Electrodeposition Patterned Copper Foam with Micro/Nanostructures for Reducing Supercooling in Water-Based Cool Storage Phase-Change Materials
Abstract
:1. Introduction
2. Experimental Section
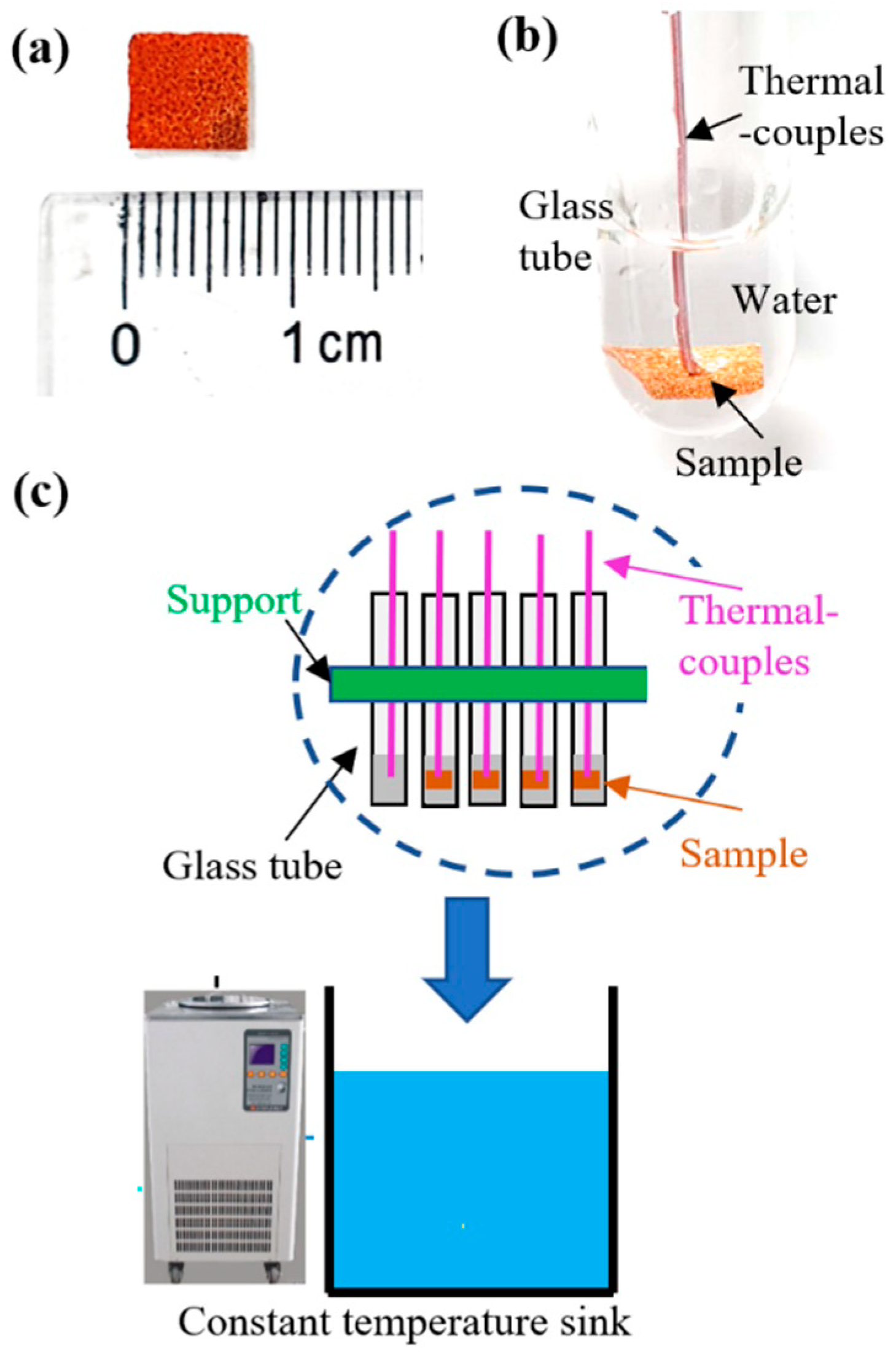
2.1. Sample Preparation and Characterizaition
2.2. Test Setup for the Cool Storage Phase-Change Process
3. Results and Discussion
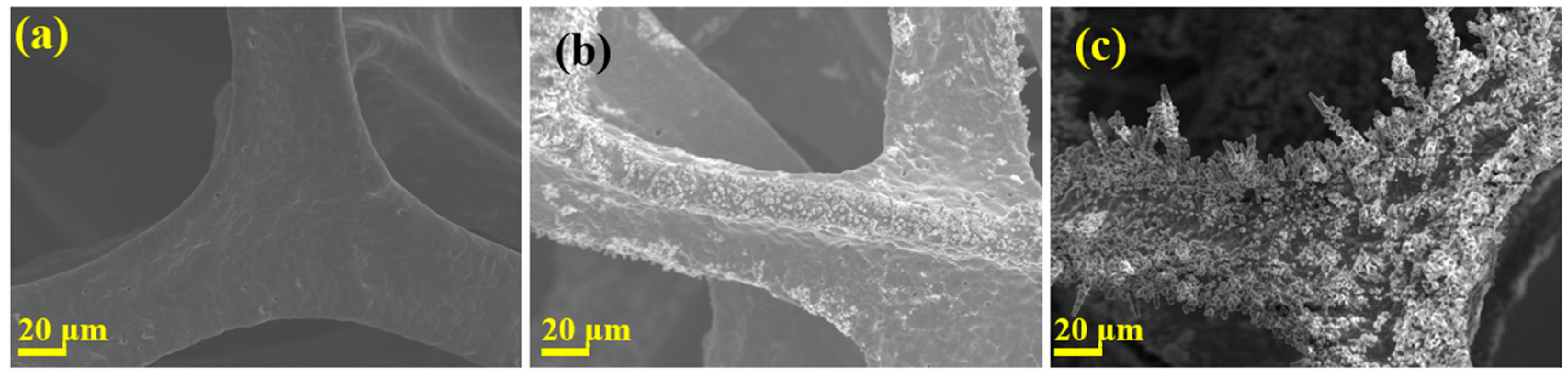
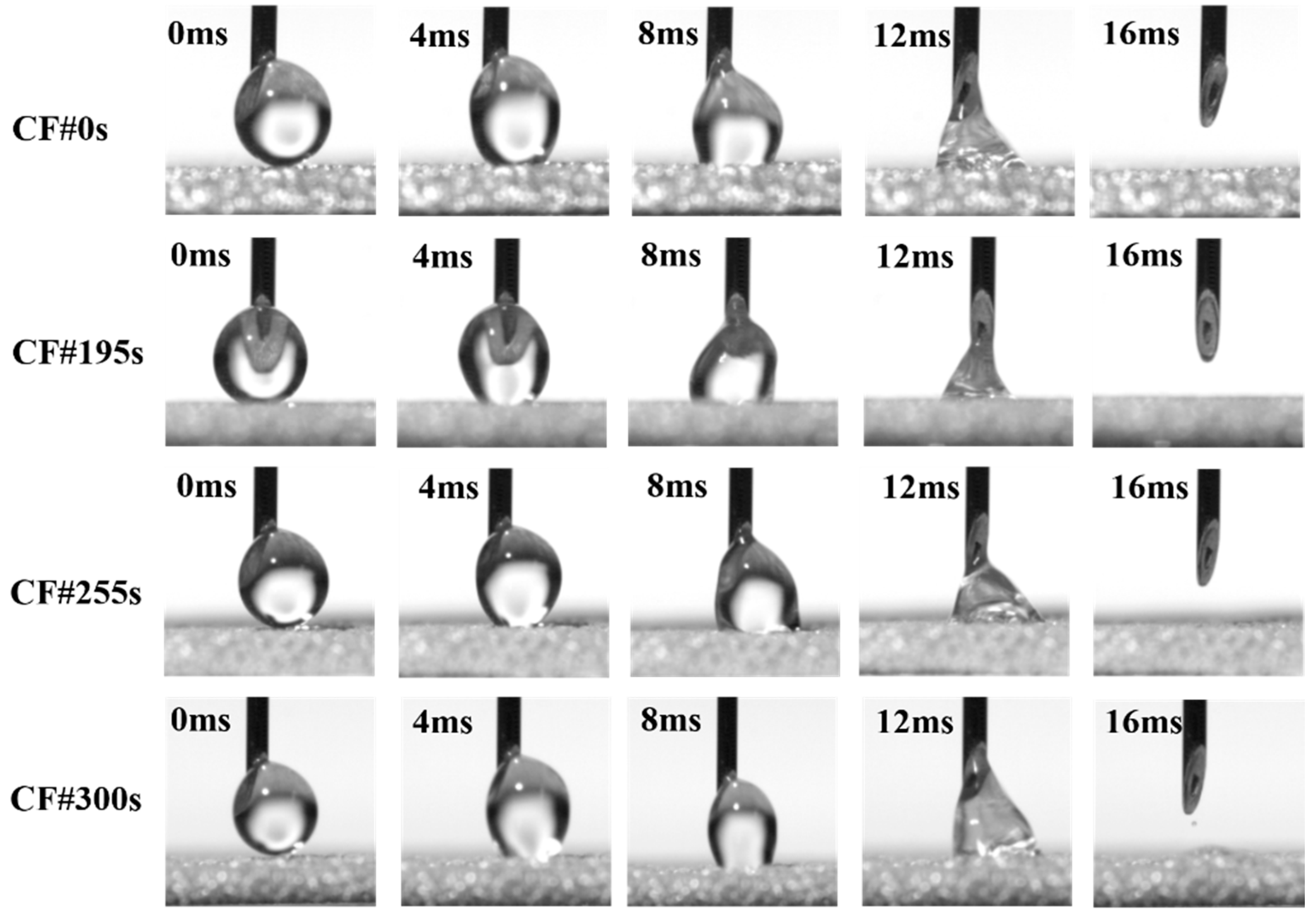
3.1. Morphology of Different Samples
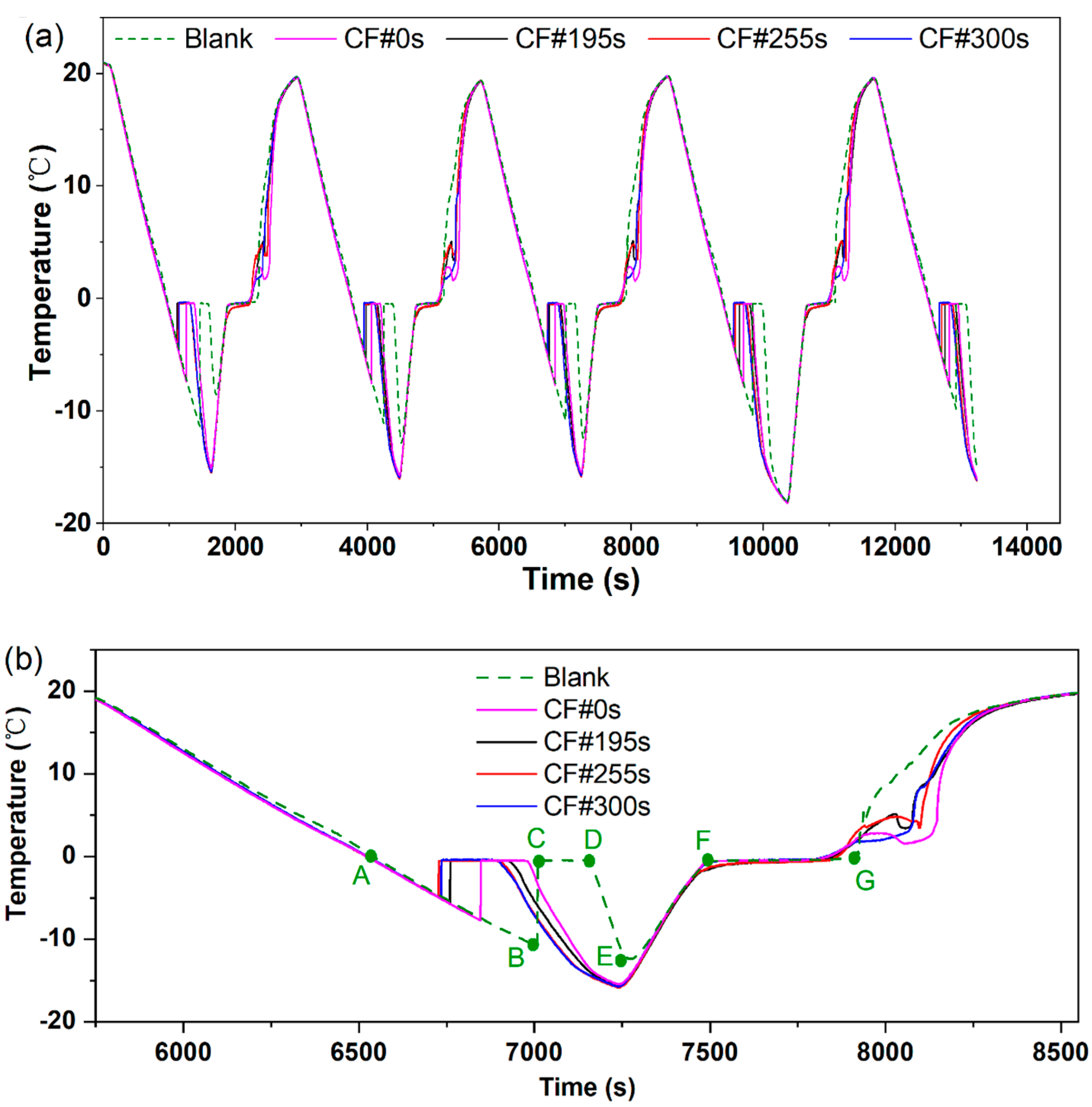
3.2. Temperature Profiles during the Cool Storage Phase-Change Process
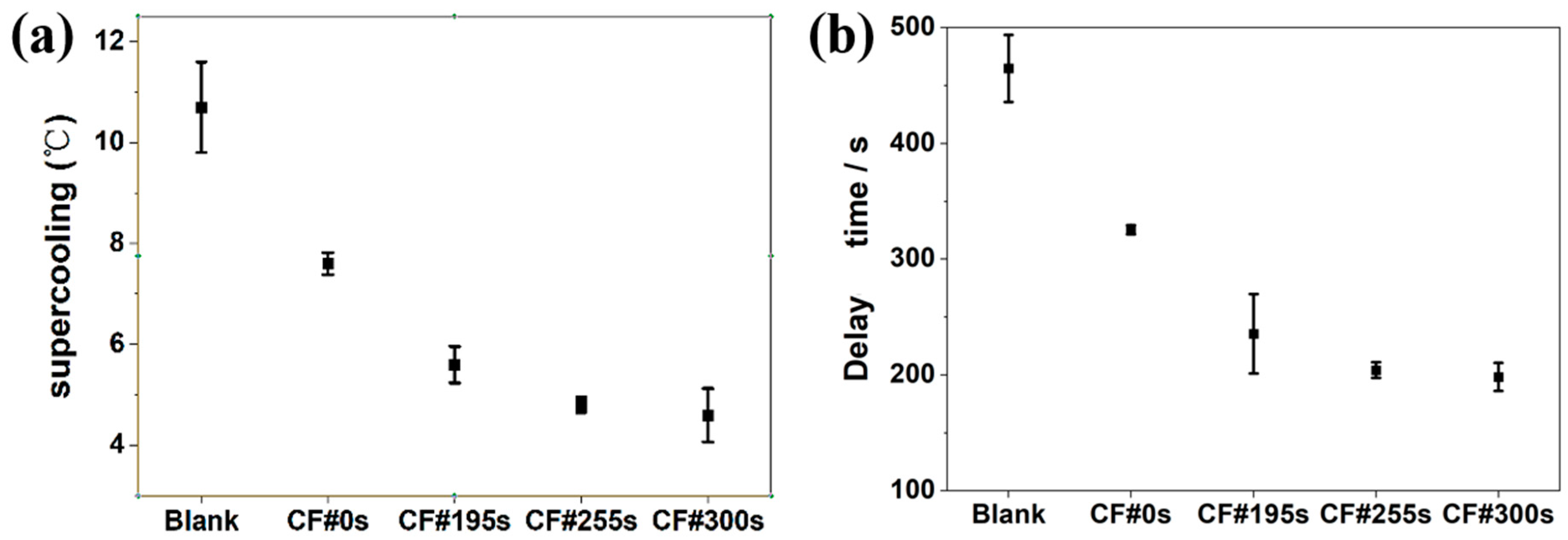
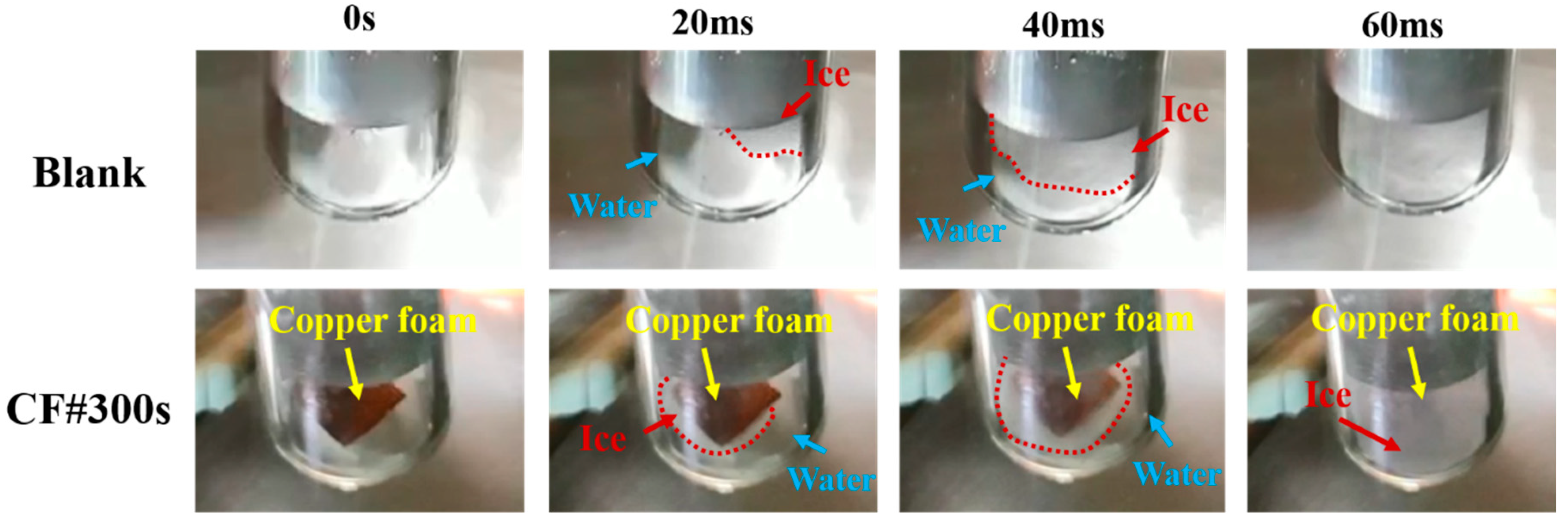
3.3. Supercooling during the Solidification Process
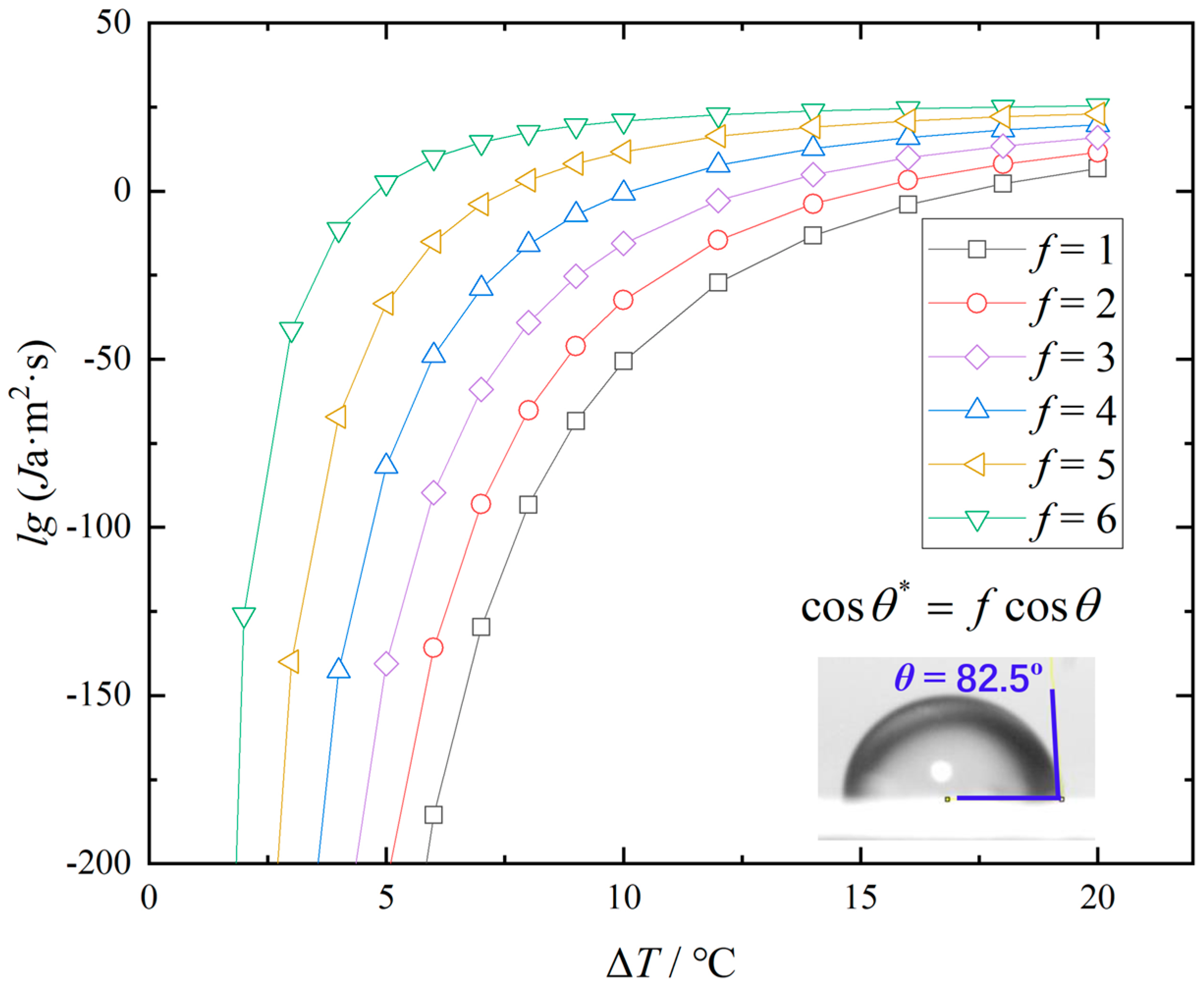
3.4. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carrera-Crespo, J.E.; Huerta-Flores, A.M.; Torres-Martínez, L.M.; Juárez-Ramírez, I. Effect of the Cu foam pretreatment in the growth and inhibition of copper oxide nanoneedles obtained by thermal oxidation and their evaluation as photocathodes. Mater. Sci. Semicond. Process. 2019, 102, 104604. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Lunin, V.V.; Devochkin, A.N.; Petrov, L.A.; Bogdanchikova, N.E.; Petranovskii, V.P. Selective oxidation of alcohols over foam-metal catalysts. Appl. Catal. A Gen. 2002, 227, 125–130. [Google Scholar] [CrossRef]
- Manetti, L.L.; Ribatski, G.; de Souza, R.R.; Cardoso, E.M. Pool boiling heat transfer of HFE-7100 on metal foams. Exp. Therm. Fluid Sci. 2020, 113, 110025. [Google Scholar] [CrossRef]
- Ji, W.-T.; Li, Z.-Y.; Qu, Z.-G.; Guo, J.-F.; Zhang, D.-C.; He, Y.-L.; Tao, W.-Q. Film condensing heat transfer of R134a on single horizontal tube coated with open cell copper foam. Appl. Therm. Eng. 2015, 76, 335–343. [Google Scholar] [CrossRef]
- Xu, Z.G.; Mou, S.; Wang, M.Q.; Gong, Q.; Qin, J. Experimental investigation on pool boiling mechanism of two-level gradient metal foams in deionized water, aqueous surfactant solutions and polymeric additive solutions. Exp. Therm. Fluid Sci. 2018, 96, 20–32. [Google Scholar] [CrossRef]
- Yang, J.; Yang, L.; Xu, C.; Du, X. Numerical analysis on thermal behavior of solid–liquid phase change within copper foam with varying porosity. Int. J. Heat Mass Transf. 2015, 84, 1008–1018. [Google Scholar] [CrossRef]
- Alhusseny, A.; Al-Zurfi, N.; Nasser, A.; Al-Fatlawi, A.; Aljanabi, M. Impact of using a PCM-metal foam composite on charging/discharging process of bundled-tube LHTES units. Int. J. Heat Mass Transf. 2020, 150, 119320. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, X.; Xu, J. Pool boiling heat transfer on copper foam covers with water as working fluid. Int. J. Therm. Sci. 2010, 49, 1227–1237. [Google Scholar] [CrossRef]
- Li, Y.; Chang, S.; Liu, X.; Huang, J.; Yin, J.; Wang, G.; Cao, D. Nanostructured CuO directly grown on copper foam and their supercapacitance performance. Electrochim. Acta 2012, 85, 393–398. [Google Scholar] [CrossRef]
- Wang, L.; Qian, Y.; Du, J.; Wu, H.; Wang, Z.; Li, G.; Li, K.; Wang, W.; Kang, D.J. Facile synthesis of cactus-shaped CdS-Cu9S5 heterostructure on copper foam with enhanced photoelectrochemical performance. Appl. Surf. Sci. 2019, 492, 849–855. [Google Scholar] [CrossRef]
- He, D.; Xing, S.; Sun, B.; Cai, H.; Suo, H.; Zhao, C. Design and construction of three-dimensional flower-like CuO hierarchical nanostructures on copper foam for high performance supercapacitor. Electrochim. Acta 2016, 210, 639–645. [Google Scholar] [CrossRef]
- He, D.; Wang, G.; Liu, G.; Bai, J.; Suo, H.; Zhao, C. Facile route to achieve mesoporous Cu(OH)2 nanorods on copper foam for high-performance supercapacitor electrode. J. Alloys Compd. 2017, 699, 706–712. [Google Scholar] [CrossRef]
- Shinde, S.K.; Yadav, H.M.; Ghodake, G.S.; Kadam, A.A.; Kumbhar, V.S.; Yang, J.; Hwang, K.; Jagadale, A.D.; Kumar, S.; Kim, D.Y. Using chemical bath deposition to create nanosheet-like CuO electrodes for supercapacitor applications. Coll. Surf. B Biointerface 2019, 181, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Jia, X.; Feng, D.; Chen, Z.; Dang, C. Wettability effect on pool boiling heat transfer using a multiscale copper foam surface. Int. J. Heat Mass Transf. 2020, 146, 118726. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Ni, Y. Grass-like Ni/Cu nanosheet arrays grown on copper foam as efficient and non-precious catalyst for hydrogen evolution reaction. Appl. Catal. B Environ. 2020, 268, 118392. [Google Scholar] [CrossRef]
- Liu, R.; Ye, K.; Gao, Y.; Long, Z.; Cheng, K.; Zhang, W.; Wang, G.; Cao, D. Preparation of three-dimensional porous Cu film supported on Cu foam and its electrocatalytic performance for hydrazine electrooxidation in alkaline medium. Mater. Sci. Eng. B 2016, 210, 51–56. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Popov, K.I.; Pavlović, L.J.; Pavlović, M.G. Morphologies of copper deposits obtained by the electrodeposition at high overpotentials. Surf. Coat. Technol. 2006, 201, 560–566. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Lyu, S.-S.; Luo, J.-L.; Luo, Z.-Y.; Fu, Y.-X.; Heng, Y.; Zhang, J.-H.; Mo, D.-C. Copper vertical micro dendrite fin arrays and their superior boiling heat transfer capability. Appl. Surf. Sci. 2017, 422, 388–393. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Luo, J.-L.; Heng, Y.; Mo, D.-C.; Lyu, S.-S. Wettability modification to further enhance the pool boiling performance of the micro nano bi-porous copper surface structure. Int. J. Heat Mass Transf. 2018, 119, 333–342. [Google Scholar] [CrossRef]
- Zhang, B.; He, Z. The preparation of AgI/Au/foam-Cu as a framework of composite for water-based cool storage phase-change material with low supercooling. Thermochim. Acta 2019, 674, 52–57. [Google Scholar] [CrossRef]
- Faucheux, M.; Muller, G.; Havet, M.; LeBail, A. Influence of surface roughness on the supercooling degree: Case of selected water/ethanol solutions frozen on aluminium surfaces. Int. J. Refrig. 2006, 29, 1218–1224. [Google Scholar] [CrossRef]
- Zhang, R.; Hao, P.; Zhang, X.; He, F. Supercooled water droplet impact on superhydrophobic surfaces with various roughness and temperature. Int. J. Heat Mass Transf. 2018, 122, 395–402. [Google Scholar] [CrossRef]
- Irajizad, P.; Nazifi, S.; Ghasemi, H. Icephobic surfaces: Definition and figures of merit. Adv. Coll. Interface Sci. 2019, 269, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, H.; Lin, X.; Huang, W. Wenzel model based investigation of heterogeneous nucleation on a coarse substrate. IOP Conf. Ser. Mater. Sci. Eng. 2012, 27, 012006. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Zheng, H.; Lin, X.; Huang, W. Investigation of the Heterogeneous Nucleation on Fractal Surfaces. J. Mater. Sci. Technol. 2012, 28, 1169–1174. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Wang, M.; Wang, X.X.; Huang, W.D. Analysis of heterogeneous nucleation on rough surfaces based on Wenzel model. Acta Phys. Sin. 2011, 60. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Yang, Z.; Jin, Z.; Zhu, Y.; Zhou, Z. A theoretical and experimental study of typical heterogeneous ice nucleation process on auto windshield under nocturnal radiative cooling and subfreezing conditions. Int. J. Heat Mass Transf. 2019, 136, 610–626. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Song, M.; Dai, B.; Sun, Z. Impacts on the solidification of water on plate surface for cold energy storage using ice slurry. Appl. Energy 2018, 227, 284–293. [Google Scholar] [CrossRef]
- Smith, R.S.; Kay, B.D. The existence of supercooled liquid water at 150 K. Nature 1999, 398, 788–791. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Su, F.; Yao, K. Facile fabrication of superhydrophobic surface with excellent mechanical abrasion and corrosion resistance on copper substrate by a novel method. ACS Appl. Mater. Interface 2014, 6, 8762–8770. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, X.; Tian, Y. Fabrication of super-hydrophobic nickel film on copper substrate with improved corrosion inhibition by electrodeposition process. Coll. Surf. A 2019, 560, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-W.; Zhang, C.-Z.; Yang, D.; Sun, B.; Hong, W.-P. Experimental investigation on flow boiling heat transfer characteristics of R141b refrigerant in parallel small channels filled with metal foam. Int. J. Heat Mass Transf. 2019, 133, 21–35. [Google Scholar] [CrossRef]

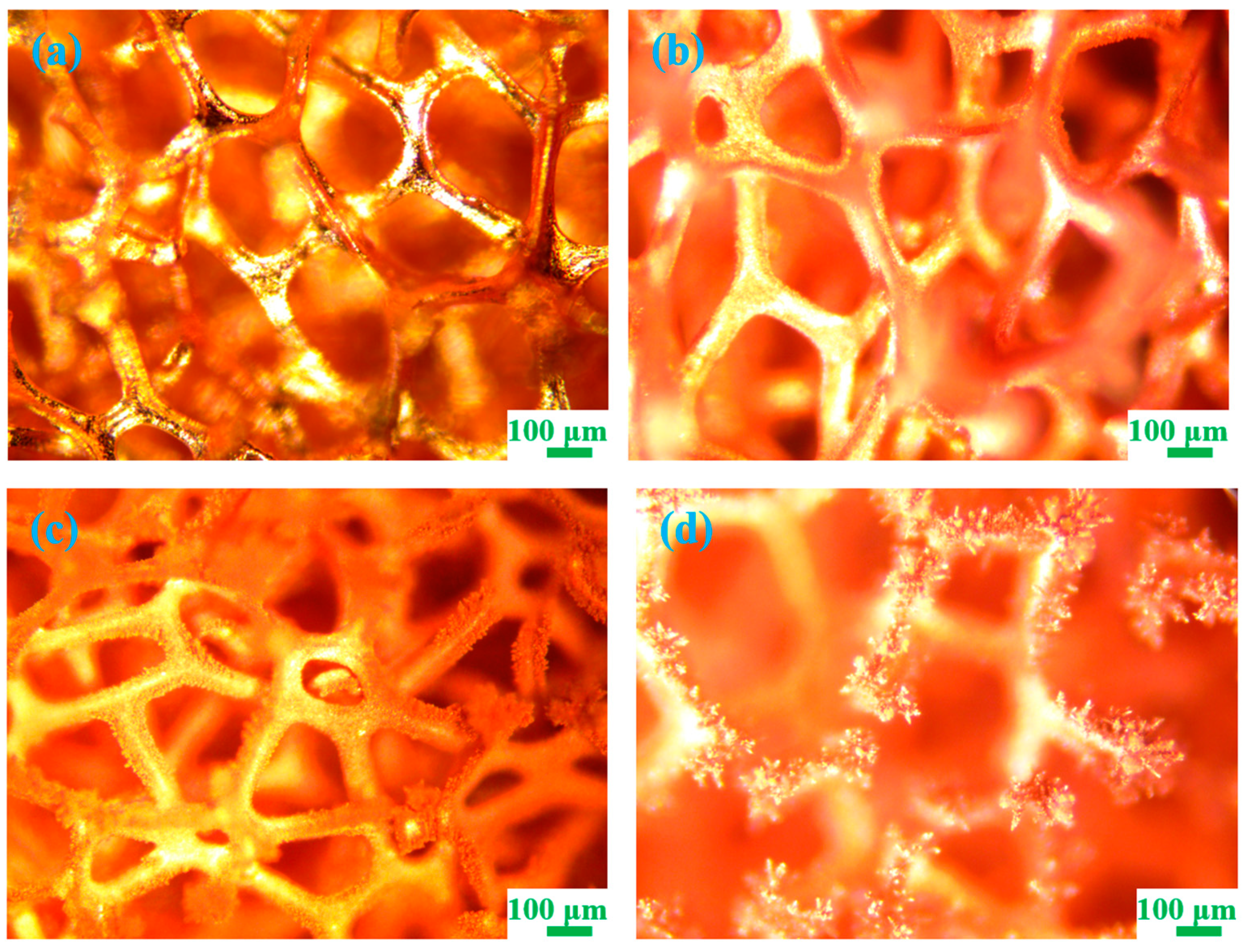
| No. | Sample | Deposition Time(s) |
|---|---|---|
| 1 | CF#0s | 0 |
| 2 | CF#195s | 195 |
| 3 | CF#255s | 255 |
| 4 | CF#300s | 300 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Chen, Y.-F.; Liang, J.-Y.; Mo, D.-C.; Lyu, S.-S. Electrodeposition Patterned Copper Foam with Micro/Nanostructures for Reducing Supercooling in Water-Based Cool Storage Phase-Change Materials. Appl. Sci. 2020, 10, 4202. https://doi.org/10.3390/app10124202
Xu M, Chen Y-F, Liang J-Y, Mo D-C, Lyu S-S. Electrodeposition Patterned Copper Foam with Micro/Nanostructures for Reducing Supercooling in Water-Based Cool Storage Phase-Change Materials. Applied Sciences. 2020; 10(12):4202. https://doi.org/10.3390/app10124202
Chicago/Turabian StyleXu, Mou, Yu-Feng Chen, Jian-Yang Liang, Dong-Chuan Mo, and Shu-Shen Lyu. 2020. "Electrodeposition Patterned Copper Foam with Micro/Nanostructures for Reducing Supercooling in Water-Based Cool Storage Phase-Change Materials" Applied Sciences 10, no. 12: 4202. https://doi.org/10.3390/app10124202




