Improvement of Bacterial Vaginosis by Oral Lactobacillus Supplement: A Randomized, Double-Blinded Trial
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Human Cervical Epithelial Cell Line
2.3. Antibacterial Activity of Lactobacilli
2.4. Adherence of Bacteria to HeLa Cells
2.5. Clinical Study Protocol and Sample Collection
2.6. RNA Extraction and Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis of Inflammatory Cytokines
2.7. DNA Extraction and Vaginal Microbiota Analysis
2.8. Statistical Analysis
3. Results
3.1. Inhibition of Vaginal Pathogenic Bacteria by Different GMNL Lactobacillus Strains
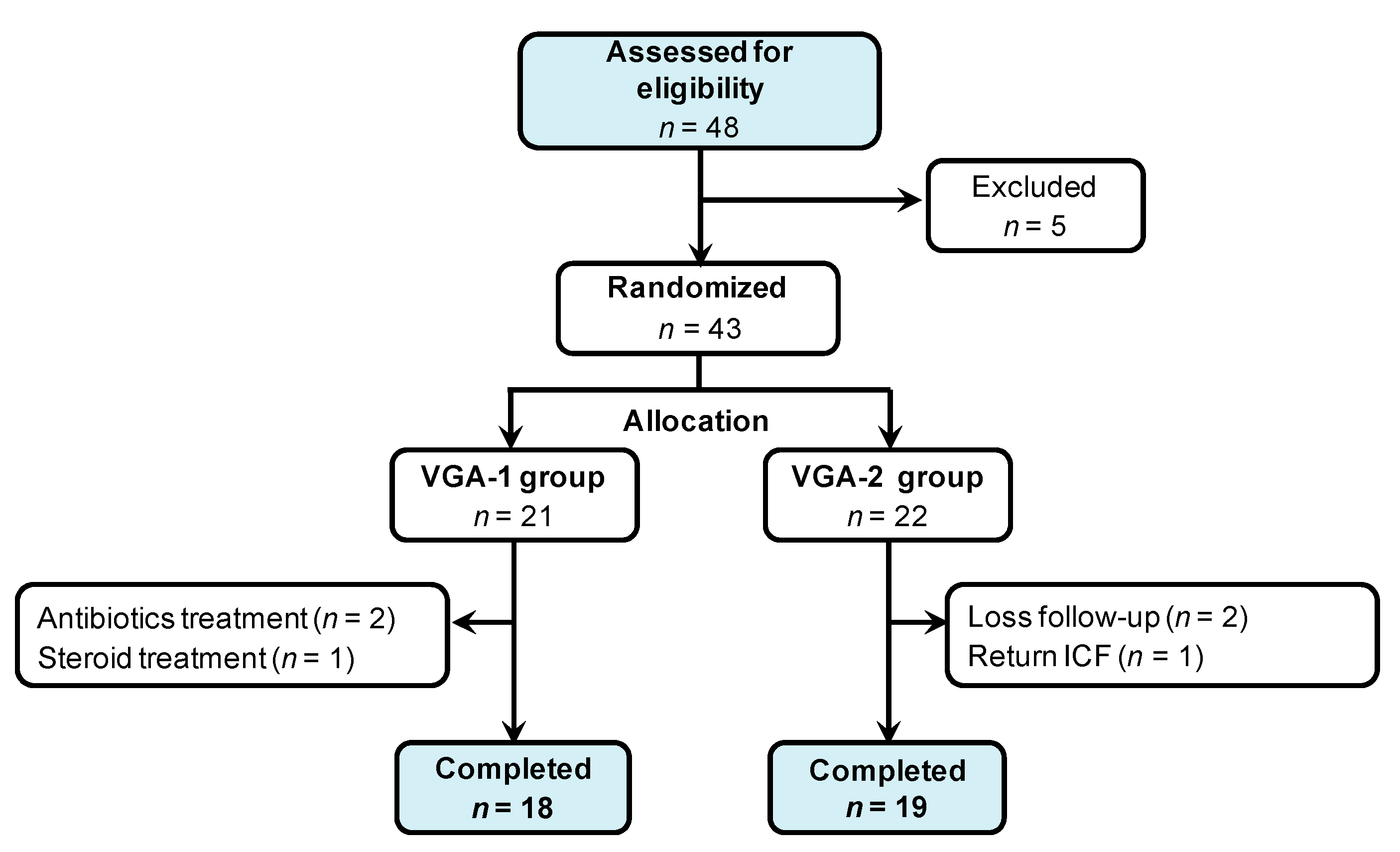
3.2. Randomized, Double-Blinded Trial of Two Formula of GMNL Lactobacillus Involving BV Patients
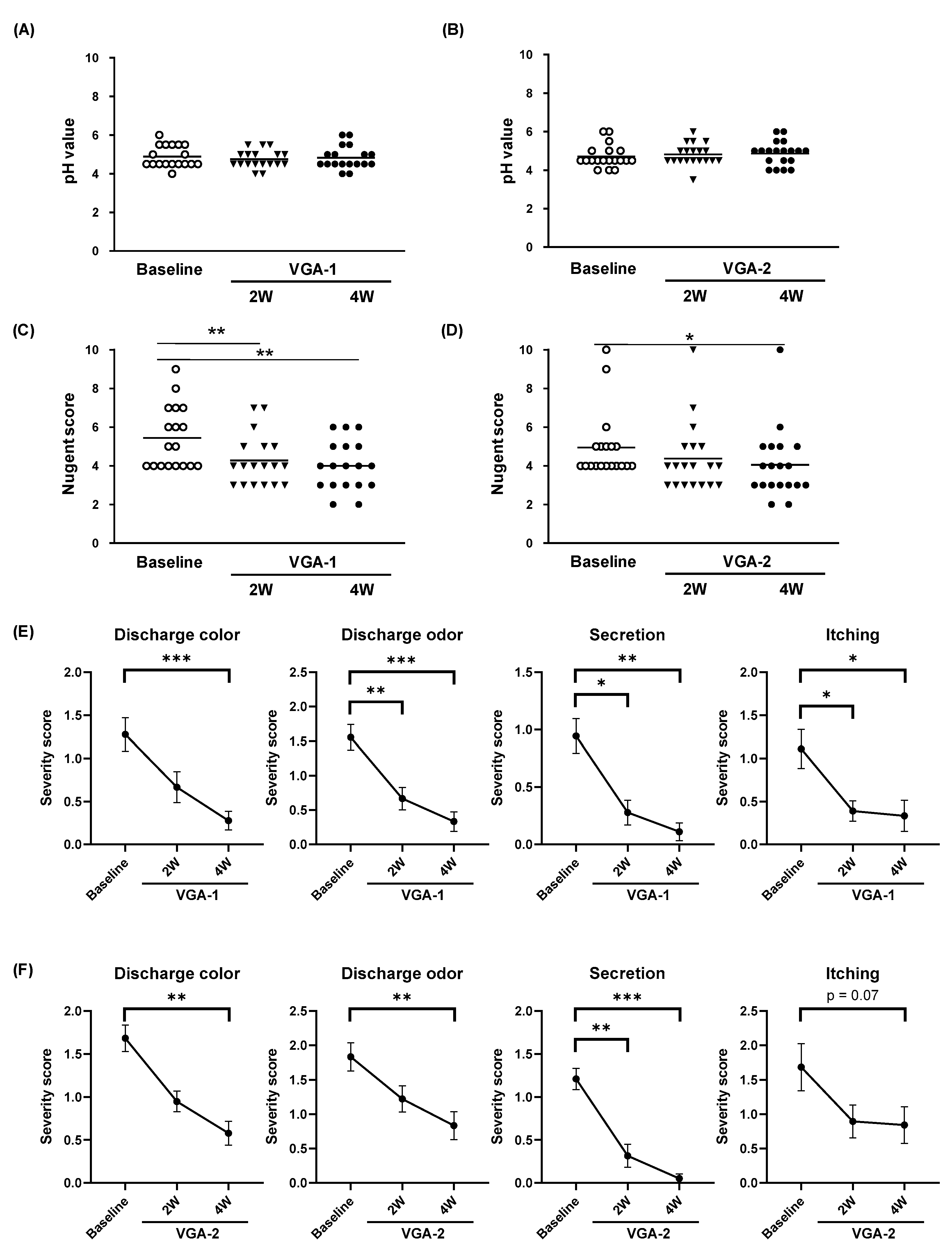
3.3. Effects of VGA-1 or VGA-2 Consumption on the Symptoms of BV Participants
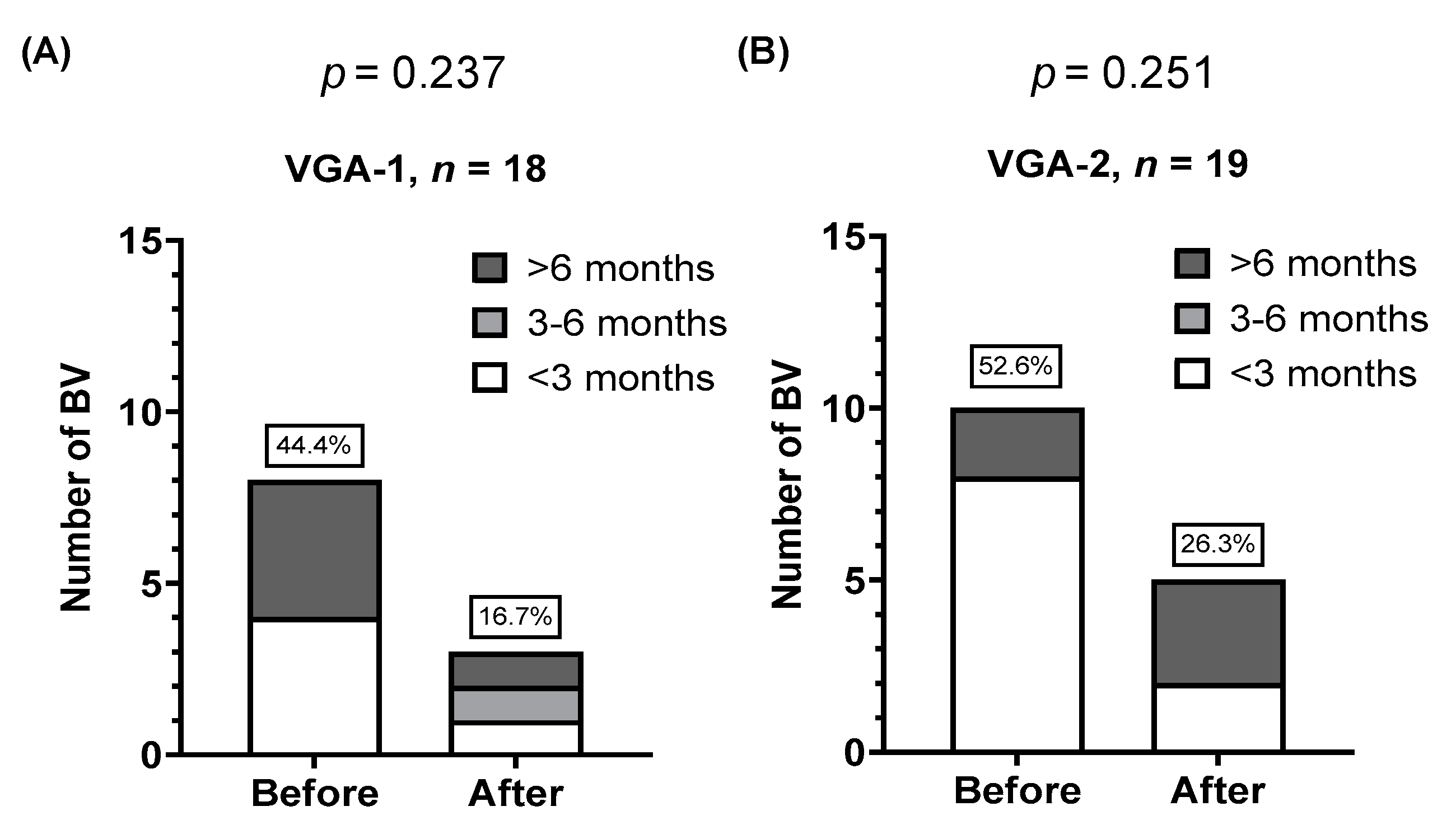
3.4. Colonization of GMNL Lactobacillus Strains among Participants of VGA-1 or VGA-2 Group
3.5. Consumption of VGA-1 or VGA-2 Reduces interleukin (IL)-6 Expression in the Vaginal Specimens of BV Patients
3.6. Changes in Vaginal Lactobacillus Species Composition in BV Patients after VGA-1 or VGA-2 Consumption
4. Discussion
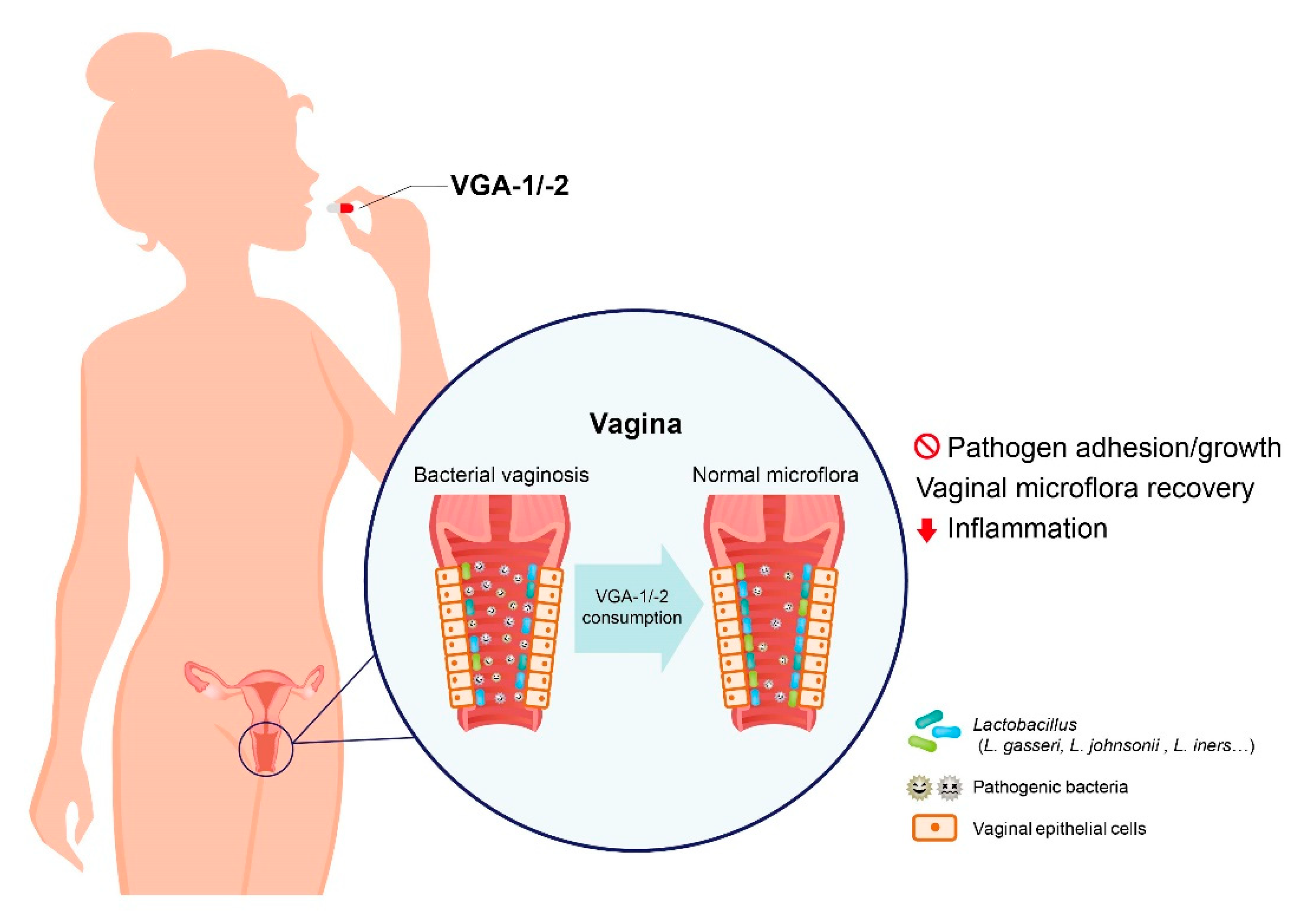
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Allsworth, J.E.; Peipert, J.F. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Obstet. Gynecol. 2007, 109, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Amsel, R.; Totten, P.A.; Spiegel, C.A.; Chen, K.C.; Eschenbach, D.; Holmes, K.K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 1983, 74, 14–22. [Google Scholar] [CrossRef]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdool Karim, S.S.; Baxter, C.; Passmore, J.S.; McKinnon, L.R.; Williams, B.L. The genital tract and rectal microbiomes: Their role in HIV susceptibility and prevention in women. J. Int. AIDS Soc. 2019, 22, e25300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillet, E.; Meys, J.F.; Verstraelen, H.; Bosire, C.; De Sutter, P.; Temmerman, M.; Broeck, D.V. Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: A meta-analysis. BMC Infect. Dis. 2011, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Peelen, M.J.; Luef, B.M.; Lamont, R.F.; de Milliano, I.; Jensen, J.S.; Limpens, J.; Hajenius, P.J.; Jorgensen, J.S.; Menon, R.; Group, P.B.W. The influence of the vaginal microbiota on preterm birth: A systematic review and recommendations for a minimum dataset for future research. Placenta 2019, 79, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Hillier, S.L.; Nugent, R.P.; Eschenbach, D.A.; Krohn, M.A.; Gibbs, R.S.; Martin, D.H.; Cotch, M.F.; Edelman, R.; Pastorek, J.G., 2nd; Rao, A.V.; et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N. Engl. J. Med. 1995, 333, 1737–1742. [Google Scholar] [CrossRef]
- Lamont, R.F.; Sobel, J.D.; Akins, R.A.; Hassan, S.S.; Chaiworapongsa, T.; Kusanovic, J.P.; Romero, R. The vaginal microbiome: New information about genital tract flora using molecular based techniques. BJOG 2011, 118, 533–549. [Google Scholar] [CrossRef] [Green Version]
- Donati, L.; Di Vico, A.; Nucci, M.; Quagliozzi, L.; Spagnuolo, T.; Labianca, A.; Bracaglia, M.; Ianniello, F.; Caruso, A.; Paradisi, G. Vaginal microbial flora and outcome of pregnancy. Arch. Gynecol. Obstet. 2010, 281, 589–600. [Google Scholar] [CrossRef]
- Aroutcheva, A.; Gariti, D.; Simon, M.; Shott, S.; Faro, J.; Simoes, J.A.; Gurguis, A.; Faro, S. Defense factors of vaginal lactobacilli. Am. J. Obstet. Gynecol. 2001, 185, 375–379. [Google Scholar] [CrossRef]
- Livengood, C.H. Bacterial vaginosis: An overview for 2009. Rev. Obstet. Gynecol. 2009, 2, 28–37. [Google Scholar] [PubMed]
- Jahic, M.; Mulavdic, M.; Nurkic, J.; Jahic, E.; Nurkic, M. Clinical characteristics of aerobic vaginitis and its association to vaginal candidiasis, trichomonas vaginitis and bacterial vaginosis. Med. Arch. 2013, 67, 428–430. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, C.S.; Morton, A.N.; Hocking, J.; Garland, S.M.; Morris, M.B.; Moss, L.M.; Horvath, L.B.; Kuzevska, I.; Fairley, C.K. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 2006, 193, 1478–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, S.; Silva, J.; Teixeira, P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2014, 289, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Charbonneau, D.; Erb, J.; Kochanowski, B.; Beuerman, D.; Poehner, R.; Bruce, A.W. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: Randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol. Med. Microbiol. 2003, 35, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Martinez, R.C.; Franceschini, S.A.; Patta, M.C.; Quintana, S.M.; Gomes, B.C.; De Martinis, E.C.; Reid, G. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: A randomized, double-blind, placebo-controlled trial. Can. J. Microbiol. 2009, 55, 133–138. [Google Scholar] [CrossRef]
- Wang, Z.; He, Y.; Zheng, Y. Probiotics for the Treatment of Bacterial Vaginosis: A Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 3859. [Google Scholar] [CrossRef] [Green Version]
- Tagg, J.R.; McGiven, A.R. Assay system for bacteriocins. Appl. Microbiol. 1971, 21, 943. [Google Scholar] [CrossRef]
- Anderson, M.R.; Klink, K.; Cohrssen, A. Evaluation of vaginal complaints. JAMA 2004, 291, 1368–1379. [Google Scholar] [CrossRef]
- Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Caspani, M.; Mondello, F.; Cresci, A. In vitro evaluation on HeLa cells of protective mechanisms of probiotic lactobacilli against Candida clinical isolates. J. Appl. Microbiol. 2015, 119, 1383–1390. [Google Scholar] [CrossRef] [Green Version]
- Reid, G.; Bruce, A.W.; Fraser, N.; Heinemann, C.; Owen, J.; Henning, B. Oral probiotics can resolve urogenital infections. FEMS Immunol. Med. Microbiol. 2001, 30, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, R.; Ramalaxmi, B.A.; Swetha, E.; Balakrishna, N.; Mastromarino, P. Evaluation of vaginal pH for detection of bacterial vaginosis. Indian J. Med. Res. 2013, 138, 354–359. [Google Scholar] [PubMed]
- Krauss-Silva, L.; Almada-Horta, A.; Alves, M.B.; Camacho, K.G.; Moreira, M.E.; Braga, A. Basic vaginal pH, bacterial vaginosis and aerobic vaginitis: Prevalence in early pregnancy and risk of spontaneous preterm delivery, a prospective study in a low socioeconomic and multiethnic South American population. BMC Pregnancy Childbirth 2014, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Ya, W.; Reifer, C.; Miller, L.E. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: A double-blind, randomized, placebo-controlled study. Am. J. Obstet. Gynecol. 2010, 203, 120.e1–120.e6. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O.C. Female Gut and Genital Tract Microbiota-Induced Crosstalk and Differential Effects of Short-Chain Fatty Acids on Immune Sequelae. Front. Immunol. 2020, 11, 2184. [Google Scholar] [CrossRef]
- El Aila, N.A.; Tency, I.; Saerens, B.; De Backer, E.; Cools, P.; dos Santos Santiago, G.L.; Verstraelen, H.; Verhelst, R.; Temmerman, M.; Vaneechoutte, M. Strong correspondence in bacterial loads between the vagina and rectum of pregnant women. Res. Microbiol. 2011, 162, 506–513. [Google Scholar] [CrossRef]
- Dillon, H.C., Jr.; Gray, E.; Pass, M.A.; Gray, B.M. Anorectal and vaginal carriage of group B streptococci during pregnancy. J. Infect. Dis. 1982, 145, 794–799. [Google Scholar] [CrossRef]
- Cauci, S.; Driussi, S.; De Santo, D.; Penacchioni, P.; Iannicelli, T.; Lanzafame, P.; De Seta, F.; Quadrifoglio, F.; de Aloysio, D.; Guaschino, S. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J. Clin. Microbiol. 2002, 40, 2147–2152. [Google Scholar] [CrossRef] [Green Version]
- Morison, L.; Ekpo, G.; West, B.; Demba, E.; Mayaud, P.; Coleman, R.; Bailey, R.; Walraven, G. Bacterial vaginosis in relation to menstrual cycle, menstrual protection method, and sexual intercourse in rural Gambian women. Sex Transm. Infect. 2005, 81, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Yefet, E.; Colodner, R.; Strauss, M.; Gam Ze Letova, Y.; Nachum, Z. A Randomized Controlled Open Label Crossover Trial to Study Vaginal Colonization of Orally Administered Lactobacillus Reuteri RC-14 and Rhamnosus GR-1 in Pregnant Women at High Risk for Preterm Labor. Nutrients 2020, 12, 1141. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.E.; Kim, J.K.; Han, S.K.; Jang, S.E.; Han, M.J.; Kim, D.H. Lactobacillus plantarum NK3 and Bifidobacterium longum NK49 Alleviate Bacterial Vaginosis and Osteoporosis in Mice by Suppressing NF-kappaB-Linked TNF-alpha Expression. J. Med. Food 2019, 22, 1022–1031. [Google Scholar] [CrossRef]
- Beekhuizen, H.; van de Gevel, J.S. Gamma interferon confers resistance to infection with Staphylococcus aureus in human vascular endothelial cells by cooperative proinflammatory and enhanced intrinsic antibacterial activities. Infect. Immun. 2007, 75, 5615–5626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, M.; Gomez, J.C.; Chugh, P.E.; Lowell, C.A.; Dinauer, M.C.; Dittmer, D.P.; Doerschuk, C.M. Interferon-gamma production by neutrophils during bacterial pneumonia in mice. Am. J. Respir. Crit. Care Med. 2011, 183, 1391–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, S.R.; Liu, X.P.; Liao, Q.P. Human defensins and cytokines in vaginal lavage fluid of women with bacterial vaginosis. Int. J. Gynaecol. Obstet. 2008, 103, 50–54. [Google Scholar] [CrossRef]
- Faure, E.; Faure, K.; Figeac, M.; Kipnis, E.; Grandjean, T.; Dubucquoi, S.; Villenet, C.; Grandbastien, B.; Brabant, G.; Subtil, D.; et al. Vaginal Mucosal Homeostatic Response May Determine Pregnancy Outcome in Women With Bacterial Vaginosis: A Pilot Study. Medicine (Baltimore) 2016, 95, e2668. [Google Scholar] [CrossRef] [PubMed]
- Goepfert, A.R.; Varner, M.; Ward, K.; Macpherson, C.; Klebanoff, M.; Goldenberg, R.L.; Mercer, B.; Meis, P.; Iams, J.; Moawad, A.; et al. Differences in inflammatory cytokine and Toll-like receptor genes and bacterial vaginosis in pregnancy. Am. J. Obstet. Gynecol. 2005, 193, 1478–1485. [Google Scholar] [CrossRef]
- Manhanzva, M.T.; Abrahams, A.G.; Gamieldien, H.; Froissart, R.; Jaspan, H.; Jaumdally, S.Z.; Barnabas, S.L.; Dabee, S.; Bekker, L.G.; Gray, G.; et al. Inflammatory and antimicrobial properties differ between vaginal Lactobacillus isolates from South African women with non-optimal versus optimal microbiota. Sci. Rep. 2020, 10, 6196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlier, C.; Cretenet, M.; Even, S.; Le Loir, Y. Interactions between Staphylococcus aureus and lactic acid bacteria: An old story with new perspectives. Int. J. Food Microbiol. 2009, 131, 30–39. [Google Scholar] [CrossRef]
- Fredricks, D.N.; Fiedler, T.L.; Thomas, K.K.; Mitchell, C.M.; Marrazzo, J.M. Changes in vaginal bacterial concentrations with intravaginal metronidazole therapy for bacterial vaginosis as assessed by quantitative PCR. J. Clin. Microbiol. 2009, 47, 721–726. [Google Scholar] [CrossRef] [Green Version]
- Hutt, P.; Lapp, E.; Stsepetova, J.; Smidt, I.; Taelma, H.; Borovkova, N.; Oopkaup, H.; Ahelik, A.; Roop, T.; Hoidmets, D.; et al. Characterisation of probiotic properties in human vaginal lactobacilli strains. Microb. Ecol. Health Dis. 2016, 27, 30484. [Google Scholar] [CrossRef]
- Pavlova, S.I.; Kilic, A.O.; Kilic, S.S.; So, J.S.; Nader-Macias, M.E.; Simoes, J.A.; Tao, L. Genetic diversity of vaginal lactobacilli from women in different countries based on 16S rRNA gene sequences. J. Appl. Microbiol. 2002, 92, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Bent, S.J.; Schneider, M.G.; Davis, C.C.; Islam, M.R.; Forney, L.J. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology (Reading) 2004, 150, 2565–2573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Wijgert, J.; Verwijs, M.C. Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: A systematic review and recommendations for future trial designs. BJOG 2020, 127, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Basavaprabhu, H.N.; Sonu, K.S.; Prabha, R. Mechanistic insights into the action of probiotics against bacterial vaginosis and its mediated preterm birth: An overview. Microb. Pathog. 2020, 141, 104029. [Google Scholar] [CrossRef] [PubMed]
- Vicariotto, F.; Mogna, L.; Del Piano, M. Effectiveness of the two microorganisms Lactobacillus fermentum LF15 and Lactobacillus plantarum LP01, formulated in slow-release vaginal tablets, in women affected by bacterial vaginosis: A pilot study. J. Clin. Gastroenterol. 2014, 48 (Suppl. 1), 106–112. [Google Scholar] [CrossRef]
- Palma, E.; Recine, N.; Domenici, L.; Giorgini, M.; Pierangeli, A.; Panici, P.B. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: A promising solution against HPV-infection. BMC Infect. Dis. 2018, 18, 13. [Google Scholar] [CrossRef] [Green Version]
- Pino, A.; Giunta, G.; Randazzo, C.L.; Caruso, S.; Caggia, C.; Cianci, A. Bacterial biota of women with bacterial vaginosis treated with lactoferrin: An open prospective randomized trial. Microb. Ecol. Health Dis. 2017, 28, 1357417. [Google Scholar] [CrossRef] [Green Version]




| Lactobacillus Strain | Antimicrobial Activity of Probiotics (A.U./mL) a | ||||
|---|---|---|---|---|---|
| E. coli (BCRC 11634) | S. aureus (BCRC 10451) | G. vaginalis (BCRC 17040) | S. agalactiae (BCRC 10787) | S. agalactiae (BCRC 10902) | |
| L. rhamnosus GMNL-74 | 19.85 ± 0.49 | 6.90 ± 2.69 d | 16.99 ± 1.15 | 17.57 ± 1.03 c | 10.39 ± 1.73 c |
| L. acidophilus GMNL-185 | 19.65 ± 4.31 | 10.05 ± 1.06 | 18.27 ± 3.02 c | 18.13 ± 0.71 | 22.25 ± 0.76 |
| L. rhamnosus GMNL-680 | 18.33 ± 0.50 | 58.77 ± 0.09 | 13.87 ± 0.11 b | 25.87 ± 0.33 a | 30.95 ± 0.02 a |
| L. plantarum GMNL-682 | 18.98 ± 0.18 | 59.85 ± 0.40 a | 14.90 ± 0.10 | 23.50 ± 0.18 | 27.52 ± 0.06 |
| Characteristics | VGA-1, n = 18 | VGA-2, n = 19 | p Value |
|---|---|---|---|
| Age (y), median (range) | 35.5 (22–51) | 38 (28–53) | 0.34 |
| <30, n (%) | 3 (17) | 1 (5) | |
| ≥30, n (%) | 15 (83) | 18 (95) | |
| pH of secretion, mean ± SD | 4.89 ± 0.557 | 4.71 ± 0.585 | |
| <4.5, n (%) | 1 (6) | 3 (16) | 0.60 |
| ≥4.5, n (%) | 17 (94) | 16 (84) | |
| Nugent score, mean ± SD | 5.44 ± 1.617 | 4.95 ± 1.715 | |
| 4–6, n (%) | 13 (72) | 17 (89) | 0.23 |
| ≥7, n (%) | 5 (28) | 2 (11) | |
| History of BV before enrollment | |||
| Yes, n (%) | 8 (44) | 10 (53) | 0.87 |
| No, n (%) | 10 (56) | 9 (47) | |
| History of vaginal symptoms last episode if history | |||
| Urinary tract infection, n (%) | 2 (11) | 1 (5) | 0.85 |
| Vaginitis, n (%) | 3 (17) | 5 (26) | |
| Vulvitis, n (%) | 2 (11) | 2 (11) | |
| No history, n (%) | 11 (61) | 11 (58) | |
| History of treatment last episode if history | |||
| Antibiotics, n (%) | 1 (5) | 1 (5) | 0.97 |
| Antifungal, n (%) | 5 (28) | 6 (32) | |
| No treatment, n (%) | 12 (67) | 12 (63) | |
| VGA-1, n = 18 | VGA-2, n = 19 | ||
|---|---|---|---|
| Patient | BV recurrence after-intervention | Patient | BV recurrence after-intervention |
| BV history, n = 8 | 1 (12.5) | BV history, n = 10 | 2 (20) |
| VGA-1, n = 18 | VGA-2, n = 19 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2W | 4W | 2W | 4W | |||||||
| 2−ΔΔCt | Mean ± SE | p-value (*) | Mean ± SE | p-value (*) | p-value (#) | Mean ± SE | p-value (*) | Mean ± SE | p-value (*) | p-value (#) |
| Lactobacilli | 24.7 ± 15.1 | 0.1845 | 12.6 ± 5.1 | 0.5094 | 0.9771 | 1.2 ± 0.3 | 0.7435 | 1.0 ± 0.1 | 0.7504 | 0.4084 |
| L. plantarum | 13.5 ± 10.6 | 0.9851 | 2.8 ± 0.8 | 0.1845 | 0.5087 | 3.3 ± 1.2 | 0.2886 | 5.5 ± 1.9 | 0.5053 | 0.7774 |
| L. rhamnosus | 2.2 ± 1.1 | 0.0014 ** | 10.2 ± 8.6 | 0.5128 | 0.8457 | 1.3 ± 0.2 | 0.1771 | 2.5 ± 0.9 | 0.5057 | 0.8308 |
| L. acidophilus | 205.0 ± 191.2 | 0.5079 | 29.1 ± 26.2 | 0.5128 | 0.8892 | 1.1 ± 0.2 | 0.1763 | 1.5 ± 0.3 | 0.1768 | 0.5452 |
| L. crispatus | 106.4 ± 97.9 | 0.1845 | 16.5 ± 13.9 | 0.1845 | 0.9778 | 2.2 ± 1.1 | 0.0397 * | 1.2 ± 0.2 | 0.5002 | 0.5959 |
| L. gasseri | 250.1 ± 222.3 | >0.9999 | 72.1 ± 39.5 | 0.0426 * | 0.2350 | 1.1 ± 0.2 | 0.7028 | 1.2 ± 0.2 | 0.4629 | 0.5121 |
| L. jensenii | 4.5 ± 2.7 | 0.3178 | 1.2 ± 0.3 | 0.0426 * | 0.7804 | 1.1 ± 0.2 | 0.1931 | 1.2 ± 0.2 | 0.1768 | 0.9646 |
| L. iners | 0.8 ± 0.1 | 0.0153 * | 43.8 ± 40.5 | 0.0041 ** | 0.8599 | 1.2 ± 0.3 | 0.5000 | 1.8 ± 0.4 | 0.0388 ** | 0.2579 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-C.; Hsu, I.-L.; Tsai, W.-H.; Chu, Y.-C.; Kuan, L.-C.; Huang, M.-S.; Yeh, W.-L.; Chen, Y.-H.; Hsu, S.-J.; Chang, W.-W. Improvement of Bacterial Vaginosis by Oral Lactobacillus Supplement: A Randomized, Double-Blinded Trial. Appl. Sci. 2021, 11, 902. https://doi.org/10.3390/app11030902
Lin T-C, Hsu I-L, Tsai W-H, Chu Y-C, Kuan L-C, Huang M-S, Yeh W-L, Chen Y-H, Hsu S-J, Chang W-W. Improvement of Bacterial Vaginosis by Oral Lactobacillus Supplement: A Randomized, Double-Blinded Trial. Applied Sciences. 2021; 11(3):902. https://doi.org/10.3390/app11030902
Chicago/Turabian StyleLin, Ta-Chin, I-Ling Hsu, Wan-Hua Tsai, Yi-Chih Chu, Lung-Ching Kuan, Min-Syuan Huang, Wen-Ling Yeh, Ya-Hui Chen, Shan-Ju Hsu, and Wen-Wei Chang. 2021. "Improvement of Bacterial Vaginosis by Oral Lactobacillus Supplement: A Randomized, Double-Blinded Trial" Applied Sciences 11, no. 3: 902. https://doi.org/10.3390/app11030902
APA StyleLin, T.-C., Hsu, I.-L., Tsai, W.-H., Chu, Y.-C., Kuan, L.-C., Huang, M.-S., Yeh, W.-L., Chen, Y.-H., Hsu, S.-J., & Chang, W.-W. (2021). Improvement of Bacterial Vaginosis by Oral Lactobacillus Supplement: A Randomized, Double-Blinded Trial. Applied Sciences, 11(3), 902. https://doi.org/10.3390/app11030902







