Insights into Anaerobic Co-Digestion of Lignocellulosic Biomass (Sugar Beet By-Products) and Animal Manure in Long-Term Semi-Continuous Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock and Inoculum Characteristics
2.2. Semi-Continuous Digesters
2.3. Analytical Methods
2.4. Biomethanation Degree Calculations
2.5. The Indirect Carbon-Related Parameter Calculations: Acidogenic Substrate as Carbon
3. Results and Discussion
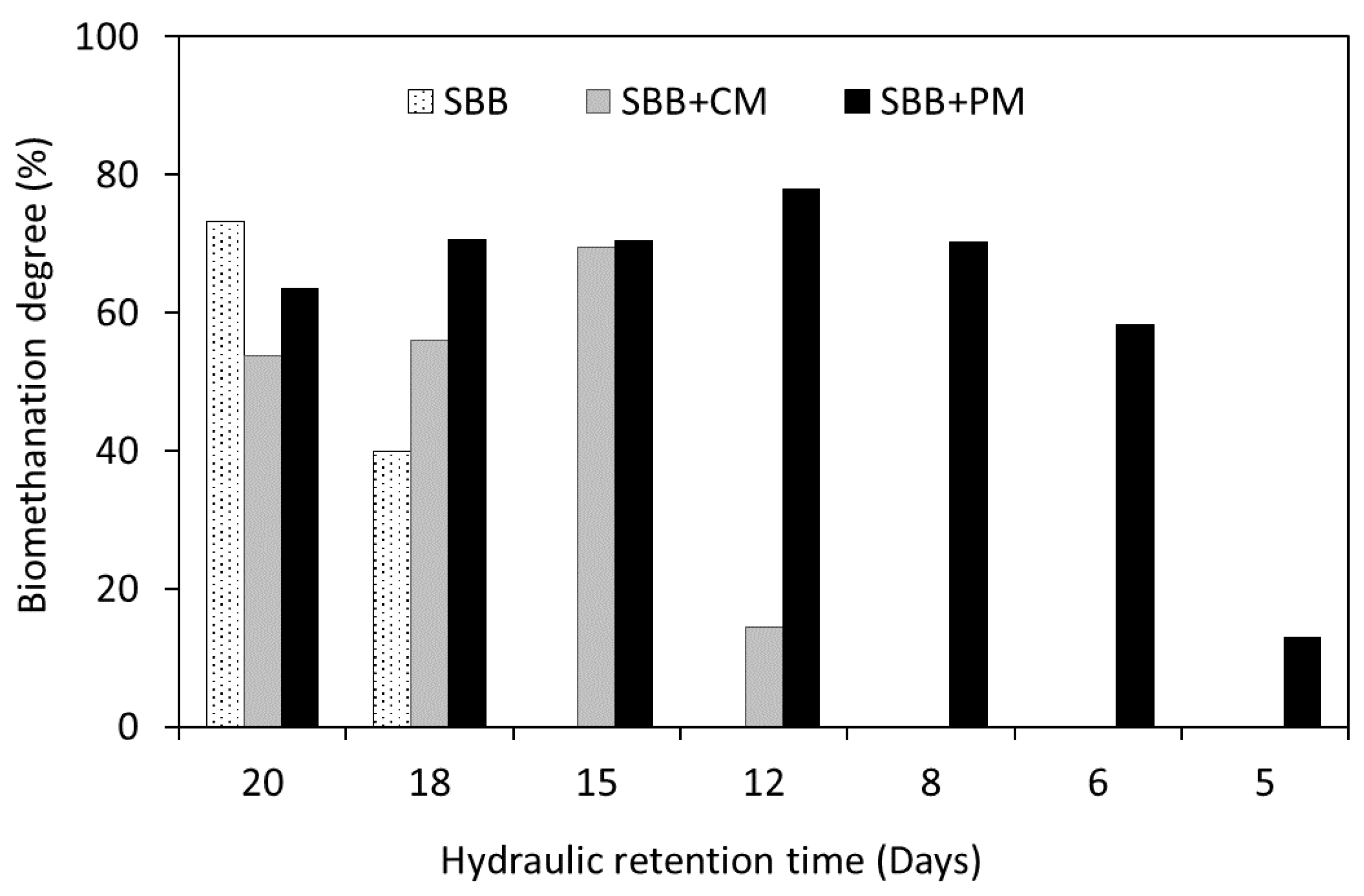
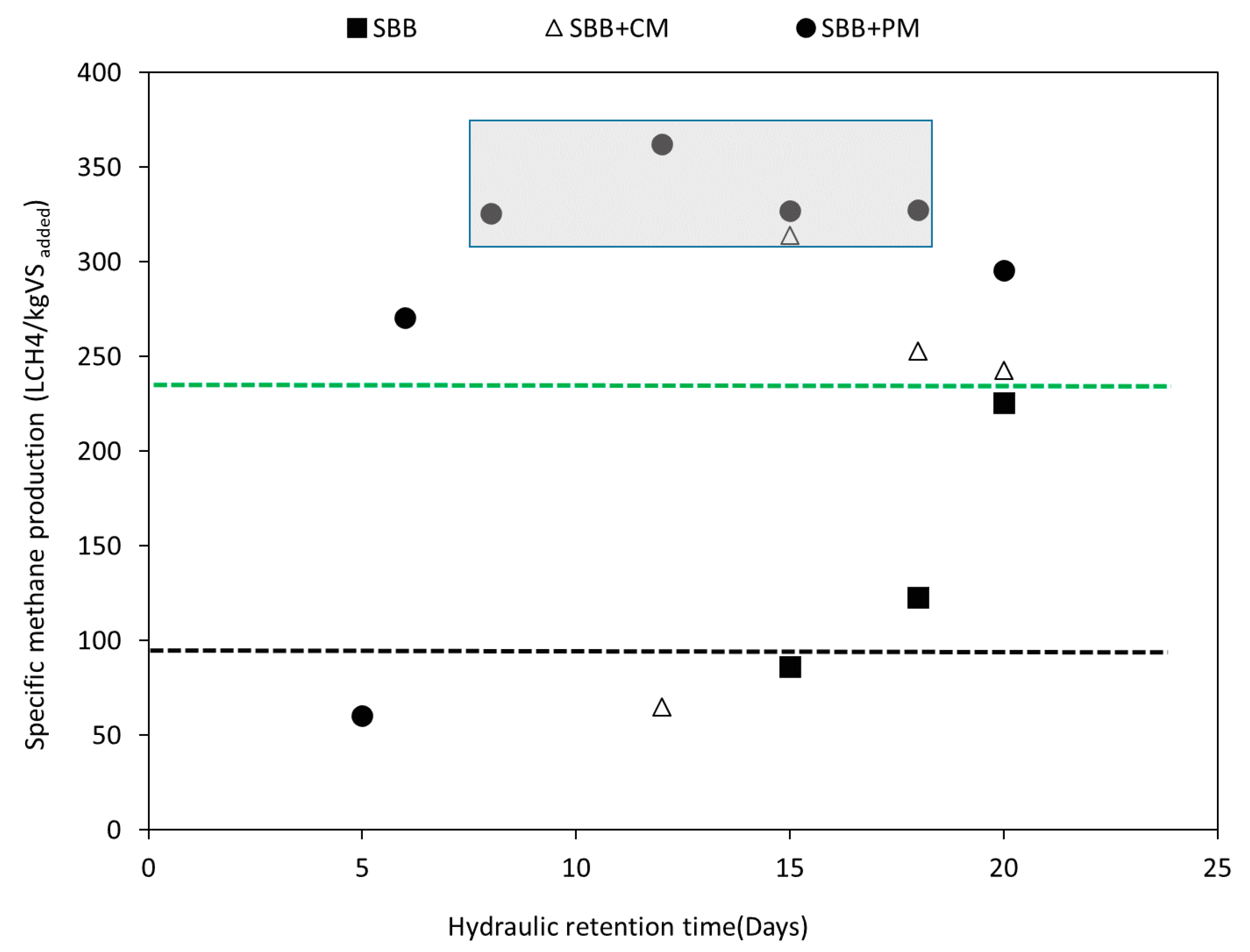
3.1. Methane Production Yields and Process Efficiency
3.2. Analysis of the Process Stability Based on the Indirect Carbon-Related Parameters
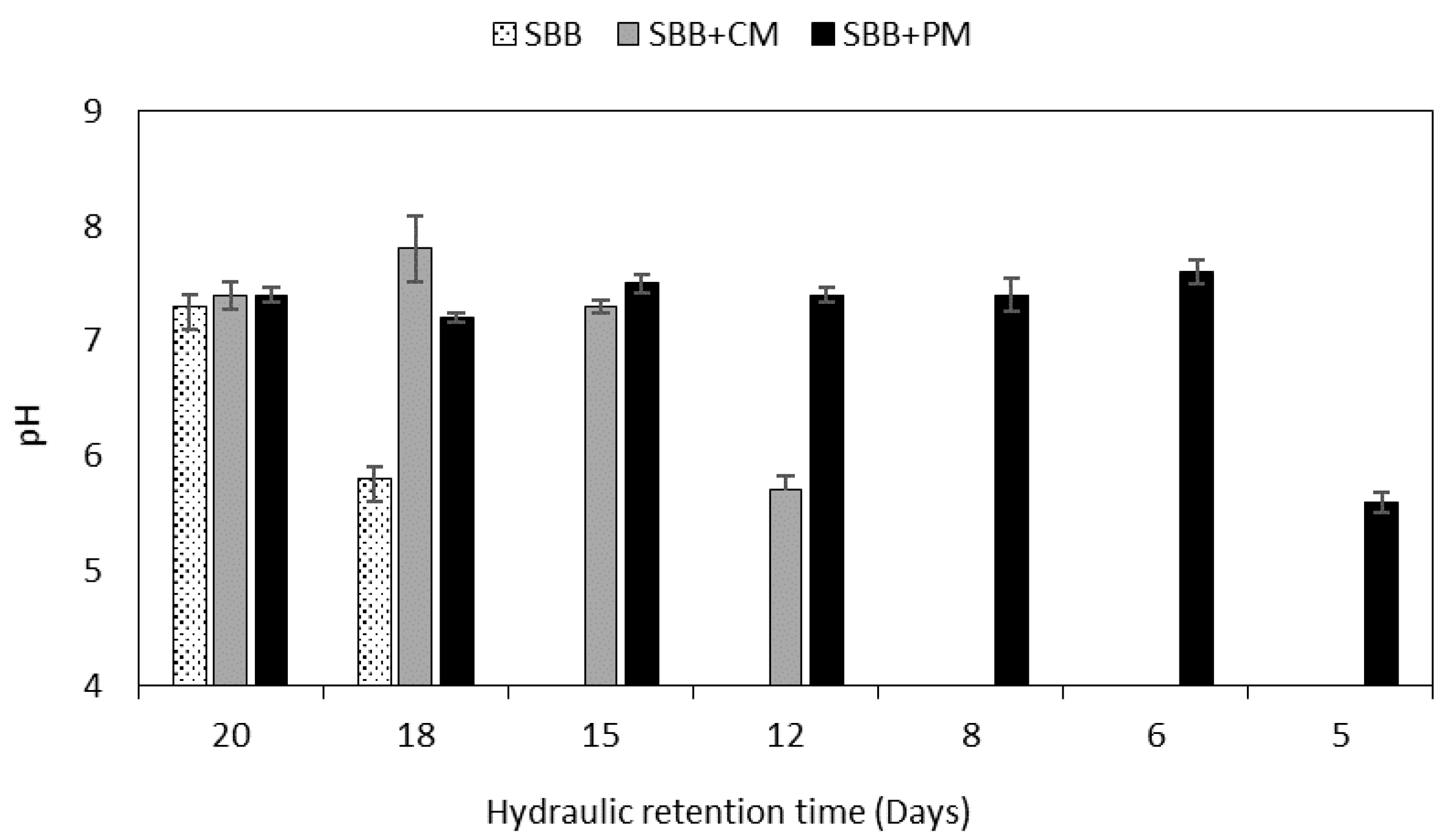
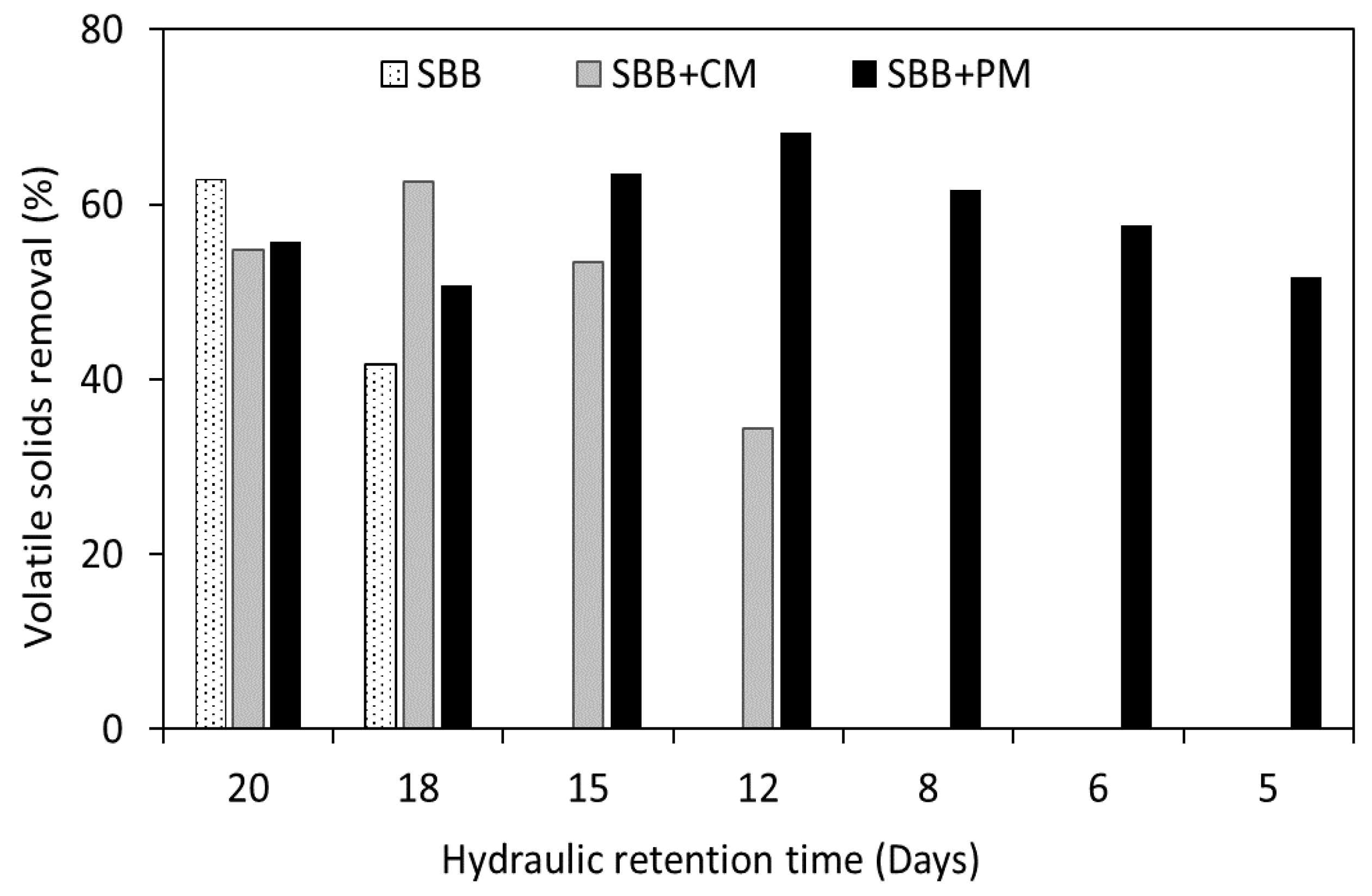
3.3. Analysis of the Process Stability Based on the Classical Parameters
4. Conclusions
- ➢
- Non-classical parameters could give more insight into the coupling/uncoupling of the AD phases and the involved microorganisms, revealing that process failure was mainly due to methanogenesis inhibition in co-digestion with PM, while for co-digestion with CM or individual digestion of SBB, both hydrolysis–acidogenesis and methanogenesis phases were affected.
- ➢
- Co-digestion with manure contributed to reducing the inhibitory effect of volatile fatty acids at high organic loading rates (OLRs), leading to increases in methane production by 70% and 31% in comparison with AD of SBB, for co-digestion with pig and cow manure, respectively.
- ➢
- Biomethanation degree (BD) refers to the maximum methane potential that can be obtained from organic wastes under specific operating conditions. SBB required a long digestion-time to achieve high biodegradability. However, short digestion-times for co-digestion assays led to high BD.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stanchev, P.; Vasilaki, V.; Egas, D.; Colon, J.; Ponsá, S.; Katsou, E. Multilevel environmental assessment of the anaerobic treatment of dairy processing effluents in the context of circular economy. J. Clean. Prod. 2020, 261, 121139. [Google Scholar] [CrossRef]
- FAO. 2019. Available online: http://www.fao.org/news/story/en/item/196402/icode/ (accessed on 15 June 2020).
- Vasco-Correa, J.; Khanal, S.; Manandhar, A.; Shah, A. Anaerobic digestion for bioenergy production: Global status, environmental and techno-economic implications, and government policies. Bioresour. Technol. 2018, 247, 1015–1026. [Google Scholar] [CrossRef]
- De Corato, U.; De Bari, I.; Viola, E.; Pugliese, M. Assessing the main opportunities of integrated biorefining from agro-bioenergy co/by-products and agroindustrial residues into high-value added products associated to some emerging markets: A review. Renew. Sustain. Energy Rev. 2018, 88, 326–346. [Google Scholar] [CrossRef]
- Joanna, B.; Michal, B.; Piotr, D.; Agnieszka, W.; Dorota, K.; Izabela, W. Sugar beet pulp as a source of valuable biotechnological products. In Advances in Biotechnology for Food Industry; Holban, A.M., Grumezescu, A.M., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 2018; pp. 359–392. [Google Scholar] [CrossRef]
- Ileleji, K.E.; Martin, C.; Jones, D. Basics of energy production through anaerobic digestion of livestock manure. In Bioenergy; Dahiya, A., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 2015; pp. 287–295. [Google Scholar] [CrossRef]
- Eurostat. 2017. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Agricultural_production_-_animals#Livestock_population (accessed on 15 June 2020).
- Wainaina, S.; Awasthi, K.M.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Kumar-Awasthi, S.; Liu, T.; Duan, Y.; et al. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef]
- Zhang, Y.; Angelidaki, I. Counteracting ammonia inhibition during anaerobic digestion by recovery using submersible microbial desalination cell. Biotechnol. Bioeng. 2015, 112, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Moestedt, J.; Müller, B.; Westerholm, M.; Schnürer, A. Ammonia threshold for inhibition of anaerobic digestion of thin stillage and the importance of organic loading rate. Microb. Biotechnol. 2015, 9, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Villegas, H.A.; Larson, R.A. Evaluating greenhouse gas emissions from dairy manure management practices using survey data and lifecycle tools. J. Clean. Prod. 2017, 143, 169–179. [Google Scholar] [CrossRef]
- Shou, Z.; Yuan, H.; Shen, Y.; Liang, J.; Zhua, N.; Gu, L. Mitigating inhibition of undissociated volatile fatty acids (VFAs) for enhanced sludge-rice bran composting with ferric nitrate amendment. Bioresour. Technol. 2017, 244, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.; Martínez, J.E.; Escapa, A.; Martínez, O.; Díez-Antolínez, R.; Gómez, X. Mitigation of volatile fatty acid build-up by the use of soft carbon felt electrodes: Evaluation of anaerobic digestion in acidic conditions. Fermentation 2018, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Boldrin, A.; Baral, K.R.; Fitamo, T.; Vazifehkhoran, A.H.; Jensen, I.G.; Kjærgaard, I.; Lyng, K.-A.; van Nguyen, Q.; Nielsen, L.S.; Triolo, J.M. Optimised biogas production from the co-digestion of sugar beet with pig slurry: Integrating energy, GHG and economic accounting. Energy 2016, 112, 606–617. [Google Scholar] [CrossRef] [Green Version]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Evaluation of methane generation and process stability from anaerobic co-digestion of sugar beet by-product and cow manure. J. Biosci. Bioeng. 2016, 121, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Alkaya, E.; Demirer, G.N. Anaerobic acidification of sugar-beet processing wastes: Effect of operational parameters. Biomass Bioenergy 2011, 35, 32–39. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Su, C. Comparison of anaerobic digestion characteristics and kinetics of four livestock manures with different substrate concentrations. Bioresour. Technol. 2015, 198, 133–140. [Google Scholar] [CrossRef]
- Vivekanand, V.; Mulat, D.G.; Eijsink, V.G.H.; Horn, S.J. Synergistic effects of anaerobic co-digestion of whey, manure and fish ensilage. Bioresour. Technol. 2018, 249, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Pagés-Díaz, J.; Sárvári-Horváth, I.; Pérez-Olmo, J.; Pereda-Reyes, I. Co-Digestion of bovine slaughterhouse wastes, cow manure, various crops and municipal solid waste at thermophilic conditions: A comparison with specific case running at mesophilic conditions. Water Sci. Technol. 2013, 67, 989–995. [Google Scholar] [CrossRef] [Green Version]
- Lehtomäki, A.; Huttunen, S.; Rintala, J.A. Laboratory investigations on co-digestion of energy crops and crop residues with cow manure for methane production: Effect of crop to manure ratio. Resour. Conserv. Recycl. 2007, 51, 591–609. [Google Scholar] [CrossRef]
- Xie, T.; Xie, S.; Sivakumar, M.; Nghiem, L.D. Relationship between the synergistic/antagonistic effect of anaerobic co-digestion and organic loading. Int. Biodeterior. Biodegrad. 2017, 124, 155–161. [Google Scholar] [CrossRef]
- Cheng, X.-Y.; Zhong, C. Effects of feed to inoculum ratio, co-digestion, and pretreatment on biogas production from anaerobic digestion of cotton stalk. Energy Fuels 2014, 28, 3157–3166. [Google Scholar] [CrossRef]
- Borowski, S.; Kucner, M.; Czyżowska, A.; Berłowska, J. Co-Digestion of poultry manure and residues from enzymatic saccharification and dewatering of sugar beet pulp. Renew. Energy 2016, 99, 492–500. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Pérez, M.; Romero, L.I. Mesophilic anaerobic digestion of the organic fraction of municipal solid waste: Optimisation of the semicontinuous process. Chem. Eng. J. 2012, 193–194, 10–15. [Google Scholar] [CrossRef]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Improvement of exhausted sugar beet cossettes anaerobic digestion process by Co-digestion with pig manure. Energy Fuels 2015, 29, 754–762. [Google Scholar] [CrossRef]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Influence of total solids concentration on the anaerobic co-digestion of sugar beet by-products and livestock manures. Sci. Total Environ. 2017, 586, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Wintsche, B.; Glaser, K.; Sträuber, H.; Centler, F.; Liebetrau, J.; Harms, H.; Kleinsteuber, S. Trace elements induce predominance among methanogenic activity in anaerobic digestion. Front. Microbiol. 2016, 7, 2034. [Google Scholar] [CrossRef] [PubMed]
- Demirel, B.; Scherer, P. Production of methane from sugar beet silage without manure addition by a single-stage anaerobic digestion process. Biomass Bioenergy 2008, 32, 203–209. [Google Scholar] [CrossRef]
- APHA-AWWA. Standards Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Cui, S.; Yu, Q.; Ma, R. Anaerobic co-digestion of animal manures with corn stover or apple pulp for enhanced biogas production. Renew. Energy 2018, 118, 335–342. [Google Scholar] [CrossRef]
- Kaparaju, P.; Rintala, J. Anaerobic co-digestion of potato tuber and its industrial by-products with pig manure. Resour. Conserv. Recycl. 2005, 43, 175–188. [Google Scholar] [CrossRef]
- Fdez-Güelfo, L.A.; Álvarez-Gallego, C.; Sales, D.; Romero, L.I. New indirect parameters for interpreting a destabilization episode in an anaerobic reactor. Chem. Eng. J. 2012, 180, 32–38. [Google Scholar] [CrossRef]
- Gómez-Quiroga, X.; Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Enhancement of methane production in thermophilic anaerobic co-digestion of exhausted sugar beet pulp and pig manure. Appl. Sci. 2019, 9, 1791. [Google Scholar] [CrossRef] [Green Version]
- Damaceno, F.M.; Chiarelotto, M.; Pires-Salcedo-Restrepo, J.C.; Buligon, E.L.; Costa, L.A.d.M.; de Lucas-Junior, J.; Costa-SilvadeMendonça, M.S. Anaerobic co-digestion of sludge cake from poultry slaughtering wastewater treatment and sweet potato: Energy and nutrient recovery. Renew. Energy 2019, 133, 489–499. [Google Scholar] [CrossRef]
- Panichnumsin, P.; Nopharatana, A.; Ahring, B.; Chaiprasert, P. Production of methane by co-digestion of cassava pulp with various concentrations of pig manure. Biomass Bioenergy 2010, 34, 1117–1124. [Google Scholar] [CrossRef]
- Smith, P.H.; Mah, R.A. Kinetics of acetate metabolism during sludge digestion. Appl. Microbiol. 1966, 14, 368–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehring, T.; Klang, J.; Niedermayr, A.; Berzio, S.; Immenhauser, A.; Klocke, M.; Wichern, M.; Lübken, M. Determination of methanogenic pathways through carbon isotope (δ13C) analysis for the two-stage anaerobic digestion of high-solids substrates. Environ. Sci. Technol. 2015, 49, 4705–4714. [Google Scholar] [CrossRef]
- Siegrist, H.; Vogt, D.; García-Heras, J.L.; Gujer, W. Mathematical model for meso- and thermophilic anaerobic sewage sludge digestion. Environ. Sci. Technol. 2002, 36, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.M.; Westerholmc, M.; Qiaoa, W.; Bia, S.J.; Wanderaa, S.M.; Fana, R.; Jianga, M.M.; Dong, R.J. An explanation of the methanogenic pathway for methane production in anaerobic digestion of nitrogen-rich materials under mesophilic and thermophilic conditions. Bioresour. Technol. 2018, 264, 42–50. [Google Scholar] [CrossRef]
- Jin, W.; Xu, X.; Yang, F.; Li, C.; Zhou, M. Performance enhancement by rumen cultures in anaerobic co-digestion of corn straw with pig manure. Biomass Bioenergy 2018, 115, 120–129. [Google Scholar] [CrossRef]
- Callaghan, F.J.; Wase, D.A.J.; Thayanithy, K.; Forster, C.F. Continuous co-digestion of cattle slurry with fruit and vegetable wastes and chicken manure. Biomass Bioenergy 2002, 22, 71–77. [Google Scholar] [CrossRef]
- Switzenbaum, M.S.; Giraldo-Gomez, E.; Hickeyt, R.F. Monitoring of the anaerobic methane fermentation process. Enzym. Microb. Technol. 1990, 12, 722–730. [Google Scholar] [CrossRef]
- Zickefoose, C.; Hayes, R.B.J.; Bryant, J.O. Anaerobic Sludge Digestion: Operations Manual; National Technical Information Service, Ed.; EPA 430=9-76-001; United States Environmental Protection Agency: Washington, DC, USA, 1976. [Google Scholar]
- Marchaim, U.; Krause, C. Propionic to acetic acid ratios in overloaded anaerobic digestion. Bioresour. Technol. 1993, 43, 195–203. [Google Scholar] [CrossRef]
- Pullammanappallil, P.C.; Chynoweth, D.P.; Lyberatos, G.; Svoronos, S.A. Stable performance of anaerobic digestion in the presence of a high concentration of propionic acid. Bioresour. Technol. 2001, 78, 165–169. [Google Scholar] [CrossRef]
- Hill, D.T.; Cobb, S.A.; Bolte, J.P. Using Volatile Fatty Acid Relationships to Predict Anaerobic Digester Failure. Trans. ASAE 1987, 30, 496. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Pagés-Díaz, J.; Westman, J.; Taherzadeh, M.J.; Pereda-Reyes, I.; Sárvári-Horváth, I. Semi-Continuous co-digestion of solid cattle slaughterhouse wastes with other waste streams: Interactions within the mixtures and methanogenic community structure. Chem. Eng. J. 2015, 273, 28–36. [Google Scholar] [CrossRef]
- González-Suárez, A.; Pereda-Reyes, I.; Oliva-Merencio, D.; Suárez-Quiñones, T.; José da Silva, A.; Zaiat, M. Bioavailability and dosing strategies of mineral in anaerobic mono-digestion of maize straw. Eng. Life Sci. 2018, 18, 562–569. [Google Scholar] [CrossRef]
- Schmidt, T.; McCabe, B.K.; Harris, P.W.; Lee, S. Effect of trace element addition and increasing organic loading rates on the anaerobic digestion of cattle slaughterhouse wastewater. Bioresour. Technol. 2018, 264, 51–57. [Google Scholar] [CrossRef]



| Parameters (Units) | SBB | CM | PM | Inoc1 | Inoc2 |
|---|---|---|---|---|---|
| pH | 5.7 ± 0.7 | 6.2 ± 1.3 | 6.5 ± 1.7 | 7.5 ± 0.7 | 7.6 ± 0.8 |
| TS (g/kg) | 880.3 ± 12.4 | 222.7 ± 11.5 | 225.3 ± 7.4 | 41.2 ± 1.3 | 36.1 ± 10.6 |
| VS (%TS) | 90.6 ± 1.8 | 77.7 ± 6.7 | 75.1 ± 5.2 | 16.3 ± 1.6 | 21.8 ± 3.3 |
| CODS (gO2/kg TS) | 64.7 ± 2.5 | 77.7 ± 13.9 | 83.0 ± 7.9 | 246.5 ± 6.1 | |
| CODT (gO2/kg TS) | 165.8 ± 5.9 | 308.5 ± 6.3 | 220.6 ± 11.1 | 321.3 ± 6.9 | |
| DOC (gC/kg TS) | 49.7 ± 5.1 | 33.7 ± 9.8 | 39.1 ± 6.6 | 150 ± 5.0 | 127.4 ± 19.4 |
| TVFA (gHAc/kg TS) | 2.5 ± 0.9 | 24.3 ± 4.9 | 20.4 ± 2.2 | 7.2 ± 2.4 | 47.1 ± 4.3 |
| Alkalinity (gCaCO3/kg TS) | 3.3 ± 1.4 | 170.2 ± 0.9 | 169.9 ± 49.3 | 116.4 ± 69.3 | |
| N-NH4+ (gN/kg TS) | 0.3 ± 0.1 | 15.7 ± 3.2 | 10.6 ± 2.6 | 30.5 ± 5.5 | |
| TN (gTN/kg TS) | 14.5 ± 1.5 | 157.6 ± 6.7 | 145.2 ± 12.4 | 174.5 ± 3.6 | |
| Ratio C/N | 38.9 ± 2.7 | 13.2 ± 1.0 | 13.5 ± 0.3 | - | 19.4 ± 2.4 |
| Hemicellulose (%) | 15.4 ± 0.3 | 13.9 ± 0.8 | 14.4 ± 0.1 | - | - |
| Cellulose (%) | 25.9 ± 0.3 | 23.2 ± 1.2 | 16.3 ± 1.2 | - | - |
| Lignin (%) | 1.6 ± 0.1 | 19.8 ± 1.1 | 16.8 ± 1.9 | - | - |
| Mineral salts (%) | 6.7 ± 0.2 | 26.2 ± 1.0 | 27.2 ± 3.1 | - | - |
| Reactors | Hydraulic Retention Times (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 20 | 18 | 15 | 12 | 8 | 6 | 5 | ||
| SBB | Organic loading rates (gVS/Lreactor*d) | 3.3 | 3.6 | |||||
| SBB + PM | 4.2 | 4.7 | 5.9 | 7.4 | 8.5 | 11.2 | 12.8 | |
| SBB + CM | 3.7 | 4.2 | 4.9 | 6.2 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboudi, K.; Gómez-Quiroga, X.; Álvarez-Gallego, C.J.; Romero-García, L.I. Insights into Anaerobic Co-Digestion of Lignocellulosic Biomass (Sugar Beet By-Products) and Animal Manure in Long-Term Semi-Continuous Assays. Appl. Sci. 2020, 10, 5126. https://doi.org/10.3390/app10155126
Aboudi K, Gómez-Quiroga X, Álvarez-Gallego CJ, Romero-García LI. Insights into Anaerobic Co-Digestion of Lignocellulosic Biomass (Sugar Beet By-Products) and Animal Manure in Long-Term Semi-Continuous Assays. Applied Sciences. 2020; 10(15):5126. https://doi.org/10.3390/app10155126
Chicago/Turabian StyleAboudi, Kaoutar, Xiomara Gómez-Quiroga, Carlos José Álvarez-Gallego, and Luis Isidoro Romero-García. 2020. "Insights into Anaerobic Co-Digestion of Lignocellulosic Biomass (Sugar Beet By-Products) and Animal Manure in Long-Term Semi-Continuous Assays" Applied Sciences 10, no. 15: 5126. https://doi.org/10.3390/app10155126
APA StyleAboudi, K., Gómez-Quiroga, X., Álvarez-Gallego, C. J., & Romero-García, L. I. (2020). Insights into Anaerobic Co-Digestion of Lignocellulosic Biomass (Sugar Beet By-Products) and Animal Manure in Long-Term Semi-Continuous Assays. Applied Sciences, 10(15), 5126. https://doi.org/10.3390/app10155126







