction rate constants of ozone with organic compounds from radical structures. Atmos. Environ. 2012, 51, 124–130. [Google Scholar] [CrossRef]
- Weschler, C.J. New Directions: Ozone-initiated reaction products indoors may be more harmful than ozone itself. Atmos. Environ. 2004, 38, 5715–5716. [Google Scholar] [CrossRef]
- Gall, E.; Rim, D. Mass accretion and ozone reactivity of idealized indoor surfaces in mechanically or naturally ventilated indoor environments. Build. Environ. 2018, 138, 89–97. [Google Scholar] [CrossRef]
- Shen, J.; Gao, Z. Ozone removal on building material surface: A literature review. Build. Environ. 2018, 134, 205–217. [Google Scholar] [CrossRef]
- Ye, W.; Won, D.; Zhang, X. A practical method and its applications to prioritize volatile organic compounds emitted from building materials based on ventilation rate requirements and ozone-initiated reactions. Indoor Built Environ. 2016, 26, 166–184. [Google Scholar] [CrossRef]
- Darling, E.; Morrison, G.C.; Corsi, R. Passive removal materials for indoor ozone control. Build. Environ. 2016, 106, 33–44. [Google Scholar] [CrossRef]
- Gall, E.; Corsi, R.L.; Siegel, J.A. Barriers and opportunities for passive removal of indoor ozone. Atmos. Environ. 2011, 45, 3338–3341. [Google Scholar] [CrossRef]
- Cros, C.J.; Morrison, G.C.; Siegel, J.A.; Corsi, R. Long-term performance of passive materials for removal of ozone from indoor air. Indoor Air 2011, 22, 43–53. [Google Scholar] [CrossRef]
- Ye, W.; Won, D.; Zhang, X. Examining the applicability of empirical models using short-term VOC emissions data from building materials to predict long-term emissions. Build. Simul. 2016, 9, 701–715. [Google Scholar] [CrossRef]
- Liang, W.; Yang, S.; Yang, X. Long-Term Formaldehyde Emissions from Medium-Density Fiberboard in a Full-Scale Experimental Room: Emission Characteristics and the Effects of Temperature and Humidity. Environ. Sci. Technol. 2015, 49, 10349–10356. [Google Scholar] [CrossRef]
- Wang, S. The Current Conditions and Developing Tendency of Building Decoration Materials of America; Development Guide to Building Materials: Kunming, China, 2003; pp. 59–66. (In Chinese) [Google Scholar]
- Shen, Y.; Wang, J. The applications and market outlooks of a couple of decoration materials of floors. Build. Decor. 2004, 40–42. (In Chinese) [Google Scholar]
- Liu, W.; Zhang, Y.; Yao, Y.; Li, J. Indoor decorating and refurbishing materials and furniture volatile organic compounds emission labeling systems: A review. Chin. Sci. Bull. 2012, 57, 2533–2543. [Google Scholar] [CrossRef]
- Ausschuss zur gesundheitlichen Bewertung von Bauprodukten. Health-related Evaluation Procedure for Volatile Organic Compounds Emissions (VVOC, VOC and SVOC) from Building Products; Ausschuss zur gesundheitlichen Bewertung von Bauprodukten: Dessau-Roßlau, Germany, 2015. [Google Scholar]
- Ye, W.; Won, D.; Zhang, X. A preliminary ventilation rate determination methods study for residential buildings and offices based on VOC emission database. Build. Environ. 2014, 79, 168–180. [Google Scholar] [CrossRef]
- CNFPIA, SFA. Report on China Wood-based Panels Industry; China National Forest Products Industry Association, State Forestry Planning and Design Institute of Forest Products Industry: Beijing, China, 2018. [Google Scholar]
- Lusztyk, E.; Yang, W.; Won, D. Methodology for the Analysis of VOCs in Emission Testing of Building Materials; National Research Council Canada: Ottawa, ON, Canada, 2005. [Google Scholar]
- Mueller, F.X.; Loeb, L.; Mapes, W.H. Decomposition rates of ozone in living areas. Environ. Sci. Technol. 1973, 7, 342–346. [Google Scholar] [CrossRef]
- AQSIQ. GB/T 31106-2014 Determination of Volatile Organic Compounds in Furniture; Standards Press of China: Beijing, China, 2014. [Google Scholar]
- Song, W.; Cao, Y.; Wang, D.; Hou, G.; Shen, Z.; Zhang, S. An Investigation on Formaldehyde Emission Characteristics of Wood Building Materials in Chinese Standard Tests: Product Emission Levels, Measurement Uncertainties, and Data Correlations between Various Tests. PLoS ONE 2015, 10, e0144374. [Google Scholar] [CrossRef] [PubMed]
- MEP. HG 683-2014 Ambient Air-Determination of Aldehyde and Ketone Compounds-High Performance Liquid Chromatography; China Environmental Science Press: Beijing, China, 2014. [Google Scholar]
- AQSIQ, NHFPC, MEP. GB/T 18883-2002 Indoor Air Quality Standard; China Standards Press: Beijing, China, 2002. [Google Scholar]
- BIFMA. ANSI/BIFMA M7.1 Standard Test Method for Determining VOC Emissions from Office Furniture Systems, Components and Seating; BIFMA International: Grand Rapids, MI, USA, 2011. [Google Scholar]
- Salthammer, T. Formaldehyde sources, formaldehyde concentrations and air exchange rates in European housings. Build. Environ. 2019, 150, 219–232. [Google Scholar] [CrossRef]
- Schripp, T.; Langer, S.; Salthammer, T. Interaction of ozone with wooden building products, treated wood samples and exotic wood species. Atmos. Environ. 2012, 54, 365–372. [Google Scholar] [CrossRef]
- Sabersky, R.H.; Sinema, D.A.; Shair, F.H. Concentrations, decay rates, and removal of ozone and their relation to establishing clean indoor air. Environ. Sci. Technol. 1973, 7, 347–353. [Google Scholar] [CrossRef]
- Nicolas, M.; Ramalho, O.; Maupetit, F. Reactions between ozone and building products: Impact on primary and secondary emissions. Atmos. Environ. 2007, 41, 3129–3138. [Google Scholar] [CrossRef]
- Grøntoft, T.; Raychaudhuri, M.R. Compilation of tables of surface deposition velocities for O3, NO2 and SO2 to a range of indoor surfaces. Atmos. Environ. 2004, 38, 533–544. [Google Scholar] [CrossRef]
- Lamble, S.; Corsi, R.; Morrison, G.C. Ozone deposition velocities, reaction probabilities and product yields for green building materials. Atmos. Environ. 2011, 45, 6965–6972. [Google Scholar] [CrossRef]
- Klenø, J.G.; Clausen, P.A.; Weschler, C.J.; Wolkoff, P. Determination of ozone removal rates by selected building products using the FLEC emission cell. Environ. Sci. Technol. 2001, 35, 2548–2553. [Google Scholar] [CrossRef]
- Abbass, O.A.; Sailor, D.J.; Gall, E. Effect of fiber material on ozone removal and carbonyl production from carpets. Atmos. Environ. 2017, 148, 42–48. [Google Scholar] [CrossRef]
- Coleman, B.K.; Destaillats, H.; Hodgson, A.T.; Nazaroff, W.W. Ozone consumption and volatile byproduct formation from surface reactions with aircraft cabin materials and clothing fabrics. Atmos. Environ. 2008, 42, 642–654. [Google Scholar] [CrossRef]
- Wang, H.; Morrison, G.C. Ozone-Initiated Secondary Emission Rates of Aldehydes from Indoor Surfaces in Four Homes. Environ. Sci. Technol. 2006, 40, 5263–5268. [Google Scholar] [CrossRef] [PubMed]
- Rim, D.; Gall, E.; Maddalena, R.L.; Nazaroff, W.W. Ozone reaction with interior building materials: Influence of diurnal ozone variation, temperature and humidity. Atmos. Environ. 2016, 125, 15–23. [Google Scholar] [CrossRef]
- Ye, W.; Little, J.C.; Won, D.; Zhang, X. Screening-level estimates of indoor exposure to volatile organic compounds emitted from building materials. Build. Environ. 2014, 75, 58–66. [Google Scholar] [CrossRef]
- MOHURD. GB 50736-2012 Code for Design of Heating Ventilation and Air Conditioning; China Architecture & Building Press: Beijing, China, 2012. [Google Scholar]
- ASHRAE. ANSI/ASHRAE Standard 62.2-2019 Ventilation for Acceptable Indoor Air Quality in Residential Buildings; ASHRA: Atlanta, GA, USA, 2019. [Google Scholar]
- BSI. BS EN 16798: 2019 Energy Performance of Buildings-Ventilation for Buildings Part 1: Indoor Environmental Input Parameters for Design and Assessment of Energy Performance of Buildings Addressing Indoor Air Quality, Thermal Environment, Lighting and Acoustics-Module M1-6; BSI Standards Limited: London, UK, 2019. [Google Scholar]
- Huang, L.; Mo, J.; Sundell, J.; Fan, Z.; Zhang, Y. Health Risk Assessment of Inhalation Exposure to Formaldehyde and Benzene in Newly Remodeled Buildings, Beijing. PLoS ONE 2013, 8, e79553. [Google Scholar] [CrossRef]









| Item | OSB with a Green-Core, i.e., Moisture-Resistant Additives | Lauan Plywood | Paint-Free Pine |
|---|---|---|---|
| Estimated age | >1 year | >1–2 years | >1–2 years |
| Pictures |   |   |   |
| Exposed area, m2 | 0.061 | 0.054 | 0.075 |
| Thickness, mm | 18.97 | 7.65 | 16.55 |
| Weight, g | >500.000 1 | 234.083 | 623.731 |
| Density, kg·m−3 | >432.1 | 566.6 | 502.5 |
| Loading ratio, m2·m−3 | 1.24 | 1.10 | 1.52 |
| Item | Flannel | Weaved Leather | Microfiber Lining | Carpet |
|---|---|---|---|---|
| Estimated age | <1 year | <1 year | <1 year | 1–2 years |
| Pictures |  |  |  |  |
| Exposed area, m2 | 0.091 | 0.088 | 0.080 | 0.064 |
| Thickness, mm | 0.90 | 0.59 | 0.80 | 4.83 |
| Weight, g | 18.327 | 70.514 | 30.727 | 200.065 |
| Density, kg·m−3 | 233.8 | 1358.1 | 480.1 | 647.2 |
| Loading ratio, m2·m−3 | 1.62 | 1.79 | 1.84 | 1.30 |
| Specimen | Number of Tests | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| None | 100 ppm | 10 | Measured | Measured | 50 L | Not applicable | Measured | Not applicable | Determined |
| 300 ppm | 10 | ||||||||
| One at a time | 100 ppm | 2–4 | 50 L minus the volume of the specimen | Measured | Measured | Determined | Based on empty-chamber tests | ||
| 300 ppm | 2–4 |
| Specimen | Chemicals | |||||
|---|---|---|---|---|---|---|
| One specimen from each material | Aromatic, aliphatic, and ester compounds | First 0, then 100 ppm | Assumed at 0 | Measured following [40] | Determined by Equation (4) | |
| First 0, then 300 ppm | ||||||
| Aldehyde and ketone compounds | First 0, then 100 ppm | Measured following [41] | ||||
| First 0, then 300 ppm | ||||||
| Parameters of the standard office | Value |
|---|---|
| Volume (m3) | 65.2 |
| Emission loading ratio (m2∙m−3) | 1.2 |
| Ceiling/flooring surface area (m2) | 23.8 |
| Wall surface area (m2) | 53.1 |
| Material | Description | Age | Chamber | ACH (h−1) | Temp. (°C) | RH (%) | Ozone Conc. (ppm) | Deposition Velocity (cm·s−1) | Source |
|---|---|---|---|---|---|---|---|---|---|
| OSB | Green-core | >1 year | 50 L | 1.52 | 23 | 20 | 100, 300 | 0.040, 0.051 | This study |
| No data | No data | 35 mL | ~1714 | 23 | <2 | 100 | 0.011–0.033 | [46] | |
| Plywood | Lauan | >1–2 years | 50 L | 1.52 | 23 | 20 | 100, 300 | 0.035, 0.026 | This study |
| One-side varnished | No data | 2.38 m3 | Decay method | 22 | 50 ± 5 | 1000, 5000 | 0.03 | [47] | |
| Pine | Paint-free | >1–2 years | 50 L | 1.52 | 23 | 20 | 100, 300 | 0.009, 0.005 | This study |
| Raw, untreated, without knots | New and unused | 17 L | 12 | 23 ± 2 | 47 ± 1 | 109 ± 1 | 0.108 | [48] | |
| 48 ± 1 | 129 ± 2 | 0.069 | |||||||
| 50 ± 1 | 167 ± 1 | 0.056 ± 0.041 | |||||||
| 41 ± 1 | 33 ± 1 | 0.553 ± 0.157 | |||||||
| 41 ± 1 | 92 ± 1 | 0.203 ± 0.184 | |||||||
| 38 ± 1 | 165 ± 1 | 0.142 ± 0.033 | |||||||
| Carpet | Synthetic | 1–2 years | 50 L | 1.52 | 23 | 20 | 100, 300 | 0.024, 0.034 | This study |
| Wool | No data | 10.9 L | 11 | 22 | 0 | 40 | 0.051 | [49] | |
| 30 | 0.062 | ||||||||
| 50 | 0.073 | ||||||||
| 70 | 0.100 | ||||||||
| 90 | 0.130 | ||||||||
| Synthetic | 0 | 0.057 | |||||||
| 30 | 0.069 | ||||||||
| 50 | 0.081 | ||||||||
| 70 | 0.113 | ||||||||
| 90 | 0.145 | ||||||||
| Nylon, GlasBac backing | Recycled | 10 L | 12 | 25 | 50 | 150–200 | 0.068 | [50] | |
| Nylon, SoftBac platinum backing | 0.054 | ||||||||
| Nylon fibers and latex backing | >1 year | 35 mL | ~1543 | 21 ± 2 | 50 ± 5 | 50 ± 1 | 0.7 ± 0.4 (sample 1) 0.032 ± 0.004 (sample 2) | [51] | |
| Rubber backing | New and unused | 17 L | 12 | 23 ± 2 | 52 ± 1 | 219 ± 3 | 0.039 | [48] | |
| Textile backing | 48 ± 1 | 106 ± 1 | 0.058 | ||||||
| Bitumen backing | 52 ± 1 | 131 ± 2 | 0.076 | ||||||
| PVC backing | 47 ± 1 | 126 ± 3 | 0.077 | ||||||
| 100% BCF (Bulked continuous filament) Triexta | Unused | 52 L | 3 | 21 ± 1 | 50 ± 2 | 120 ± 2 | 0.147 | [52] | |
| 75% BCF Polyester, 25%BCF Triexta | 0.111 | ||||||||
| 100% Polypropylene | 0.158 | ||||||||
| 100% PET BCF polyester | 0.058 | ||||||||
| 100% Nylon | 0.083 | ||||||||
| 100% Wool | 0.186 | ||||||||
| Eight samples | New | 10.5 L | 17 | 23 ± 1 | 10 ± 1 | 105 ± 5 | 0.14–0.38 | [53] | |
| Two samples | Used | 0.18 | |||||||
| Nylon cut pile | Used | 4.25 L | 28 | Room temp. | 50 ± 5 | 100–150 | 0.040–0.150 | [54] | |
| Wool rugs | Used | 0.125 | |||||||
| Polypropylene | Used | 0.090, 0.250 | |||||||
| Nylon loop pile | No data | 48 L | 2 | 50 ± 2 | 147 ± 10 | 0.053–0.083 | [29] | ||
| 100% BCF synthetic fiber with PVC backing | New | 10.7 L | 10 | 22, 28 | 25, 50, 75 | 60–62 | 0.110–0.140 | [55] |
| Sources | This Study | NRC Database | ||||||
|---|---|---|---|---|---|---|---|---|
| Materials | OSB | Plywood | Pine | Carpet | OSB | Plywood | Pine | Carpet |
| Chemicals | Emission rates with no intentional ozone exposure, μg·m−2·h−1 (Emission rates with exposure to ozone at concentrations around 100 ppm, μg·m−2·h−1) | |||||||
| Formaldehyde | 111.6 (120.4) | 58.0 (43.6) | 30.3 (34.6) | 0 (0) | 5.6–10.4 | 0 | 0 | 1.5–2.2 |
| Acetaldehyde | 0 (1.2) | 0 (1.8) | 0 (0) | 0 (0) | 34.3–66.1 | 0 | 0 | 0.7–3.7 |
| Acetone | 0 (0) | 0 (6.5) | 0 (2.5) | 0 (0) | 2.1–98.4 | 2.2–5.1 | 1.1–1.3 | 0.2–41.3 |
| Hexanal | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 10.9–927.8 | 7.9–18.2 | 3.2 | 0.1–0.5 |
| Benzene | 5.5 (0) | 0 (0) | 6.0 (0) | 6.5 (0) | 0.1–0.9 | 0–0.3 | 0.2 | 0–0.2 |
| Toluene | 9.9 (0) | 2.6 (1.1) | 18.0 (35.6) | 15.0 (35.6) | 0.2–83.9 | 0.4–0.5 | 0.5–0.7 | 0–1.7 |
| Ethylbenzene | 0 (3.7) | 0 (8.6) | 0 (0) | 0 (9.1) | 0–1.7 | 0–0.1 | 0.1 | 0–31.5 |
| Styrene | 0 (0) | 0 (9.6) | 0 (0) | 0 (0) | 0–0.1 | 0 | 0 | 0–1.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, W.; Wang, H.; Chen, Z.; Zhang, X. Ozone Deposition on Free-Running Indoor Materials and the Corresponding Volatile Organic Compound Emissions: Implications for Ventilation Requirements. Appl. Sci. 2020, 10, 4146. https://doi.org/10.3390/app10124146
Ye W, Wang H, Chen Z, Zhang X. Ozone Deposition on Free-Running Indoor Materials and the Corresponding Volatile Organic Compound Emissions: Implications for Ventilation Requirements. Applied Sciences. 2020; 10(12):4146. https://doi.org/10.3390/app10124146
Chicago/Turabian StyleYe, Wei, Hao Wang, Zean Chen, and Xu Zhang. 2020. "Ozone Deposition on Free-Running Indoor Materials and the Corresponding Volatile Organic Compound Emissions: Implications for Ventilation Requirements" Applied Sciences 10, no. 12: 4146. https://doi.org/10.3390/app10124146
APA StyleYe, W., Wang, H., Chen, Z., & Zhang, X. (2020). Ozone Deposition on Free-Running Indoor Materials and the Corresponding Volatile Organic Compound Emissions: Implications for Ventilation Requirements. Applied Sciences, 10(12), 4146. https://doi.org/10.3390/app10124146





