Tryptophan and Membrane Mobility as Conditioners and Brokers of Gut–Brain Axis in Depression
Abstract
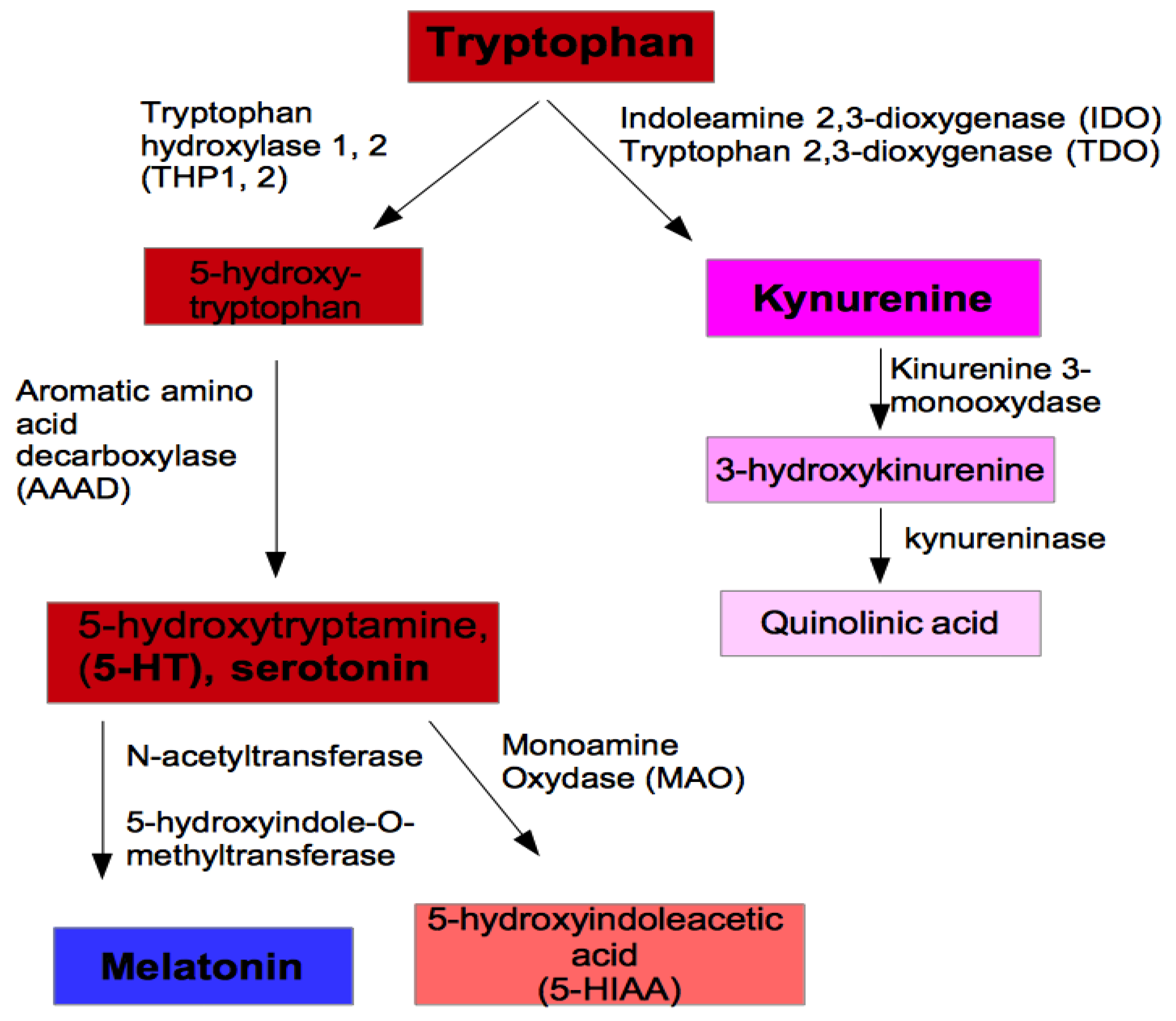
1. Introduction
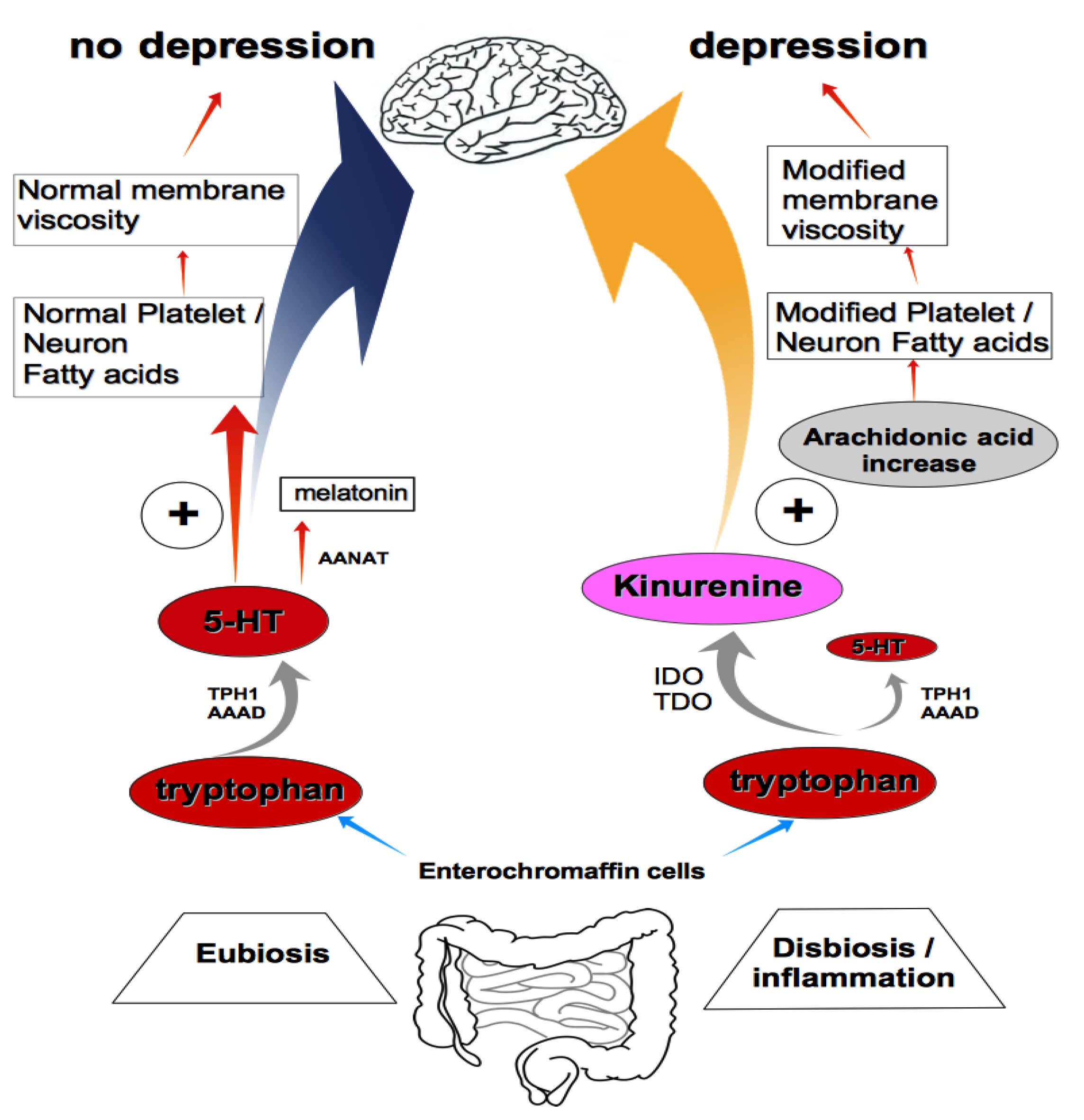
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| BD | bipolar disorder |
| CNS | central nervous system |
| DHA | docosahexaenoic acid |
| 5-HT | 5-hydroxytryptamine, serotonin |
| IBS | irritable bowel syndrome |
| IDO | indoleamine 2,3-dioxygenase |
| MD | major depression |
| REM | rapid eye movement |
| TDO | tryptophan 2,3-dioxygenase |
References
- Lapin, I.P.; Oxenkrug, G.F. Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet 1969, 1, 32–39. [Google Scholar] [CrossRef]
- Frazer, A.; Pandey, G.N.; Mendels, J. Metabolism of Tryptophan in Depressive Disease. Arch. Gen. Psychiatry 1973, 29, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Cowen, P.J.; Parry-Billings, M.; Newsholme, E.A. Decreased plasma tryptophan levels in major depression. J. Affect. Disord. 1989, 16, 27–31. [Google Scholar] [CrossRef]
- Gabbay, V.; Ely, B.A.; Babb, J.; Liebes, L. The possible role of the kynurenine pathway in anhedonia in adolescents. J. Neural. Transm. 2012, 119, 253–260. [Google Scholar] [CrossRef]
- Zhang, X.; Gainetdinov, R.R.; Beaulieu, J.M.; Sotnikova, T.D.; Burch, L.H.; Williams, R.B.; Schwartz, D.A.; Krishnan, R.R.; Caron, M.G. Loss-of-Function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron 2005, 45, 11–16. [Google Scholar] [CrossRef]
- Lemonde, S.; Turecki, G.; Bakish, D.; Du, L.; Hrdina, P.D.; Bown, C.D.; Sequeira, A.; Kushwaha, N.; Morris, S.J.; Basak, A.; et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J. Neurosci. 2003, 23, 8788–8799. [Google Scholar] [CrossRef]
- Hendriksen, E.; van Brgeijk, D.; Oosting, R.S.; Redegeld, F.A. Mast cells in neuroinflammation and brain disorders. Neurosci. Biobehav. Rev. 2017, 79, 119–133. [Google Scholar] [CrossRef]
- Traina, G. Mast cells in the brain- Old cells, new target. J. Integr. Neurosci. 2017, 16, S69–S83. [Google Scholar] [CrossRef]
- Traina, G. Mast cells in gut and brain and their potential role as an emerging therapeutic target for neural diseases. Front. Cell. Neurosci. 2019, 13, 345. [Google Scholar] [CrossRef]
- Takahashi, S. Reduction of Blood Platelet Serotonin Levels in Manic and Depressed Patients. Folia Psych. Neurol. Jap. 1976, 30, 476–486. [Google Scholar] [CrossRef]
- Stahl, S.M. The Human Platelet. A Diagnostic and Research Tool for the Study of Biogenic Amines in Psychiatric and Neurologic Disorders. Arch. Gen. Psychiatry 1977, 34, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Pletscher, A.; Laubscher, A. Blood Platelets as Models for Neurons: Uses and Limitations. J. Neural Transm. Suppl. 1980, 16, 7–16. [Google Scholar]
- Da Prada, A.M.; Cesura, J.M.; Launay, J.G.; Richards, J.G. Platelets as a Model for Neurones? Cell. Mol. Life Sci. 1988, 44, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Plaisant, O.; Leboyer, M.; Gay, C.; Kamal, L.; Devynck, M.A.; Meyer, P. Reduction of Platelet Serotonin in Major Depression (Endogenous Depression). Comptes Rendus Seances Acad. Sci. III 1982, 295, 619–622. [Google Scholar]
- Musselman, D.L.; Tomer, A.; Manatunga, A.K.; Knight, B.T.; Porter, M.R.; Kasey, S.; Marzec, U.; Harker, L.A.; Nemeroff, C.B. Exaggerated Platelet Reactivity in Major Depression. Am. J. Psychiatry 1996, 153, 1313–1317. [Google Scholar]
- Camacho, A.; Dimsdale, J.E. Platelets and Psychiatry: Lessons Learned from Old and New Studies. Psychos. Med. 2000, 62, 326–336. [Google Scholar] [CrossRef]
- Plein, H.; Berk, M. The Platelet as a Peripheral Marker in Psychiatric Illness. Hum. Psychopharm. 2001, 16, 229–236. [Google Scholar] [CrossRef]
- Cocchi, M.; Tonello, L.S.; Tsaluchidu, S.; Puri, B.K. The use of Artificial Neural Networks to Study Fatty Acids in Neuropsychiatric Disorders. BMC Psychiatry 2008, 8, S3. [Google Scholar] [CrossRef]
- Cocchi, M.; Tonello, L. Bio Molecular Considerations in Major Depression and Ischemic Cardiovascular Disease. Cent. Nerv. Syst. Agents Med. Chem. 2010, 10, 97–107. [Google Scholar] [CrossRef]
- Benedetti, S.; Bucciarelli, S.; Canestrari, F.; Catalani, S.; Mandolini, S.; Marconi, V.; Mastrogiacomo, A.; Silvestri, R.; Tagliamonte, M.; Venanzini, R.; et al. Platelet’s Fatty Acids and Differential Diagnosis of Major Depression and Bipolar Disorder through the use of an Unsupervised Competitive- Learning Network Algorithm (SOM). Open J. Depress. 2014, 3, 52–73. [Google Scholar] [CrossRef][Green Version]
- Mammadova-Bach, E.; Mauler, M.; Braun, A.; Duerschmied, D. Immuno-Thrombotic Effects of Platelet Serotonin; IntechOpen: London, UK, 2017. [Google Scholar]
- Walther, D.J.; Peter, J.U.; Winter, S.; Höltje, M.; Paulmann, N.; Grohmann, M.; Vowinckel, J.; Alamo-Bethencourt, V.; Wilhelm, C.S.; Ahnert-Hilger, G.; et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 2003, 115, 851–862. [Google Scholar] [CrossRef]
- Cloutier, N.; Allaeys, I.; Marcoux, G.; Machlus, K.R.; Mailhot, B.; Zufferey, A.; Levesque, T.; Becker, Y.; Tessandier, N.; Melki, I.; et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc. Nat. Acad. Sci. USA 2018, 115, E1550–E1559. [Google Scholar] [CrossRef]
- Zubenko, G.S.; Kopp, U.; Seto, T.; Firestone, L.L. Platelet Membrane Fluidity Individuals at Risk for Alzheimer’s Disease: A Comparison of Results From Fluorescence Spectroscopy and Electron Spin Resonance Spectroscopy. Psychopharmacology 1999, 145, 175–180. [Google Scholar] [CrossRef]
- Zubenko, G.S.; Cohen, B.M.; Reynolds, C.F.; Boller, F.; Malinakova, I.; Keefe, N. Platelet membrane fluidity in Alzheimer's disease and major depression. Am. J. Psychiatry 1987, 144, 860–868. [Google Scholar] [PubMed]
- van Rensburg, S.J.; Carstens, M.E.; Potocnik, F.C.V.; Aucamp, A.K.; Taljaard, J.J.F.; Koch, K.R. Membrane fluidity of platelets and erythrocytes in patients with Alzheimer's disease and the effect of small amounts of aluminium on platelet and erythrocyte membranes. Neurochem. Res. 1992, 17, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Leiter, O.; Walker, T.L. Platelets in Neurodegenerative Conditions—Friend or Foe? Front. Immunol. 2020, 11, 747. [Google Scholar] [CrossRef]
- Padmakumar, M.; Van Raes, E.; Van Geet, C.; Freson, K. Blood platelet research in autism spectrum disorders: In search of biomarkers. Res. Pract. Thromb. Haemost. 2019, 3, 566–577. [Google Scholar] [CrossRef]
- Tonello, L.; Cocchi, M. The Cell Membrane: Is it a bridge from psychiatry to quantum consciousness? NeuroQuantology 2010, 1, 54–60. [Google Scholar] [CrossRef][Green Version]
- Heron, D.S.; Shinitzky, M.; Hershkowitz, M.; Samuel, D. Lipid Fluidity Markedly Modulates the Binding of Serotonin to Mouse Brain Membranes. Proc. Natl. Acad. Sci. USA 1980, 77, 7463–7467. [Google Scholar] [CrossRef]
- Traina, G.; Cocchi, M. Mast Cells, Astrocytes, Arachidonic Acid: Do They Play a Role in Depression? Appl. Sci. 2020, 10, 3455. [Google Scholar] [CrossRef]
- Horrocks, L.A.; Farooqui, A.A. Docosahexaenoic acid in the diet: Its importance in maintenance and restoration of neural membrane function. Prostag. Leukotr. Essent. Fat. Acids 2004, 70, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Palmer, J.W.; Davis, J.M. Are disturbances in lipid-protein interactions by phospholipase-A2 a predisposing factor in affective illness? Biol. Psychiatry 1989, 25, 945–961. [Google Scholar] [CrossRef]
- Walsh, M.T.; Dinan, T.G.; Condren, R.M.; Ryan, M.; Kenny, D. Depression is associated with an increase in the expression of the platelet adhesion receptor glycoprotein Ib. Life Sci. 2002, 70, 3155–3165. [Google Scholar] [CrossRef]
- Piletz, J.E.; Zhu, H.; Madakasira, S.; Pazzaglia, P.; DeVane, C.L.; Goldman, N.; Halaris, A. Elevated P-selectin on platelets in depression: Response to bupropion. J. Psychiatr. Res. 2000, 34, 397–404. [Google Scholar] [CrossRef]
- Müller, C.P.; Reichel, M.; Muhle, C.; Rhein, C. Brain membrane lipids in major depression and anxiety disorders. Bioch. Biophys. Acta 2015, 1851, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Watala, C.; Golański, J.; Boncler, M.A.; Pietrucha, T.; Gwoździński, K. Membrane Lipid Fluidity of Blood Platelets: A Common Denominator That Underlies the Opposing Actions of Various Agents that Affect Platelet Activation in Whole Blood. Platelets 1998, 9, 315–327. [Google Scholar]
- Kalipatnapu, S.; Pucadyil, T.J.; Chattopadhyay, A. Membrane Organization and Dynamics of the Serotonin1A Receptor Monitored Using Fluorescence Microscopic Approaches. In Serotonin Receptors in Neurobiology; Chattopadhyay, A., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007; Chapter 3. [Google Scholar]
- Cocchi, M.; Tonello, L.; Amato, P.; De Lucia, A. Platelet and Brain Fatty acid transfer: Hypothesis on Arachidonic Acid and its relationship to Major Depression. J. Biol. Res. 2009, 82, 47–53. [Google Scholar] [CrossRef]
- Green, P.; Gispan-Herman, I.; Yadid, G. Increased Arachidonic Acid Concentration in the Brain of Flinders Sensitive Line Rats, an Animal Model of Epression. J. Lipid. Res. 2005, 46, 1093–1096. [Google Scholar] [CrossRef]
- Cocchi, M.; Tonello, L.; Lercker, G. Fatty acids, membrane viscosity, serotonin and ischemic heart disease. Lipids Health Dis. 2010, 9, 97. [Google Scholar] [CrossRef]
- Cocchi, M.; Tonello, L.; Gabrielli, F.; Pregnolato, M. Depression, osteoporosis, serotonin and cell membrane viscosity between biology and philosophical anthropology. Ann. Gen. Psychiatry 2011, 10, 9. [Google Scholar] [CrossRef]
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Melancholic microbes: A link between gut microbioma and depression? Neurogastroenterol. Motil. 2013, 25, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. The impact of gut microbiota on brain and behaviour: Implications for psychiatry. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 552–558. [Google Scholar] [CrossRef]
- Conte, C.; Sichetti, M.; Traina, G. Gut–Brain Axis: Focus on Neurodegeneration and Mast Cells. Appl. Sci. 2020, 10, 1828. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan's Metabolites in Exercise, Inflammation, and Mental Health. Science 2017, 357, 9794. [Google Scholar] [CrossRef]
- Banskota, S.; Ghia, J.E.; Khan, W.I. Serotonin in the gut: Blessing or a curse. Biochimie 2019, 161, 56–64. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Kaur, H.; Bose, C.; Mande, S.S. Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in silico Analysis. Front. Neurosci. 2019, 13, 1365. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine Pathway Metabolism and the Microbiota-Gut-Brain Axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Berger, M.; Riemann, D.; Höchli, D.; Spiegel, R. The rapid-movement cholinergic sleep induction test of the eye with RS-86. Indicator of state or trait of depression? Arch. Gen. Psychiatry 1989, 46, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, Y.; Fan, F.; Zhang, B. The Role of Microbiome in Insomnia, Circadian Disturbance and Depression. Front. Psychiatry 2018, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef] [PubMed]
- Lukic, I.; Getselter, D.; Koren, O.; Elliott, E. Role of Tryptophan in Microbiota-Induced Depressive-Like Behavior: Evidence from Tryptophan Depletion Study. Front. Behav. Neurosci. 2019, 13, 123. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocchi, M.; Traina, G. Tryptophan and Membrane Mobility as Conditioners and Brokers of Gut–Brain Axis in Depression. Appl. Sci. 2020, 10, 4933. https://doi.org/10.3390/app10144933
Cocchi M, Traina G. Tryptophan and Membrane Mobility as Conditioners and Brokers of Gut–Brain Axis in Depression. Applied Sciences. 2020; 10(14):4933. https://doi.org/10.3390/app10144933
Chicago/Turabian StyleCocchi, Massimo, and Giovanna Traina. 2020. "Tryptophan and Membrane Mobility as Conditioners and Brokers of Gut–Brain Axis in Depression" Applied Sciences 10, no. 14: 4933. https://doi.org/10.3390/app10144933
APA StyleCocchi, M., & Traina, G. (2020). Tryptophan and Membrane Mobility as Conditioners and Brokers of Gut–Brain Axis in Depression. Applied Sciences, 10(14), 4933. https://doi.org/10.3390/app10144933





