Effects of Inspiratory Muscles Training Plus Rib Cage Mobilization on Chest Expansion, Inspiratory Accessory Muscles Activity and Pulmonary Function in Stroke Patients
Abstract
:1. Introduction
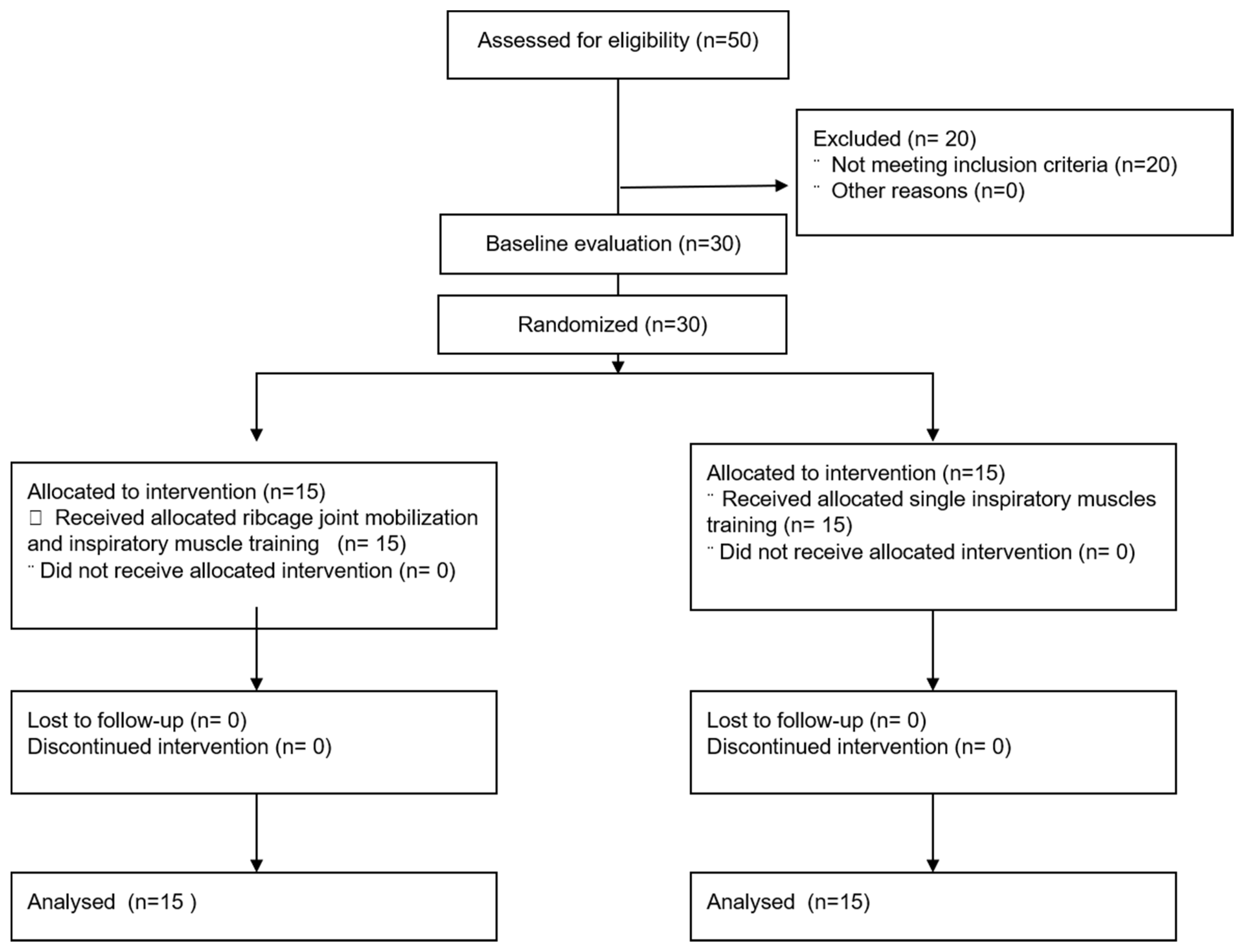
2. Subject and Methods
2.1. Participants
2.2. Sample Size
2.3. Randomization
2.4. Intervention Methods
2.5. Rib Cage Joint Mobilization
2.6. Inspiratory Muscle Training
2.7. Assessment Methods
2.7.1. Primary Outcome
2.7.2. Secondary Outcome
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Funding
Conflicts of Interest
References
- Rochester, C.L.; Mohsenin, V. Respiratory complications of stroke. In Seminars in Respiratory and Critical Care Medicine; Thieme Medical Publishers, Inc.: New York, NY, USA, 2002; pp. 248–260. [Google Scholar]
- Wannamethee, S.G.; Shaper, A.G.; Ebrahim, S.J.S. Respiratory function and risk of stroke. Stroke 1995, 26, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Katzan, I.L.; Cebul, R.D.; Husak, S.; Dawson, N.; Baker, D.J.N. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology 2003, 60, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Menezes, K.K.; Nascimento, L.R.; Ada, L.; Polese, J.C.; Avelino, P.R.; Teixeira-Salmela, L.F. Respiratory muscle training increases respiratory muscle strength and reduces respiratory complications after stroke: A systematic review. J. Physiother. 2016, 62, 138–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanini, B.; Bianchi, R.; Romagnoli, I.; Coli, C.; Binazzi, B.; Gigliotti, F.; Pizzi, A.; Grippo, A.; Scano, G. Chest wall kinematics in patients with hemiplegia. Am. J. Respir. Crit. Care Med. 2003, 168, 109–113. [Google Scholar] [CrossRef]
- Troyer, A.D.; De Beyl, D.Z.; Thirion, M. Function of the respiratory muscles in acute hemiplegia. Am. Rev. Respir. Dis. 1981, 123, 631–632. [Google Scholar]
- Khedr, E.; El Shinawy, O.; Khedr, T.; Aziz Ali, Y.; Awad, E.M. Assessment of corticodiaphragmatic pathway and pulmonary function in acute ischemic stroke patients. Eur. J. Neurol. 2000, 7, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, M.; Polese, J.; Faria, C.; Machado, G.; Parreira, V.; Britto, R.R.; Teixeira-Salmela, L.F. Inspiratory muscular weakness is most evident in chronic stroke survivors with lower walking speeds. Eur. J. Phys. Rehabil. Med. 2014, 50, 301–307. [Google Scholar]
- Fugl-Meyer, A.R.; Linderholm, H.; Wilson, A.F. Restrictive ventilatory dysfunction in stroke: Its relation to locomotor function. Supplement 1983, 9, 118–124. [Google Scholar]
- Rattes, C.; Campos, S.L.; Morais, C.; Gonçalves, T.; Sayão, L.B.; Galindo-Filho, V.C.; Parreira, V.; Aliverti, A.; de Andrade, A.D. Neurobiology. Respiratory muscles stretching acutely increases expansion in hemiparetic chest wall. Respir. Physiol. Neurobiol. 2018, 254, 16–22. [Google Scholar] [CrossRef]
- Britto, R.R.; Rezende, N.R.; Marinho, K.C.; Torres, J.L.; Parreira, V.F.; Teixeira-Salmela, L.F. Inspiratory muscular training in chronic stroke survivors: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2011, 92, 184–190. [Google Scholar] [CrossRef]
- Ogiwara, S.; Ogura, K. Antero-posterior excursion of the hemithorax in hemiplegia. J. Phys. Ther. Sci. 2001, 13, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Ezeugwu, V.E.; Olaogun, M.; Mbada, C.E.; Adedoyin, R. Comparative lung function performance of stroke survivors and age-matched and sex-matched controls. Physiother. Res. Int. 2013, 18, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Caro, C.G.; Butler, J.; DuBois, A.B. Some effects of restriction of chest cage expansion on pulmonary function in man: An experimental study. J. Clin. Investig. 1960, 39, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Kaltenborn, F.; Evjenth, O.; Kaltenborn, T.; Morgan, D.; Vollowitz, E.J.O.N. Manual Mobilization of the Joints, Vol. II: The Spine; Extrem: Oslo, Norli, 2003. [Google Scholar]
- Park, S.-J.; Kim, S.-H.; Min, K.-O. The immediate effects of rib cage joint mobilization and chest wall stretch on muscle tone and stiffness of respiratory muscles and chest expansion ability in patients with chronic stroke. J. Phys. Ther. Sci. 2017, 29, 1960–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yelvar, G.D.Y.; Çirak, Y.; Demir, Y.P.; Dalkilinc, M.; Bozkurt, B. Immediate effect of manual therapy on respiratory functions and inspiratory muscle strength in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.-H.; Bang, H.-S. Effect of thoracic and cervical joint mobilization on pulmonary function in stroke patients. J. Phys. Ther. Sci. 2016, 28, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Lee, E.; Lee, S.J.T.; Care, H. Effectiveness of mid-thoracic spine mobilization versus therapeutic exercise in patients with subacute stroke: A randomized clinical trial. Technol. Health Care 2019, 27, 149–158. [Google Scholar] [CrossRef]
- Wilson, T.; Rehder, K.; Krayer, S.; Hoffman, E.; Whitney, C.; Rodarte, J.R. Geometry and respiratory displacement of human ribs. J. Appl. Physiol. 1987, 62, 1872–1877. [Google Scholar] [CrossRef]
- Christensen, H.W.; Vach, W.; Vach, K.; Manniche, C.; Haghfelt, T.; Hartvigsen, L.; Høilund-Carlsen, P.F. Palpation of the upper thoracic spine: An observer reliability study. J. Manip. Physiol. Ther. 2002, 25, 285–292. [Google Scholar] [CrossRef]
- Bockenhauer, S.E.; Chen, H.; Julliard, K.N.; Weedon, J. Measuring thoracic excursion: Reliability of the cloth tape measure technique. J. Am. Osteopath. Assoc. 2007, 107, 191–196. [Google Scholar] [PubMed]
- Fry, D.K.; Pfalzer, L.A.; Chokshi, A.R.; Wagner, M.T.; Jackson, E.S. Randomized control trial of effects of a 10-week inspiratory muscle training program on measures of pulmonary function in persons with multiple sclerosis. J. Neurol. Phys. Ther. 2007, 31, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-E.; Lee, H.-J.; Kim, M.-K.; Lee, W.-H. The improvement in respiratory function by inspiratory muscle training is due to structural muscle changes in patients with stroke: A randomized controlled pilot trial. Top. Stroke Rehabil. 2018, 25, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Jang, S.-H. The effects of the neck stabilization exercise on the muscle activity of trunk respiratory muscles and maximum voluntary ventilation of chronic stroke patients. J. Back Musculoskelet. Rehabil. 2019, 32, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.S.; Tavares, J.M.R. Applications. Surface electromyographic amplitude normalization methods: A review. In Electromyography: New Developments, Procedures and Applications; Nova Science Publishers: Harpark, NY, USA, 2012. [Google Scholar]
- Park, B.-S.; Noh, J.-W.; Kim, M.-Y.; Lee, L.-K.; Yang, S.-M.; Lee, W.-D.; Shin, Y.-S.; Kim, J.-H.; Lee, J.-U.; Hwang, B.-Y.J.N.; et al. Randomized controlled pilot trial of truncal exercises after stroke to improve gait and muscle activity. Neurosci. Med. 2016, 7, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Joo, S.; Lee, Y.; Song, C.-H. Immediate effects of thoracic spinal manipulation on pulmonary function in stroke patients: A preliminary study. J. Manip. Physiol. Ther. 2018, 41, 602–608. [Google Scholar] [CrossRef]
- Leelarungrayub, D.; Pothongsunun, P.; Yankai, A.; Pratanaphon, S. Acute clinical benefits of chest wall-stretching exercise on expired tidal volume, dyspnea and chest expansion in a patient with chronic obstructive pulmonary disease: A single case study. J. Bodyw. Mov. Ther. 2009, 13, 338–343. [Google Scholar] [CrossRef]
- Wang, J.-S. of joint mobilization and stretching on respiratory function and spinal movement in very severe COPD with thoracic kyphosis. J. Phys. Ther. Sci. 2015, 27, 3329–3331. [Google Scholar] [CrossRef] [Green Version]
- Liebler, E.J.; Tufano-Coors, L.; Douris, P.; Makofsky, H.W.; McKenna, R.; Michels, C.; Rattray, S. The effect of thoracic spine mobilization on lower trapezius strength testing. J. Man. Manip. Ther. 2001, 9, 207–212. [Google Scholar] [CrossRef]
- Debouche, S.; Pitance, L.; Robert, A.; Liistro, G.; Reychler, G. Reliability and reproducibility of chest wall expansion measurement in young healthy adults. J. Manip. Physiol. Ther. 2016, 39, 443–449. [Google Scholar] [CrossRef]
| Classification | Experimental Group (n = 15) | Control Group (n = 15) | p-Value c | p-Value d |
|---|---|---|---|---|
| Gender (male/female) | 10/5 | 10/5 | 0.100 | |
| Paretic side (left/right) | 7/8 | 7/8 | 0.100 | |
| Pathogenesis (hemorrhages/infarction) | 2/13 | 4/11 | 0.651 | |
| Disease duration (months) a | 15 ± 4 | 15 ± 5 | 0.723 | |
| Age (years) a | 63 ± 7 | 64 ± 9 | 0.911 | |
| Weight (kg) a | 65 ± 8 | 63 ± 8 | 0.639 | |
| Height (cm) a | 168 ± 6 | 165 ± 6 | 0.351 | |
| K-MMSE e (point) a | 26 ± 2 | 26 ± 1 | 0.241 | |
| K-NIHSS f (point) a | 10 ± 2 | 11 ± 2 | 0.932 | |
| Baseline maximal inspiratory pressure | ||||
| Maximal inspiratory pressure (MIP, mmHg) b | 47.00 ± 10.83 | 45.73 ± 10.53 | 0.748 | |
| MIP predicted normal absolute values b | 78.47 ± 17.97 | 78.27 ± 18.53 | 0.976 | |
| % of MIP predicted normal values b | 62.07 ± 16.82 | 62.00 ± 20.32 | 0.992 | |
| Baseline pulmonary function | ||||
| Forced vital capacity (FVC, ℓ) b | 2.89 ± 0.81 | 2.65 ± 0.80 | 0.418 | |
| FVC predicted normal values b | 3.08 ± 0.45 | 2.96 ± 0.45 | 0.472 | |
| % of FVC predicted normal values b | 92.47 ± 18.65 | 90.40 ± 28.14 | 0.936 | |
| Forced expiratory volume in 1 s (FEV1, ℓ) b | 2.32 ± 0.65 | 2.19 ± 0.68 | 0.596 | |
| FEV1 predicted normal absolute values b | 2.83 ± 0.55 | 2.69 ± 0.53 | 0.512 | |
| % of FEV1 predicted normal values b | 82.07 ± 18.53 | 82.73 ± 26.14 | 0.936 | |
| Peak expiratory flow (PEF, ℓ/min) b | 276.67 ± 72.54 | 255.33 ± 75.52 | 0.437 | |
| PEF predicted normal absolute values b | 432.40 ± 81.85 | 420.80 ± 85.54 | 0.707 | |
| % of PEF predicted normal values b | 64.67 ± 15.90 | 64.53 ± 29.24 | 0.988 | |
| Measure/Group | Baseline Test a | Post-Test a | Within-Subjects Change b | Between Group Change b |
|---|---|---|---|---|
| Primary outcome | ||||
| Upper chest (cm) | ||||
| Experimental group | 1.10 ± 0.65 | 2.52 ± 0.73 | 1.43 (1.09, 1.76) * | 0.79 (0.37, 1.21) ** |
| Control group | 1.05 ± 0.42 | 1.69 ± 0.73 | 0.64 (0.35, 0.93) * | |
| Lower chest (cm) | ||||
| Experimental group | 1.74 ± 0.95 | 3.32 ± 0.44 | 1.58 (1.18, 1.98) * | 0.84 (0.40, 1.29) ** |
| Control group | 1.70 ± 0.61 | 2.43 ± 0.55 | 0.73 (0.50, 0.97) * | |
| Secondary outcome | ||||
| UT (%RVC) | ||||
| Experimental group | 102.98 ± 2.29 | 139.91 ± 16.78 | 36.93 (27.68, 46.17) * | 22.14 (11.35, 32.93) ** |
| Control group | 102.66 ± 2.65 | 117.44 ± 11.98 | 14.79 (8.29, 21.28) * | |
| LD (%RVC) | ||||
| Experimental group | 103.99 ± 2.91 | 143.20 ± 17.86 | 39.21 (28.71, 49.71) * | 24.15 (11.93, 36.36) ** |
| Control group | 103.55 ± 2.75 | 118.61 ± 13.79 | 15.06 (7.90, 22.22) * | |
| FEV1 (ℓ) | ||||
| Experimental group | 2.32 ± 0.65 | 2.65 ± 0.61 | 0.33 (0.21, 0.45) * | 0.12 (−0.06, 0.29) |
| Control group | 2.19 ± 0.68 | 2.40 ± 0.77 | 0.22 (0.08, 0.35) * | |
| FVC (ℓ) | ||||
| Experimental group | 2.89 ± 0.81 | 3.34 ± 0.71 | 0.45 (0.29, 0.60) * | 0.18 (−0.33, 0.39) |
| Control group | 2.65 ± 0.80 | 2.92 ± 0.90 | 0.27 (0.10, 0.43) * | |
| PEF (ℓ/min) | ||||
| Experimental group | 276.67 ± 72.54 | 359.73 ± 79.83 | 83.07 (37.56, 128.57) * | 10.87 (−50.93, 72.67) |
| Control group | 255.33 ± 75.52 | 327.53 ± 93.51 | 72.20(26.20, 118.20) * | |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.J. Effects of Inspiratory Muscles Training Plus Rib Cage Mobilization on Chest Expansion, Inspiratory Accessory Muscles Activity and Pulmonary Function in Stroke Patients. Appl. Sci. 2020, 10, 5178. https://doi.org/10.3390/app10155178
Park SJ. Effects of Inspiratory Muscles Training Plus Rib Cage Mobilization on Chest Expansion, Inspiratory Accessory Muscles Activity and Pulmonary Function in Stroke Patients. Applied Sciences. 2020; 10(15):5178. https://doi.org/10.3390/app10155178
Chicago/Turabian StylePark, Shin Jun. 2020. "Effects of Inspiratory Muscles Training Plus Rib Cage Mobilization on Chest Expansion, Inspiratory Accessory Muscles Activity and Pulmonary Function in Stroke Patients" Applied Sciences 10, no. 15: 5178. https://doi.org/10.3390/app10155178
APA StylePark, S. J. (2020). Effects of Inspiratory Muscles Training Plus Rib Cage Mobilization on Chest Expansion, Inspiratory Accessory Muscles Activity and Pulmonary Function in Stroke Patients. Applied Sciences, 10(15), 5178. https://doi.org/10.3390/app10155178





