Microgravity, Bone Homeostasis, and Insulin-Like Growth Factor-1
Abstract
:1. Introduction
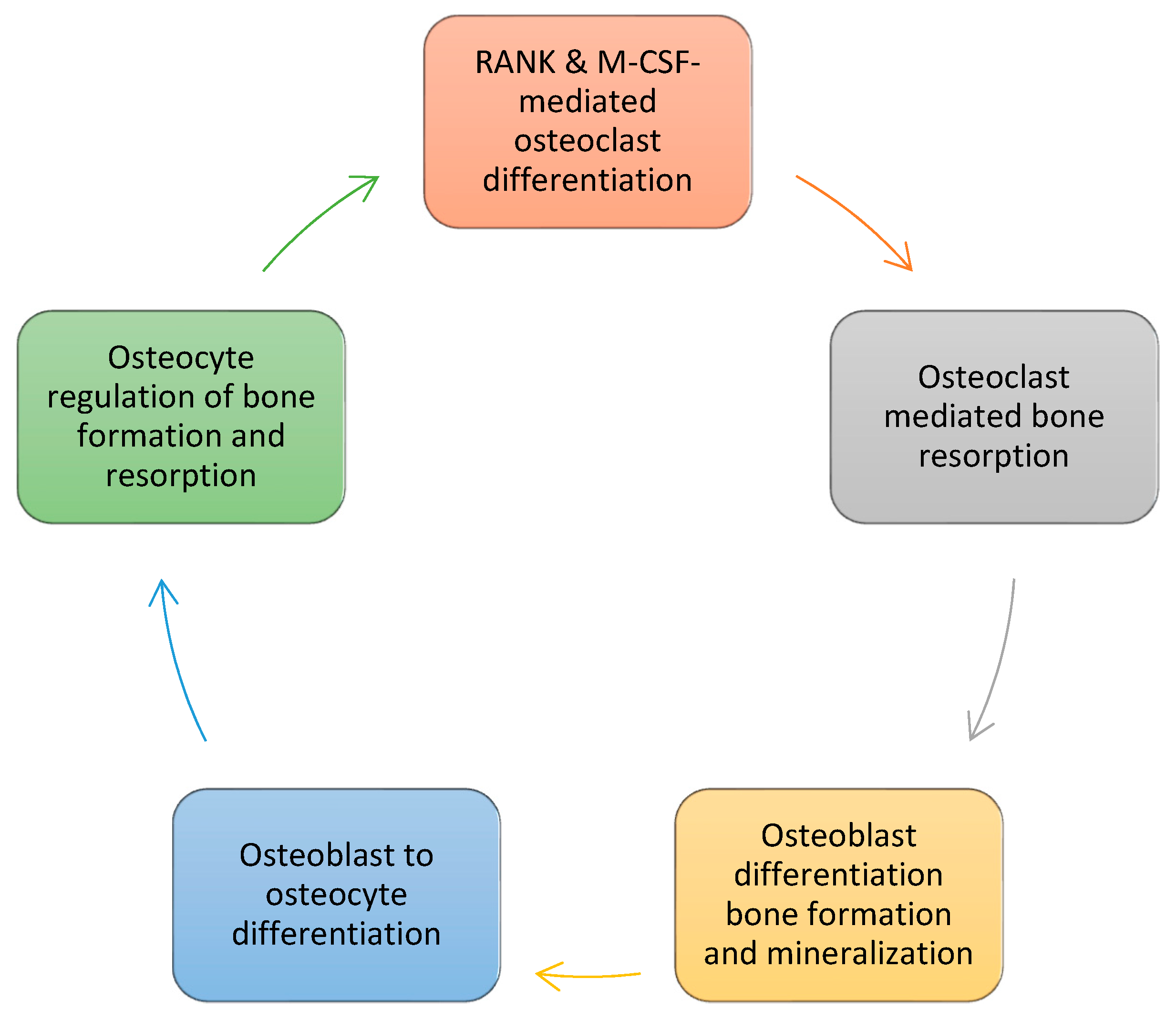
2. Bone Formation
3. Mechanotransduction
3.1. Osteocytes
3.2. Osteoblasts
3.3. Osteoclasts
4. Mechanotransduction in Space (1 × 10−6 g)
4.1. Osteocytes
4.2. Osteoblasts
4.3. Osteoclasts
5. Mechanotransduction in Simulated Microgravity (g ≥ 10−3)
5.1. Osteocytes
5.2. Osteoblasts
5.3. Osteoclasts
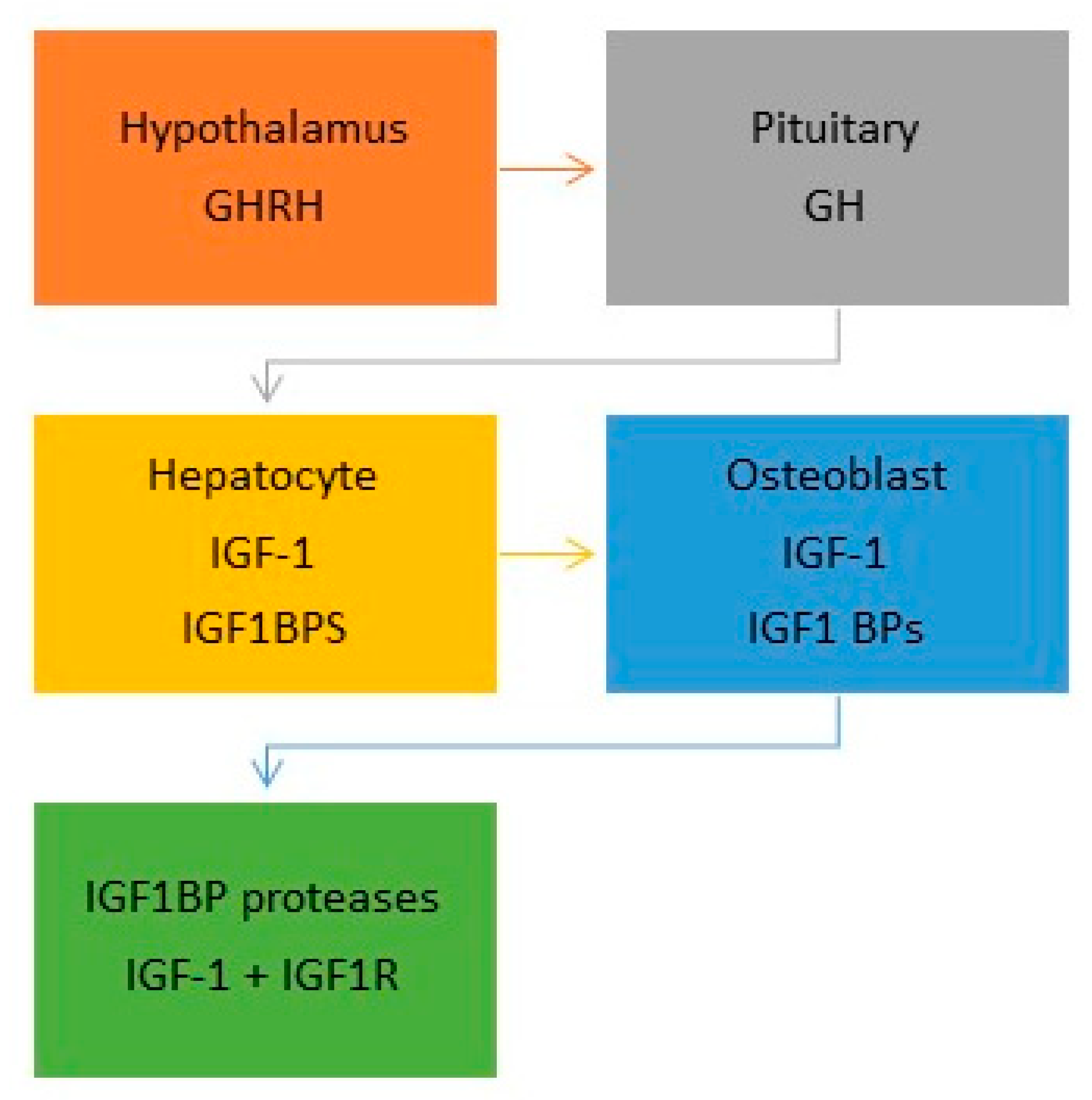
6. The Somatotrophic Axis
6.1. Insulin-Like Growth Factor-1 (IGF-1)
6.2. Insulin-Like Growth Factor-1 Receptor (IGF-1R)
7. IGF-1 and Bone Homeostasis
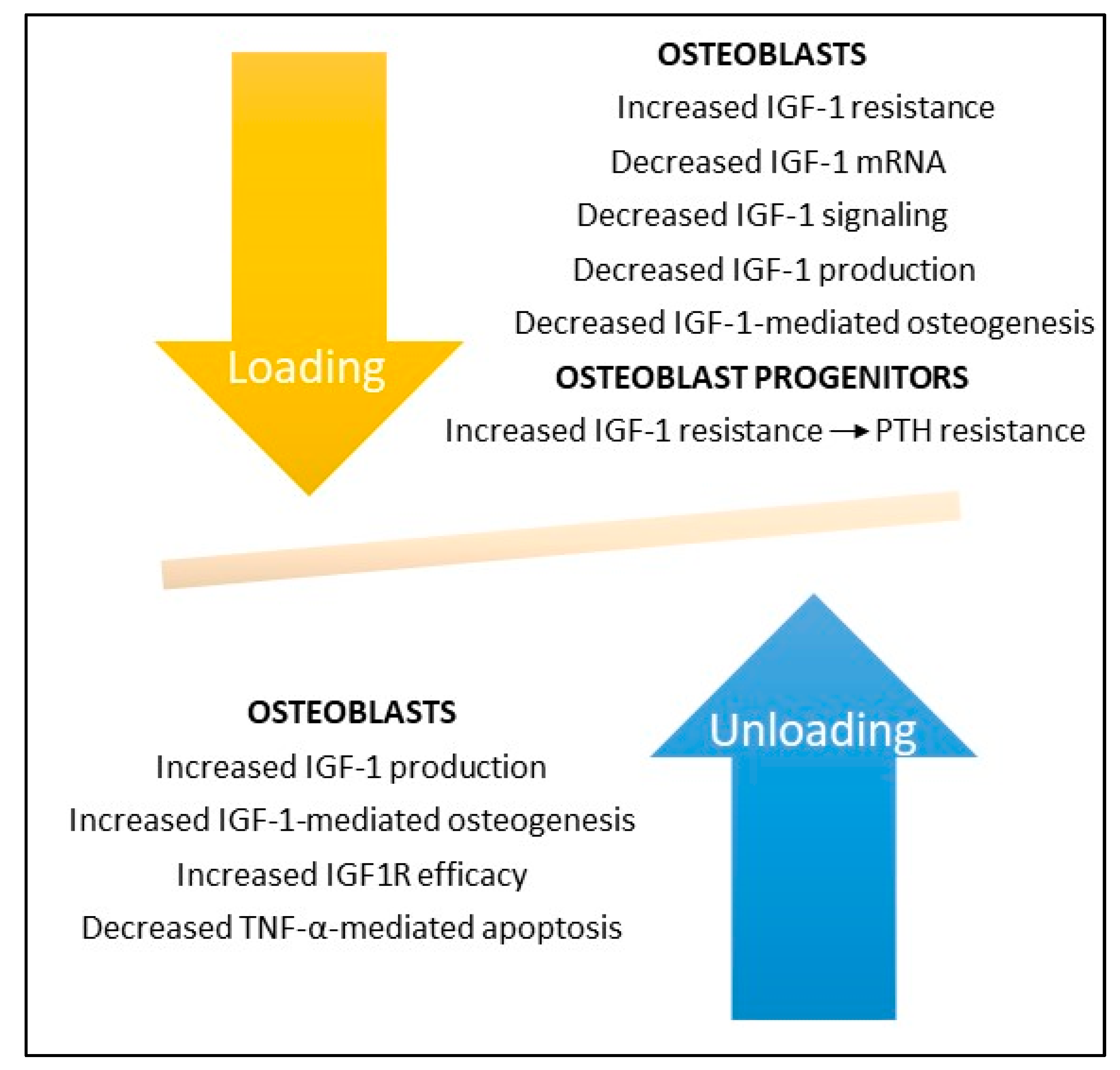
8. IGF-1 and Mechanical Loading
9. IGF-1 and Mechanical Unloading
10. Discussion
10.1. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)
10.2. Syncytin-A
10.3. Sclerostin
10.4. IGF-1
11. Conclusions
- By tradition bone formation is divided into four phases: 1. Osteoclast differentiation; 2. Osteoclast-mediated bone resorption; 3. Osteoblast-mediated bone formation/mineralization; and 4. Osteoblast to osteocyte transformation and establishment of bone homeostasis.
- Zero and modeled microgravity adversely affect all four phases of bone formation, increasing the maturation and the resorptive activities of osteoclasts, decreasing the differentiation, maturation, and bone forming abilities of osteoblasts, and causing apoptotic-mediated death of osteocytes with consequent dysregulation of bone homeostasis.
- Microgravity-associated changes in osteoblast microstructure include extended cell shapes, fragmented and condensed nuclei, shortened microtubules, smaller and fewer focal adhesions, and even complete collapse of the cytoskeleton due to microfilament and/or microtubular failure. Osteocyte death is reflected by increases in lacunar vacancies and volumes and in the production of sclerostin, a mechanosensitive protein that inhibits bone formation and enhances bone resorption.
- IGF-1 and its receptor IGF1R play critical roles in all phases of bone formation, promoting both radial and linear bone growth. Osteocyte and osteoblast responses to mechanostressors is IGF-1/IGF1R dependent and deletion of IGF-1 or IGF1R genes abates their responses to mechanical unloading and reloading. Treatment with recombinant IGF-1 (rIGF1) reverses these defects when IGF1R is intact, raising the prospect that rIGF-1 may prove useful in preventing bone loss associated with space travel or, more likely, in the microgravity of the Moon. Another potential candidate to prevent bone loss in space or on the Moon is romosozumab, a humanized antibody against sclerostin which has been approved by the FDA for treatment of osteoporosis.
Funding
Acknowledgments
Conflicts of Interest
References
- Svitkina, T. The actin cytoskeleton and actin-based motility. Cold Spring Harb. Perspect. Biol. 2018, 10, a018267. [Google Scholar] [CrossRef] [Green Version]
- LaFlamme, S.E.; Mathew-Steiner, S.; Singh, N.; Colello-Borges, D.; Nieves, B. Integrin and microtubule crosstalk in the regulation of cellular processes. Cell Mol. Life Sci. 2018, 75, 4177–4185. [Google Scholar] [CrossRef] [PubMed]
- Burke, B. Chain reaction: LINC complexes and nuclear positioning. F1000Research 2019, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.T.; Navajas, P.L.; Lietha, D. FAK Structure and regulation by membrane interactions and force in focal adhesions. Biomolecules 2020, 10, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bérut, A.; Chauvet, H.; Legué, V.; Moulia, B.; Pouliquen, O.; Forterre, Y. Gravisensors in plant cells behave like an active granular liquid. Proc. Natl. Acad. Sci. USA 2018, 115, 5123–5128. [Google Scholar] [CrossRef] [Green Version]
- Howard, R.; Scheiner, A.; Cunningham, J.; Gatenby, R. Cytoplasmic convection currents and intracellular temperature gradients. PLoS Comput. Biol. 2019, 15. [Google Scholar] [CrossRef] [Green Version]
- Yakar, S.; Werner, H.; Rosen, C.J. Insulin-like growth factors: Actions on the skeleton. J. Mol. Endocrinol. 2018, 61, T115–T137. [Google Scholar] [CrossRef]
- Wittkowske, C.; Reilly, G.C.; Lacroix, D.; Perrault, C.M. In vitro bone cell models: Impact of fluid shear stress on bone formation. Front. Bioeng. Biotechnol. 2016, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Issack, P.S.; Helfet, D.L.; Lane, J.M. Role of Wnt signaling in bone remodeling and repair. HSS J. 2008, 4, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Sakai, A. Spaceflight/bedrest immobilization and bone. Osteocyte as a sensor of mechanical stress and Wnt signal. Clin. Calcium. 2012, 22, 1829–1835. [Google Scholar]
- Aventaggiato, M.; Barreca, F.; Vernucci, E.; Bizzarri, M.; Ferretti, E.; Russo, M.A.; Tafani, M. Putative receptors for gravity sensing in mammalian cells: The effects of microgravity. Appl. Sci. 2020, 10, 2028. [Google Scholar] [CrossRef] [Green Version]
- Hillsley, M.V.; Frangos, J.A. Bone tissue engineering: Role of interstitial fluid flow. Biotechnol. Bioeng. 1994, 43, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, Y.; Cheung, W.-Y.; Gandhi, R.; Wang, L.; Lidan, Y. Effects of cyclic hydraulic pressure on osteocytes. Bone 2010, 46, 1449–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einhorn, T.A. The role of cyclooxygenase-2 in bone repair. Arthritis Res. Ther. 2003, 5, 5–7. [Google Scholar] [CrossRef]
- Young, S.R.L.; Hum, J.M.; Rodenberg, E.; Turner, C.H.; Pavalko, F.M. Non-overlapping functions for Pyk2 and FAK in osteoblasts during fluid stress-induced mechanotransduction. PLoS ONE 2011, 6, e16026. [Google Scholar] [CrossRef] [Green Version]
- Ban, Y.; Wu, Y.; Yu, T.; Geng, N.; Wang, Y.; Liu, X.; Gong, P. Response of osteoblasts to low fluid shear stress is time dependent. Tissue Cell 2011, 43, 311–317. [Google Scholar] [CrossRef]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biochem. 2008, 283, 5866–5875. [Google Scholar]
- Gerbaix, M.; Gnyubkin, V.; Farlay, D.; Olivier, C.; Ammann, P.; Courbon, G.; Laroche, N.; Genthial, R.; Follet, H.; Peyrin, F.; et al. One-month spaceflight compromises the bone microstructure, tissue-level mechanical properties, osteocyte survival and lacunae volume in mature mice skeletons. Sci. Rep. 2017, 7, 2659. [Google Scholar] [CrossRef]
- Rodionova, N.V.; Organov, V.S.; Zolotova, N.V. Ultrastructure changes in osteocytes in microgravity conditions. Adv. Space Res. 2002, 30, 765–770. [Google Scholar] [CrossRef]
- Carmeliet, G.; Bouillon, R. The effect of microgravity on morphology and gene expression of osteoblasts in vitro. FASEB J. 1999, 13, S129–S134. [Google Scholar] [CrossRef] [Green Version]
- Nabavi, N.; Khandani, A.; Camirand, A.; Harrison, R.E. Effects of microgravity on osteoclast bone resorption and on osteoblast cytoskeletal organization and adhesion. Bone 2011, 49, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Hughs-Fulford, M. Function of the cytoskeleton in gravisensing during spaceflight. Adv. Space Res. 2003, 32, 1583–1593. [Google Scholar] [CrossRef]
- Hughs-Fulford, M.; Tjandrawinata, R.; Fitzgerald, J.; Gasuad, K.; Gilbertson, V. Effects of microgravity on osteoblast growth. Gravit. Space Biol. Bull. 1998, 11, 51–60. [Google Scholar]
- Guignandon, A.; Lafage-Proust, M.H.; Usson, Y.; Laroche, N.; Caillot-Augusseau, A.; Alexandre, C.; Vico, L. Cell cycling determines integrin-mediated adhesion in osteoblastic ROS 17/2.8 cells exposed to space-related conditions. FASEB J. 2001, 15, 2036–2038. [Google Scholar] [PubMed] [Green Version]
- Tamma, R.; Colaianni, G.; Camerino, C.; Di Benedetto, A.; Greco, G.; Strippoli, M.; Vergari, R.; Grano, A.; Mancini, L.; Mori, G.; et al. Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. FASEB J. 2009, 23, 2549–2554. [Google Scholar] [CrossRef]
- Lin, C.; Jiang, X.; Dai, Z.; Guo, X.; Weng, T.; Wang, J.; Li, Y.; Feng, G.; Gao, X.; He, L. Sclerostin mediates bone resorption response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J. Bone Miner. Res. 2009, 24, 1651–1661. [Google Scholar] [CrossRef]
- Saxena, R.; Pan, G.; McDonald, J.M. Osteoblast and osteoclast differentiation in modeled microgravity. Ann. N. Y. Acad. Sci. 2007, 1116, 494–498. [Google Scholar] [CrossRef]
- Ontiveros, C.; McCabe, L.R. Simulated microgravity suppresses osteoblast phenotype, Runx2 levels and AP-1 transactivation. J. Cell. Biochem. 2003, 88, 427–437. [Google Scholar] [CrossRef]
- Patel, M.J.; Liu, W.; Sykes, M.C.; Ward, N.E.; Risin, S.A.; Risin, D.; Jo, H. Identification of mechanosensitive genes in osteoblasts by comparative microarray studies using the rotating wall vessel and the random positioning machine. J. Cell. Biochem. 2007, 101, 587–599. [Google Scholar] [CrossRef]
- Shi, W.; Xie, Y.; He, J.; Zhou, J.; Gao, Y.; Wei, W.; Ding, N.; Ma, H.; Xian, C.J.; Chen, K.; et al. Microgravity induces inhibition of osteoblastic differentiation and mineralization through abrogating primary cilia. Sci. Rep. 2017, 7, 1866. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Zhou, H.; Cai, M.; Liu, J.; Gao, S.; Yang, J.; Tong, L.; Wang, J.; Zhou, S.; et al. Simulated microgravity reduces intracellular-free calcium concentration by inhibiting calcium channels in primary mouse osteoblasts. J. Cell. Biochem. 2019, 120, 4009–4020. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.Q.; Wang, R.; Ling, S.K.; Wan, Y.M.; Li, Y.H. Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells. Cell. Prolif. 2007, 40, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, C.; Chen, J.L.; Hong, F.F.; Chen, P.; Wang, J.F. Effects of simulated microgravity on the expression profiles of RNA during osteogenic differentiation of human bone marrow mesenchymal stem cells. Cell. Prolif. 2019, 52, e12539. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, Q.; Lin, C.; Song, G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells through down regulating the transcriptional co-activator TAZ. Biochem. Biophys. Res. Commun. 2015, 468, 21–26. [Google Scholar] [CrossRef]
- Michaletti, A.; Gioia, M.; Tarantino, U.; Zolla, L. Effects of microgravity on osteoblast mitochondria: A proteomic and metabolomics profile. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Sambandam, Y.; Townsend, M.T.; Pierce, J.J.; Lipman, C.M.; Haque, A.; Bateman, T.A.; Reddy, S.V. Microgravity control of autophagy modulates osteoclastogenesis. Bone 2014, 61, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Sambandam, Y.; Baird, K.L.; Stroebel, M.; Kowai, E.; Balasubramanian, S.; Reddy, S.V. Microgravity induction of TRAIL expression in preosteoclast cells enhances osteoclast differentiation. Sci. Rep. 2016, 6, 25143. [Google Scholar] [CrossRef] [Green Version]
- Ethiraj, P.; Link, J.R.; Sinkway, J.M.; Brown, G.D.; Parler, W.A.; Reddy, S.V. Microgravity modulation of syncytin-A expression enhance osteoclast formation. J. Cell. Biochem. 2018, 119, 5696–5703. [Google Scholar] [CrossRef]
- Rucci, N.; Rufo, A.; Alamanou, M.; Teti, A. Modeled microgravity stimulates osteoclastogenesis and bone resorption by increasing osteoblast RANKL/OPG ratio. J. Cell. Biochem. 2007, 100, 464–473. [Google Scholar] [CrossRef]
- Saxena, R.; Pan, G.; Dohm, E.D.; McDonald, J.M. Modeled microgravity and hindlimb unloading sensitize osteoclast precursors to RANKL-mediated osteoclastogenesis. J. Bone Miner. Metab. 2011, 29, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Choi, E.; Yu, H.; Bai, X.C. Structural basis of the activation of type 1 insulin-like growth factor receptor. Nat. Commun. 2019, 10, 4567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, D.; Sun, J.; Wang, Y.; Ouyang, O.; Wang, T. Visualization of ligand-bound ectodomain assembly in the full-length human IGF-1 receptor by cryo-EM single-particle analysis. Structure 2020, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.A. New insights from IGF-1R stimulating activity analysis: Pathological considerations. Cells 2020, 9, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakar, S.; Rosen, C.J.; Beamer, W.G.; Ackert-Bicknell, C.L.; Wu, Y.; Liu, J.-L.; Ooi, G.T.; Setser, J.; Frystyk, J.; Boisclair, Y.R.; et al. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Invest. 2002, 110, 771–781. [Google Scholar] [CrossRef]
- Crane, J.L.; Cao, X. Function of matrix IGF-1 in coupling bone resorption and formation. J. Mol. Med. 2014, 92, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Clarmatori, S.; Kiepe, D.; Haarmann, A.; Huegel, U.; Tonshoff, B. Signaling mechanisms leading to regulation of proliferation and differentiation of the mesenchymal chondrogenic cell line. J. Mol. Endocrinol. 2007, 38, 493–508. [Google Scholar] [CrossRef] [Green Version]
- Xian, L.; Wu, X.; Pang, L.; Lou, M.; Rosen, C.; Qiu, T.; Crane, J.; Frassica, F.; Zhang, L.; Rodriguez, J.P.; et al. Matrix IGF-1 regulates bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 2012, 18, 1095–1101. [Google Scholar] [CrossRef] [Green Version]
- Kostenuik, P.J.; Harris, J.; Halloran, B.P.; Turner, R.T.; Morey-Holton, E.R.; Bikle, D.D. Skeletal unloading causes resistance of osteoprogenitor cells to parathyroid hormone and to insulin-like growth factor-1. J. Bone Miner. Res. 1999, 14, 21–31. [Google Scholar] [CrossRef]
- Wang, Y.; Nishida, S.; Elalieh, H.Z.; Long, R.K.; Halloran, B.P.; Bikle, D.D. Role of IGF-1 signaling in regulating osteoclastogenesis. J. Bone Min. Res. 2006, 21, 1350–1358. [Google Scholar] [CrossRef] [Green Version]
- Pavalko, F.M.; Gerard, R.L.; Ponik, S.M.; Gallagher, P.J.; Jin, Y.; Norvell, S.M. Fluid shear stress inhibits TNF-alpha-induced apoptosis in osteoblasts: A role for fluid shear stress-induced activation of PI3-kinase and inhibition of caspase-3. J. Cell. Physiol. 2003, 194, 194–205. [Google Scholar] [CrossRef]
- Triplett, J.W.; O’Riley, R.; Tekulve, K.; Norvell, S.M.; Pavalko, F.M. Mechanical loading by fluid shear stress enhances IGF-1 receptor signaling in osteoblasts in a PKCzeta-dependent manner. Mol. Cell. Biomech. 2007, 4, 13–25. [Google Scholar] [PubMed]
- Tian, F.; Wang, Y.; Bickle, D.D. IGF-1 signaling mediated cell-specific skeletal mechano-transduction. J. Orthop. Res. 2018, 36, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Kumei, Y.; Nakamura, H.; Morita, S.; Akiyama, H.; Hirano, M.; Ohya, K.; Shinomiya, K.I.; Shimokawa, H. Space flight and insulin-like growth factor signaling in rat osteoblasts. Ann. N. Y. Acad. Sci. 2002, 973, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Wang, Y.; Halloran, B.P.; Elalieh, H.Z.; Cao, J.; Bikle, D.D. Skeletal unloading induces resistance to insulin-like growth factor-1 (IGF-1) by inhibiting activation of the IGF-1 signaling pathways. J. Bone Miner. Res. 2004, 19, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Moriwake, T.; Matsuoka, Y.; Nakamura, T.; Seino, Y. Potential role of rhIGF-1/IGFBP-3 in maintaining skeletal mass in space. Bone 1998, 22, 145S–147S. [Google Scholar] [CrossRef]
- Bateman, T.A.; Zimmerman, R.J.; Ayers, R.A.; Ferguson, V.L.; Chapes, S.K.; Simske, S.J. Histomorphometric, physical, and mechanical effects of spaceflight and insulin-like growth factor on rat long bones. Bone 1998, 23, 527–535. [Google Scholar] [CrossRef]
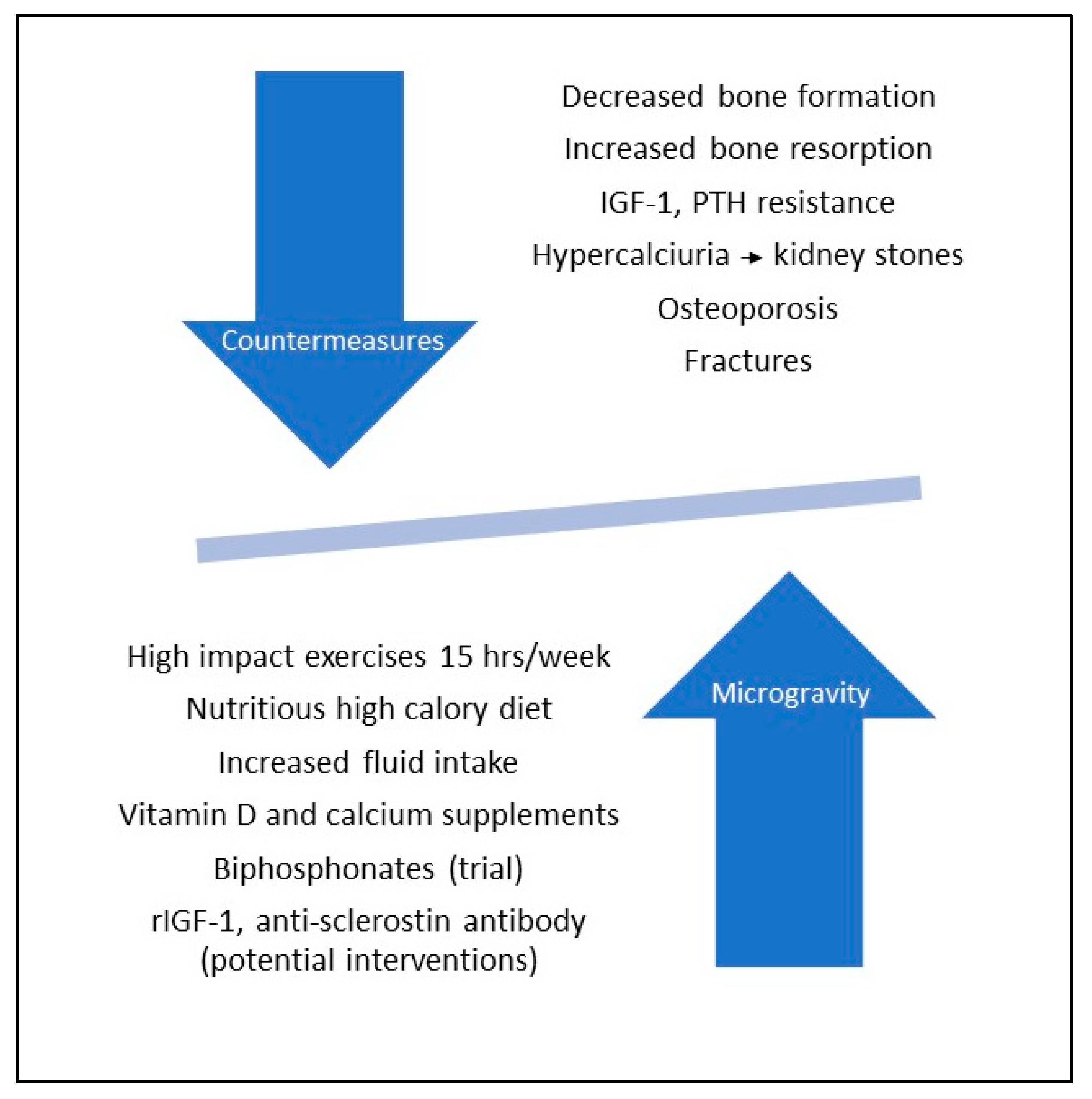
- Iwamoto, J.; Takeda, T.; Sato, Y. Interventions to prevent bone loss in astronauts during space flight. Keio J. Med. 2005, 54, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Cavanagh, P.R.; Licata, A.A.; Rice, A.J. Exercise and pharmacological countermeasures for bone loss during long-duration space flight. Gravit. Space Biol. Bull. 2005, 18, 39–58. [Google Scholar]
- Smith, S.M.; McCoy, T.; Gazda, D.; Morgan, J.L.L.; Heer, M.; Zwart, S.R. Space flight calcium: Implications for astronaut health, spaceflight operations, and Earth. Nutrients 2012, 4, 2047–2068. [Google Scholar] [CrossRef] [Green Version]
- Leblanc, A.; Matsumoto, T.; Jones, J.; Shapiro, J.; Lang, T.; Shackelford, L.; Smith, S.M.; Evans, H.; Spector, E.; Ploutz-Snyder, R.; et al. Biphosphonates as a supplement to exercise to protect bone during long-duration spaceflight. Osteoporos. Int. 2013, 24, 2105–2114. [Google Scholar] [CrossRef]
- NASA—Food For Space Flight. Available online: https:/www.nasa.gov/specials/apollo50thy/index.html (accessed on 12 June 2020).
- Micheau, O. Regulation of TNF-related apoptosis-inducing ligand signaling by glycosylation. Int. J. Mol. Sci. 2018, 19, 715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walczak, H. Biochemical analysis of the native TRAIL death-inducing signaling complex. Methods Mol. Biol. 2008, 414, 221–239. [Google Scholar] [PubMed]
- Dupressoir, A.; Lavialle, G.; Heidmann, T. From ancestral infectious retroviruses to bone fide cellular genes: Role of the captured syncytins in placentation. Placenta 2012, 33, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Pan, J.; Gong, R.; Liu, Y.; Kang, S.; Feng, H.; Qiu, G.; Guo, D.; Tien, P.; Xiao, G. Functional characterization of syncytin-A, a newly murine endogenous virus protein. Implication for its fusion mechanism. J. Biol. Chem. 2007, 282, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Moller, A.M.; Delaissé, J.M.; Soe, K. Osteoclast fusion: Time-lapse reveals involvement of CD47 and syncytin-1 at different stages of nuclearity. J. Cell. Physiol. 2017, 232, 1396–1403. [Google Scholar] [CrossRef]
- De Maré, A.; D’Haese, P.C.; Verhulst, A. The role of sclerostin in bone and ectopic calcification. Int. J. Mol. Sci. 2020, 21, 3199. [Google Scholar] [CrossRef]
- Bandeira, L.; Lewiecki, E.M.; Bilezikian, J.P. Romosozumab for the treatment of osteoporosis. Expert Opin. Biol. Ther. 2017, 17, 255–263. [Google Scholar] [CrossRef]
- Shakeri, A.; Adanty, C. Romosozumab (sclerostin monoclonal antibody) for the treatment of osteoporosis in postmenopausal women: A review. J. Popul. Ther. Clin. Pharmacol. 2020, 27, e25–e31. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.F.; Fawcett, J.R.; Thome, R.G.; DeFor, T.A.; Frey, W.H., 2nd. Intranasal administration of insulin-like growth factor-1 bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J. Neurol. Sci. 2001, 187, 91–97. [Google Scholar] [CrossRef]
- Misra, M.; McGrane, J.; Miller, K.K.; Goldstein, M.A.; Ebrahimi, S.; Weigel, T.; Klibanski, A. Effects of rhIGF-1 administration on surrogate markers of bone turnover in adolescents with anorexia nervosa. Bone 2009, 45, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Mohamed-Ali, V.; Pinkney, J. Therapeutic potential of insulin-like growth factor-1 in patients with diabetes mellitus. Treat. Endocrinol. 2002, 1, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.K. IL-6 and the dysregulation of immune, bone, muscle, and metabolic homeostasis during spaceflight. npj Microgravity 2018, 4, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Mechanostressors | G x 1 | G ≥ 10−3 | G = 10−6 | |
|---|---|---|---|---|
| Osteocytes * | Fluid shear stress Hydrostatic pressure [8,9,10,11,12,13,14] | Increased Wnt/beta-catenin, calcium, COX-2 signaling, RANKL/OPG mRNA ratios. Cytoskeletal reorganization [8,9,10,11,12,13,14] | Increased Sost expression, sclerostin production. Decreased Wnt/beta-catenin signaling, bone formation [17,26] | Apoptosis Sclerostin release [18,19] |
| Osteoblasts | Bone loading via FAK Fluid shear stress [15,16]. | Increased bone formation via enhanced expression of COX-2, c-Fos, osteopontin E2 [15,16] | Decreased expression of mechanosensitive genes & markers of bone formation. Calcium channel inhibition [27,28,29,30,31,32,33,34] | Loss of focal adhesions, FAK. F-actin micro-filament & microtubular failure with cytoskeleton implosion [20,21,22,23,24] |
| Osteoclasts | As per osteocytes & osteoblasts | Resorption inhibited by osteoblast production of OPG and by reduced production of sclerostin by osteocytes [17,26] | Increased osteoclastogenesis and bone resorption secondary to increased RANKL/OPG ratios [36,37,38,39,40] | Increased expression of genes involved in osteoclast maturation and bone resorption [25] |
| Mesenchymal stem cells | Decreased osteoblast differentiation, maturation & ciliogenesis. Increased adipogenesis [27,32,33,34] | |||
| Hematopoietic stem cells | Enhanced maturation. Increased expression of syncytin-A. Increased response to RANKL-mediated osteoclasto-genesis [36,37,38,39,40] |
| Exercise | Nutritious High Calory Diet | Vitamins/Minerals | Bone Sparing Agents |
|---|---|---|---|
| 2.5 h/day x 6 days a week of combined aerobic & resistance exercises | Calories: 2700 to 3700/day * Frozen foods: Most entrees, vegetable and fruit items. Refrigerated foods: Fresh and fresh treated fruits, vegetables and dairy products. Ambient foods: Thermostabilized, aseptic-fill, shelf-stable natural form foods and rehydrated beverages | Recommended daily allowance (RDA) of vitamins & minerals. 1000–1200 mg calcium & 800 IU vitamin D/day (Smith et al.) | † Biphosphonates |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, J.K. Microgravity, Bone Homeostasis, and Insulin-Like Growth Factor-1. Appl. Sci. 2020, 10, 4433. https://doi.org/10.3390/app10134433
Smith JK. Microgravity, Bone Homeostasis, and Insulin-Like Growth Factor-1. Applied Sciences. 2020; 10(13):4433. https://doi.org/10.3390/app10134433
Chicago/Turabian StyleSmith, John Kelly. 2020. "Microgravity, Bone Homeostasis, and Insulin-Like Growth Factor-1" Applied Sciences 10, no. 13: 4433. https://doi.org/10.3390/app10134433
APA StyleSmith, J. K. (2020). Microgravity, Bone Homeostasis, and Insulin-Like Growth Factor-1. Applied Sciences, 10(13), 4433. https://doi.org/10.3390/app10134433




