Influences of High-Sulphur Fly Ash on the Properties of Lightweight Cement-Treated Materials Subjected to Sulphate Corrosion
Abstract
:1. Introduction
2. Materials
2.1. Test Materials
2.2. Test Preparation
2.3. Test Method
3. Results and Discussion
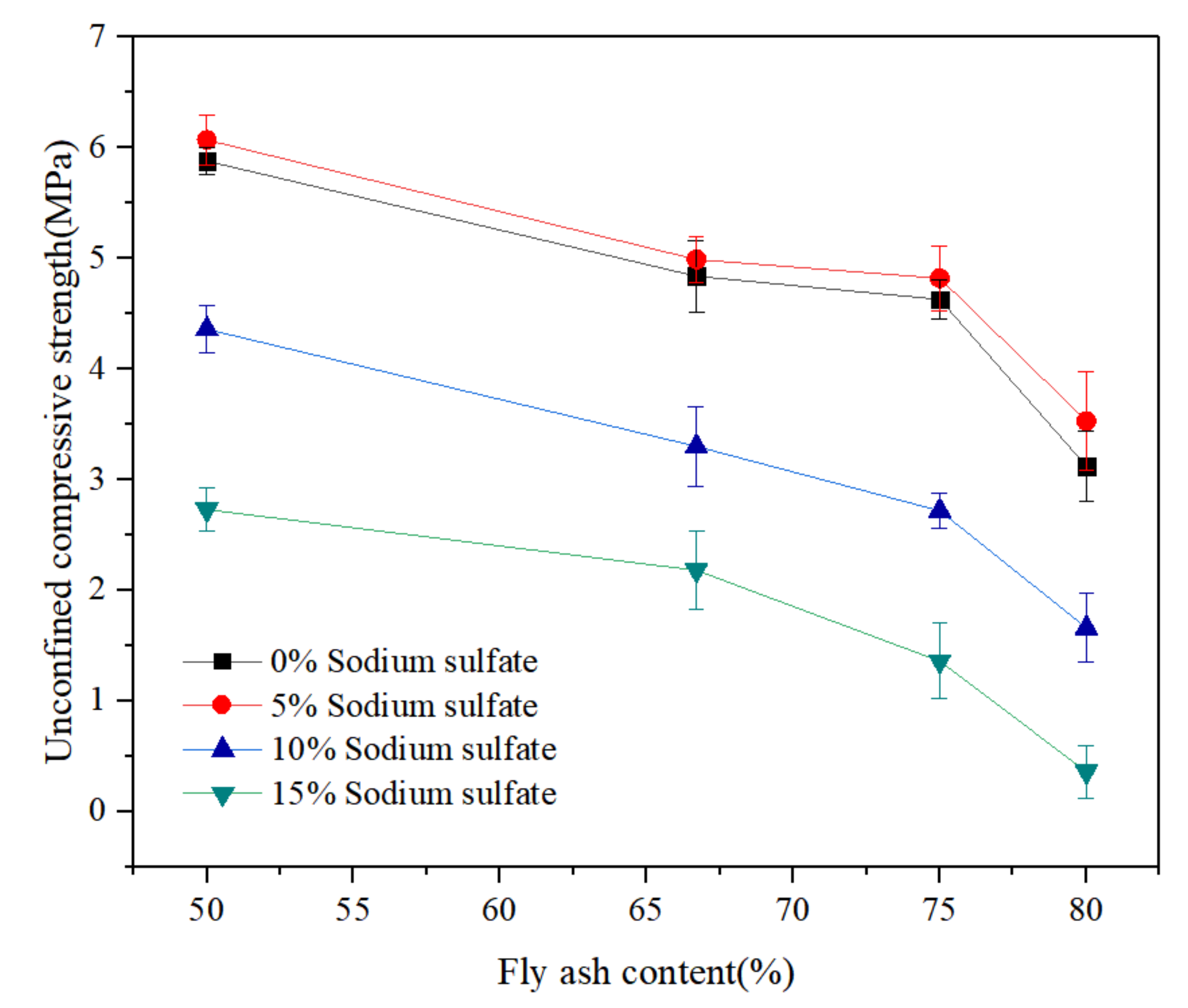
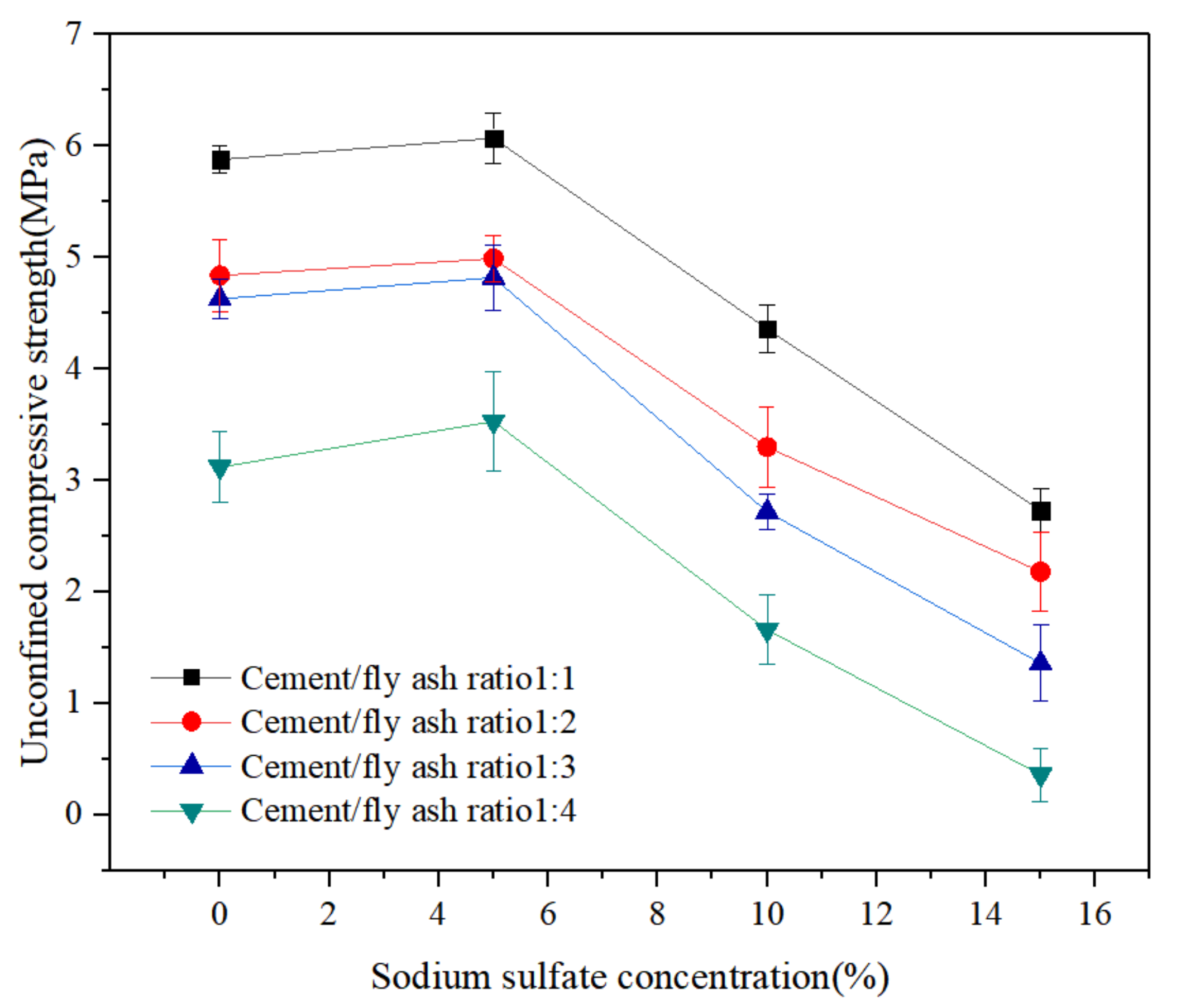
3.1. Mechanical Properties of LCMs under Sodium Sulphate Corrosion
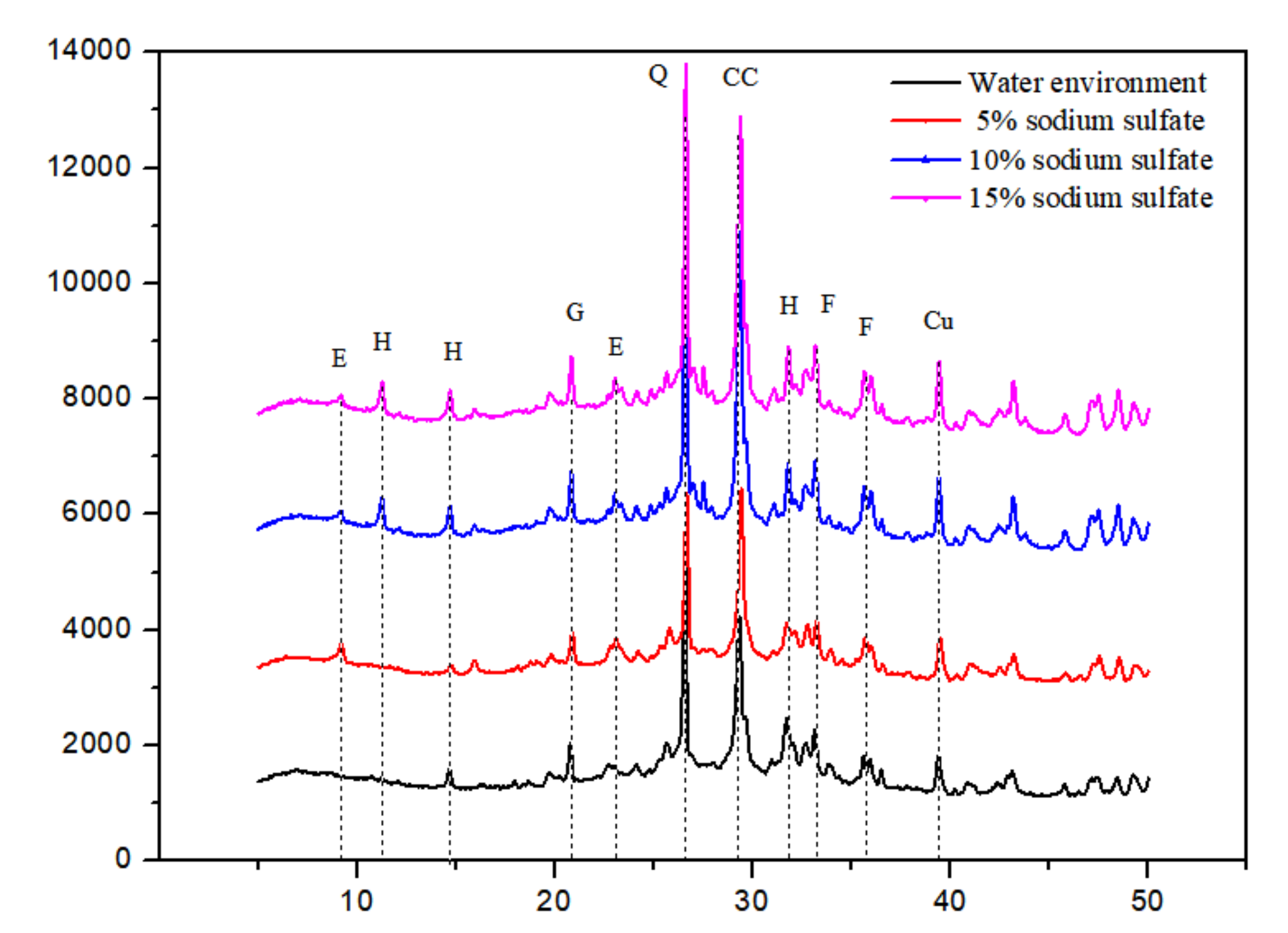
3.2. X-ray Diffraction Analysis
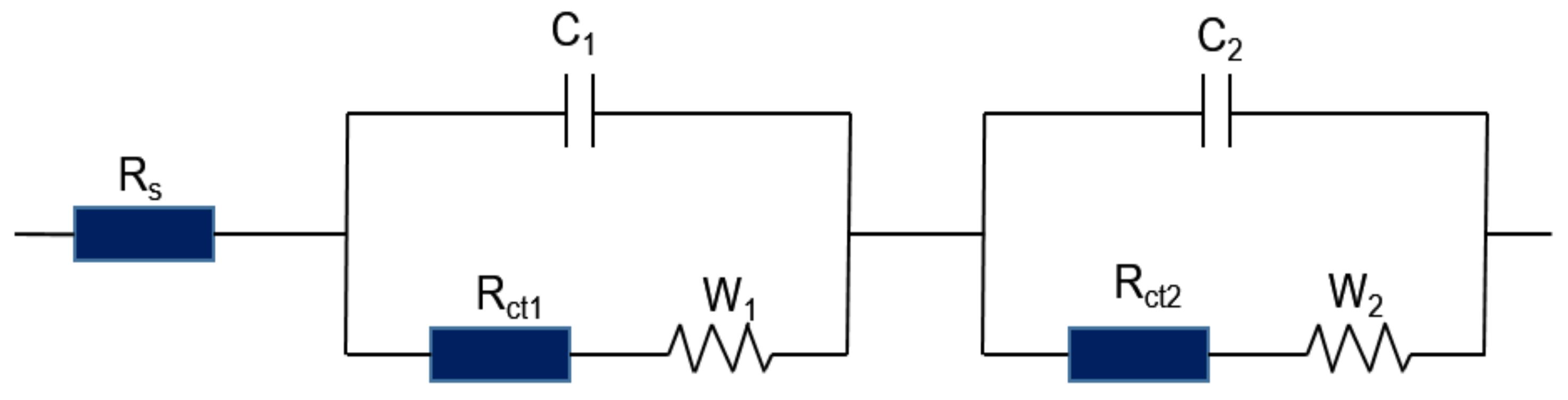
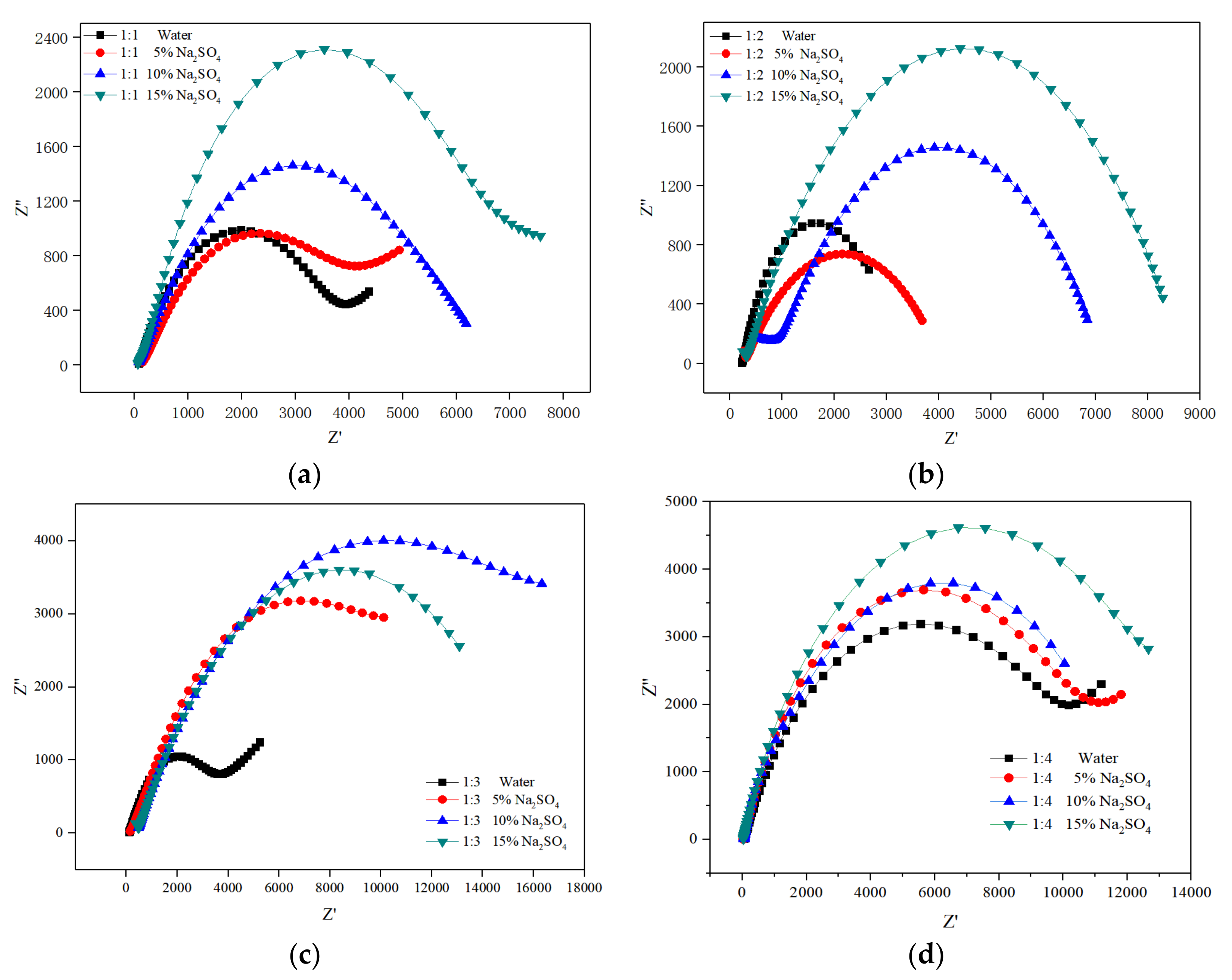
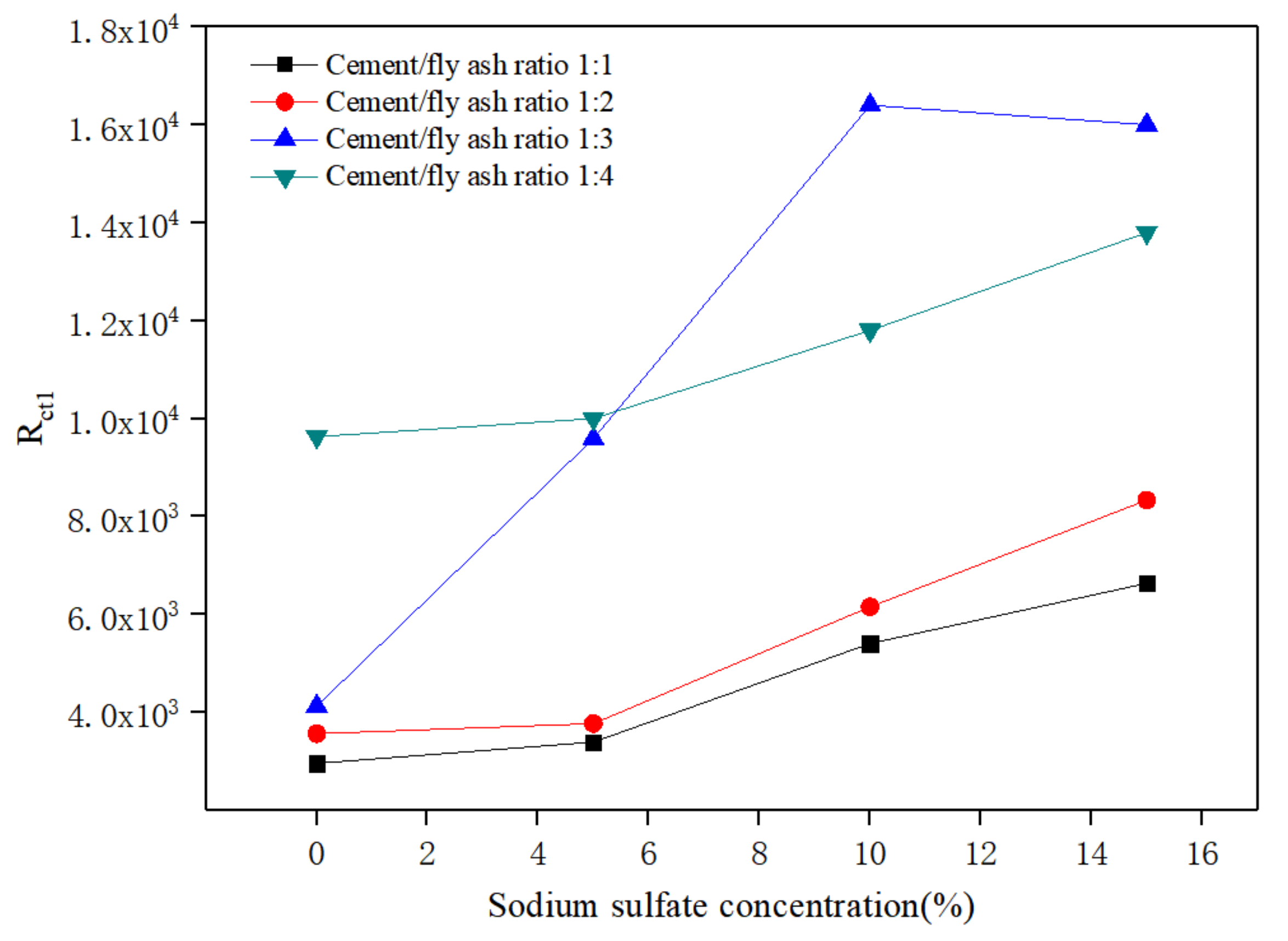
3.3. Electrochemical Impedance Spectroscopy Analysis
| Rs | Pore solution resistance |
| C1 | Pore capacitance |
| Rct1 | Material ion transfer resistance |
| W1 | Diffusion impedance of pore solution |
| C2 | Dual capacitance on the electrode/material surface |
| Rct2 | Surface ion transfer resistance of electrode plate |
| W2 | Diffusion impedance at the electrode plate |
3.4. SEM Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chauhan, M.S.; Mittal, S.; Mohanty, B. Performance evaluation of silty sand subgrade reinforced with fly ash and fibre. Geotext. Geomembr. 2008, 26, 429–435. [Google Scholar] [CrossRef]
- Phetchuay, C.; Horpibulsuk, S.; Suksiripattanapong, C.; Chinkulkijniwat, A.; Arulrajah, A.; Disfani, M.M. Calcium carbide residue: Alkaline activator for clay-fly ash geopolymer. Constr. Build. Mater. 2014, 69, 285–294. [Google Scholar] [CrossRef]
- Kua, T.-A.; Arulrajah, A.; Horpibulsuk, S.; Du, Y.-J.; Shen, S.-L. Strength assessment of spent coffee grounds-geopolymer cement utilizing slag and fly ash precursors. Constr. Build. Mater. 2016, 115, 565–575. [Google Scholar] [CrossRef]
- Wei, H.; Jiao, Y.; Liu, H. Effect of freeze-thaw cycles on mechanical property of silty clay modified by fly ash and crumb rubber. Cold Reg. Sci. Technol. 2015, 116, 70–77. [Google Scholar] [CrossRef]
- Kua, T.-A.; Arulrajah, A.; Mohammadinia, A.; Horpibulsuk, S.; Mirzababaei, M. Stiffness and deformation properties of spent coffee grounds based geopolymers. Constr. Build. Mater. 2017, 138, 79–87. [Google Scholar] [CrossRef]
- Jiang, N.-J.; Du, Y.-J.; Liu, K. Durability of lightweight alkali-activated ground granulated blast furnace slag (GGBS) stabilized clayey soils subjected to sulfate attack. Appl. Clay Sci. 2018, 161, 70–75. [Google Scholar] [CrossRef]
- Solanki, P.; Zaman, M.M. Laboratory Performance Evaluation of Subgrade Soils Stabilized with Sulfate-Bearing Cementitious Additives. J. Test. Eval. 2010, 38, 1–12. [Google Scholar]
- Knopp, J.; Moormann, C. Ettringite Swelling in the Treatment of Sulfate-Containing Soils Used as Subgrade for Road Constructions. Procedia Eng. 2016, 143, 128–137. [Google Scholar]
- Islam, M.A.; Golrokh, A.J.; Lu, Y. Chemomechanical Modeling of Sulfate Attack-Induced Damage Process in Cement-Stabilized Pavements. J. Eng. Mech. 2019, 145. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Gao, L. Effect of low-calcium fly ash on sulfate resistance of cement paste under different exposure conditions. Adv. Concr. Constr. 2019, 7, 175–181. [Google Scholar]
- Neramitkornburi, A.; Horpibulsuk, S.; Shen, S.L.; Chinkulkijniwat, A.; Arulrajah, A.; Disfani, M.M. Durability against wetting-drying cycles of sustainable Lightweight Cellular Cemented construction material comprising clay and fly ash wastes. Constr. Build. Mater. 2015, 77, 41–49. [Google Scholar] [CrossRef]
- Neramitkornburi, A.; Horpibulsuk, S.; Shen, S.L.; Arulrajah, A.; Disfani, M.M. Engineering properties of lightweight cellular cemented clay-fly ash material. Soils Found. 2015, 55, 471–483. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, M.A.; Prakash, S.S. Behavior of hybrid-synthetic fiber reinforced cellular lightweight concrete under uniaxial tension—Experimental and analytical studies. Constr. Build. Mater. 2018, 162, 857–870. [Google Scholar] [CrossRef]
- Huang, J.-J.; Su, Q.; Zhao, W.-H.; Li, T.; Zhang, X.-X. Experimental study on use of lightweight foam concrete as subgrade bed filler of ballastless track. Constr. Build. Mater. 2017, 149, 911–920. [Google Scholar] [CrossRef]
- Kim, Y.T.; Kim, H.J.; Lee, G.H. Mechanical behavior of lightweight soil reinforced with waste fishing net. Geotext. Geomembr. 2008, 26, 512–518. [Google Scholar] [CrossRef]
- Kraemer, C.; Schauerte, M.; Kowald, T.L.; Trettin, R.H.F. Three-phase-foams for foam concrete application. Mater. Charact. 2015, 102, 173–179. [Google Scholar] [CrossRef]
- She, W.; Du, Y.; Zhao, G.; Feng, P.; Zhang, Y.; Cao, X. Influence of coarse fly ash on the performance of foam concrete and its application in high-speed railway roadbeds. Constr. Build. Mater. 2018, 170, 153–166. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Farzadnia, N.; Ali, A.A.A. Properties and applications of foamed concrete; A review. Constr. Build. Mater. 2015, 101, 990–1005. [Google Scholar] [CrossRef]
- Kadela, M.; Kozłowski, M.; Kukiełka, A. Application of Foamed Concrete in Road Pavement—Weak Soil System. Procedia Eng. 2017, 193, 439–446. [Google Scholar] [CrossRef]
- Kang, S.-H.; Jeong, Y.; Kim, M.O.; Moon, J. Pozzolanic reaction on alkali-activated Class F fly ash for ambient condition curable structural materials. Constr. Build. Mater. 2019, 218, 235–244. [Google Scholar] [CrossRef]
- Wang, T.; Ishida, T. Multiphase pozzolanic reaction model of low-calcium fly ash in cement systems. Cem. Concr. Res. 2019, 122, 274–287. [Google Scholar] [CrossRef]
- Gadouri, H.; Harichane, K.; Ghrici, M. Assessment of sulphates effect on pH and pozzolanic reactions of soil-lime-natural pozzolana mixtures. Int. J. Pavement Eng. 2019, 20, 761–774. [Google Scholar] [CrossRef]
- Kan, L.; Zhang, L.; Shi, H. Hydration Kinetics of Municipal Solid Wastes Incineration (MSWI) Fly Ash-Cement. J. Wuhan Univ. Technol. -Mater. Sci. Ed. 2019, 34, 596–603. [Google Scholar] [CrossRef]
- Liao, W.; Sun, X.; Kumar, A.; Sun, H.; Ma, H. Hydration of Binary Portland Cement Blends Containing Silica Fume: A Decoupling Method to Estimate Degrees of Hydration and Pozzolanic Reaction. Front. Mater. 2019, 6. [Google Scholar] [CrossRef]
- Ramanathan, S.; Moon, H.; Croly, M.; Chung, C.-W.; Suraneni, P. Predicting the degree of reaction of supplementary cementitious materials in cementitious pastes using a pozzolanic test. Constr. Build. Mater. 2019, 204, 621–630. [Google Scholar] [CrossRef]
- Contrafatto, L. Recycled Etna volcanic ash for cement, mortar and concrete manufacturing. Constr. Build. Mater. 2017, 151, 704–713. [Google Scholar] [CrossRef]
- Contrafatto, L.; Danzuso, C.L.; Gazzo, S.; Greco, L. Physical, mechanical and thermal properties of lightweight insulating mortar with recycled Etna volcanic aggregates. Constr. Build. Mater. 2020, 240, 117917. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, F.; Liu, R.; Zhang, R.; Liu, Z.; Liu, H. Effects of pozzolanic and non-pozzolanic nanomaterials on cement-based materials. Constr. Build. Mater. 2019, 213, 1–9. [Google Scholar] [CrossRef]
- Pourkhorshidi, A.R.; Najimi, M.; Parhizkar, T.; Jafarpour, F.; Hillemeier, B. Applicability of the standard specifications of ASTM C618 for evaluation of natural pozzolans. Cem. Concr. Compos. 2010, 32, 794–800. [Google Scholar] [CrossRef]
- Celik, K.; Hay, R.; Hargis, C.W.; Moon, J. Effect of volcanic ash pozzolan or limestone replacement on hydration of Portland cement. Constr. Build. Mater. 2019, 197, 803–812. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, J.; Liu, C.; Chen, X.; Xu, Z. The particle-size effect of waste clay brick powder on its pozzolanic activity and properties of blended cement. J. Clean. Prod. 2020, 242, 118521. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; Palkovic, S.D.; Bumajdad, A.; Soriano, C.; Büyüköztürk, O. Use of silica fume and natural volcanic ash as a replacement to Portland cement: Micro and pore structural investigation using NMR, XRD, FTIR and X-ray microtomography. Constr. Build. Mater. 2018, 158, 574–590. [Google Scholar] [CrossRef]
- Kalakada, Z.; Doh, J.H.; Zi, G. Utilisation of coarse glass powder as pozzolanic cement—A mix design investigation. Constr. Build. Mater. 2020, 240, 117916. [Google Scholar] [CrossRef]
- Hossain, K.M.A.; Lachemi, M. Performance of volcanic ash and pumice based blended cement concrete in mixed sulfate environment. Cem. Concr. Res. 2006, 36, 1123–1133. [Google Scholar] [CrossRef]
- Puthipad, N.; Ouchi, M.; Attachaiyawuth, A. Effects of fly ash, mixing procedure and type of air-entraining agent on coalescence of entrained air bubbles in mortar of self-compacting concrete at fresh state. Constr. Build. Mater. 2018, 180, 437–444. [Google Scholar] [CrossRef]
- Chen, X.; Yan, Y.; Liu, Y.; Hu, Z. Utilization of circulating fluidized bed fly ash for the preparation of foam concrete. Constr. Build. Mater. 2014, 54, 137–146. [Google Scholar] [CrossRef]
- Jones, M.R.; McCarthy, A. Utilising unprocessed low-lime coal fly ash in foamed concrete. Fuel 2005, 84, 1398–1409. [Google Scholar] [CrossRef]
- Posi, P.; Ridtirud, C.; Ekvong, C.; Chammanee, D.; Janthowong, K.; Chindaprasirt, P. Properties of lightweight high calcium fly ash geopolymer concretes containing recycled packaging foam. Constr. Build. Mater. 2015, 94, 408–413. [Google Scholar] [CrossRef]
- Gokce, H.S.; Hatungimana, D.; Ramyar, K. Effect of fly ash and silica fume on hardened properties of foam concrete. Constr. Build. Mater. 2019, 194, 1–11. [Google Scholar] [CrossRef]
- Falliano, D.; de Domenico, D.; Ricciardi, G.; Gugliandolo, E. Experimental investigation on the compressive strength of foamed concrete: Effect of curing conditions, cement type, foaming agent and dry density. Constr. Build. Mater. 2018, 165, 735–749. [Google Scholar] [CrossRef]
- Hussin, M.W.; Muthusamy, K.; Zakaria, F. Effect of Mixing Constituent toward Engineering Properties of POFA Cement-Based Aerated Concrete. J. Mater. Civ. Eng. 2010, 22, 287–295. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Shrinkage behavior of structural foam lightweight concrete containing glycol compounds and fly ash. Mater. Des. 2011, 32, 723–727. [Google Scholar] [CrossRef]
- Ma, C.; Chen, B. Experimental study on the preparation and properties of a novel foamed concrete based on magnesium phosphate cement. Constr. Build. Mater. 2017, 137, 160–168. [Google Scholar] [CrossRef]
- Jones, M.R.; McCarthy, A. Heat of hydration in foamed concrete: Effect of mix constituents and plastic density. Cem. Concr. Res. 2006, 36, 1032–1041. [Google Scholar] [CrossRef]
- Farhan, N.A.; Sheikh, M.N.; Hadi, M.N.S. Investigation of engineering properties of normal and high strength fly ash based geopolymer and alkali-activated slag concrete compared to ordinary Portland cement concrete. Constr. Build. Mater. 2019, 196, 26–42. [Google Scholar] [CrossRef]
- Uthaman, S.; Vishwakarma, V.; George, R.P.; Ramachandran, D.; Kumari, K.; Preetha, R.; Premila, M.; Rajaraman, R.; Mudali, U.K.; Amarendra, G. Enhancement of strength and durability of fly ash concrete in seawater environments: Synergistic effect of nanoparticles. Constr. Build. Mater. 2018, 187, 448–459. [Google Scholar] [CrossRef]
- Li, J.; Ji, Y.; Zhang, L.; Liu, B. Resistance to sulfate attack of magnesium phosphate cement-coated concrete. Constr. Build. Mater. 2019, 195, 156–164. [Google Scholar]
- Sirisawat, I.; Baingam, L.; Saengsoy, W.; Krammart, P.; Tangtermsirikul, S. Sodium and Magnesium Sulfate Resistance of Mortars with Interground Limestone and Limestone Powder Replacing Cements. J. Adv. Concr. Technol. 2014, 12, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ji, Y.; Huang, G.; Zhang, L. Microstructure Evolution of a Magnesium Phosphate Protective Layer on Concrete Structures in a Sulfate Environment. Coatings 2018, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Tixier, R.; Mobasher, B. Modeling of Damage in Cement-Based Materials Subjected to External Sulfate Attack. II: Comparison with Experiments. J. Mater. Civ. Eng. 2003, 15, 314–322. [Google Scholar] [CrossRef]
- Haufe, J.; Vollpracht, A. Tensile strength of concrete exposed to sulfate attack. Cem. Concr. Res. 2019, 116, 81–88. [Google Scholar] [CrossRef]
- Siad, H.; Kamali-Bernard, S.; Mesbah, H.A.; Escadeillas, G.; Mouli, M.; Khelafi, H. Characterization of the degradation of self-compacting concretes in sodium sulfate environment: Influence of different mineral admixtures. Constr. Build. Mater. 2013, 47, 1188–1200. [Google Scholar] [CrossRef]
- Sun, H.; Ren, Z.; Memon, S.A.; Zhao, D.; Zhang, X.; Li, D.; Xing, F. Investigating drying behavior of cement mortar through electrochemical impedance spectroscopy analysis. Constr. Build. Mater. 2017, 135, 361–368. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Zhang, Z.; Yao, Y. Electrochemical impedance spectroscopy (EIS) of hydration process and drying shrinkage for cement paste with W/C of 0.25 affected by high range water reducer. Constr. Build. Mater. 2017, 131, 536–541. [Google Scholar] [CrossRef]
- Dong, B.; Li, G.; Zhang, J.; Liu, Y.; Xing, F.; Hong, S. Non-destructive tracing on hydration feature of slag blended cement with electrochemical method. Constr. Build. Mater. 2017, 149, 467–473. [Google Scholar] [CrossRef]
- Dong, B.; Gu, Z.; Qiu, Q.; Liu, Y.; Ding, W.; Xing, F.; Hong, S. Electrochemical feature for chloride ion transportation in fly ash blended cementitious materials. Constr. Build. Mater. 2018, 161, 577–586. [Google Scholar] [CrossRef]
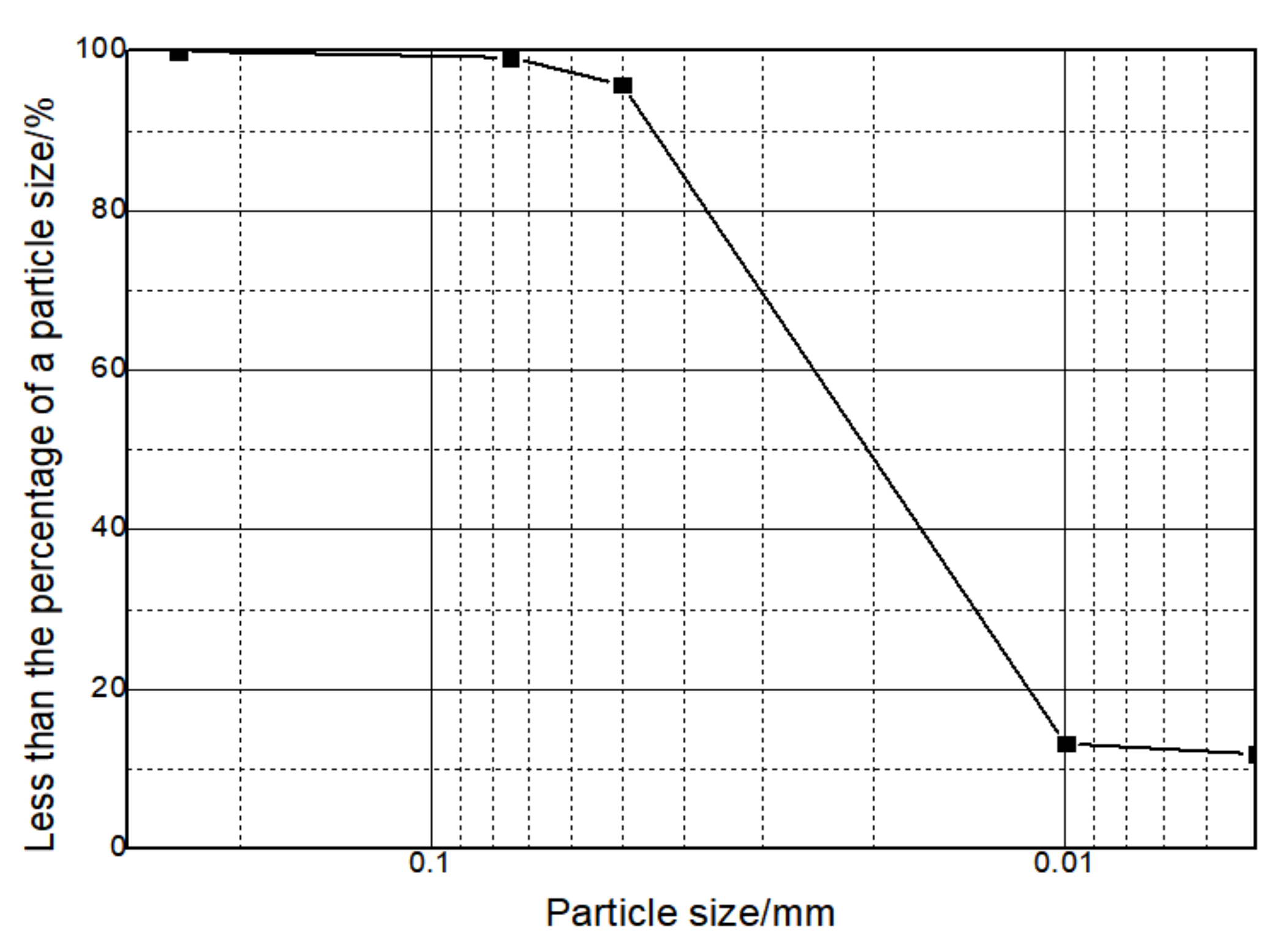
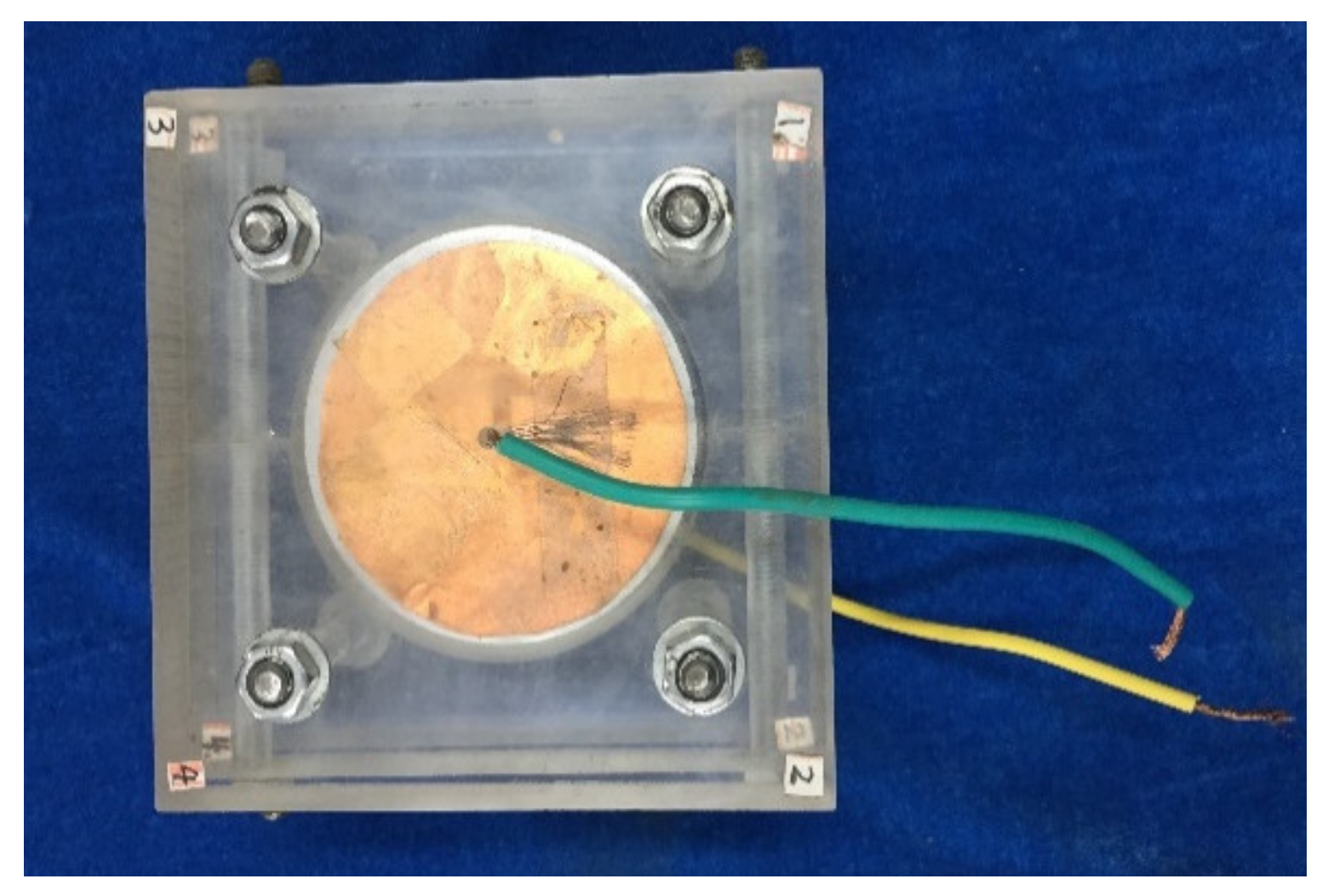




| Component | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | Na2O | K2O | Loss |
|---|---|---|---|---|---|---|---|---|---|
| Content (by mass %) | 66.35 | 18.81 | 5.86 | 3.34 | 1.04 | 2.53 | 0.31 | 0.41 | 0.45 |
| Fineness | Water Requirement Ratio | Loss on Ignition | Sulphur Trioxide | Water Content | Free Calcium Oxide |
|---|---|---|---|---|---|
| 10.2 | 107 | 5.21 | 6.99 | 0.32 | 2.45 |
| Component | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | f-CaO | Loss |
|---|---|---|---|---|---|---|---|---|
| Content (by mass %) | 23.57 | 34.6 | 18.57 | 7.66 | 3.41 | 6.99 | 2.45 | 2.75 |
| Number | Cement (kg/m3) | High-Sulphur Fly Ash (kg/m3) | Water (kg/m3) | Early Strength Agent (kg/m3) | Foam (L/m3) | Dry Density of Mixture (kg/m3) |
|---|---|---|---|---|---|---|
| A1-1 | 465 | 465 | 650 | 18.6 | 1200 | 816 |
| A1-2 | 310 | 620 | 650 | 18.6 | 1200 | 874 |
| A1-3 | 235 | 695 | 650 | 18.6 | 1200 | 860 |
| A1-4 | 190 | 740 | 650 | 18.6 | 1200 | 758 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Han, P.; Dong, X.; Li, X.; Bai, X.; He, B.; Niu, S.; Sun, F. Influences of High-Sulphur Fly Ash on the Properties of Lightweight Cement-Treated Materials Subjected to Sulphate Corrosion. Appl. Sci. 2020, 10, 5217. https://doi.org/10.3390/app10155217
Wang X, Han P, Dong X, Li X, Bai X, He B, Niu S, Sun F. Influences of High-Sulphur Fly Ash on the Properties of Lightweight Cement-Treated Materials Subjected to Sulphate Corrosion. Applied Sciences. 2020; 10(15):5217. https://doi.org/10.3390/app10155217
Chicago/Turabian StyleWang, Xiaoyuan, Pengju Han, Xiaoqiang Dong, Xiangyu Li, Xiaohong Bai, Bin He, Shiwei Niu, and Funan Sun. 2020. "Influences of High-Sulphur Fly Ash on the Properties of Lightweight Cement-Treated Materials Subjected to Sulphate Corrosion" Applied Sciences 10, no. 15: 5217. https://doi.org/10.3390/app10155217




