Distribution of Harmful Algal Growth-Limiting Bacteria on Artificially Introduced Ulva and Natural Macroalgal Beds
Abstract
:1. Introduction
2. Materials and Methods
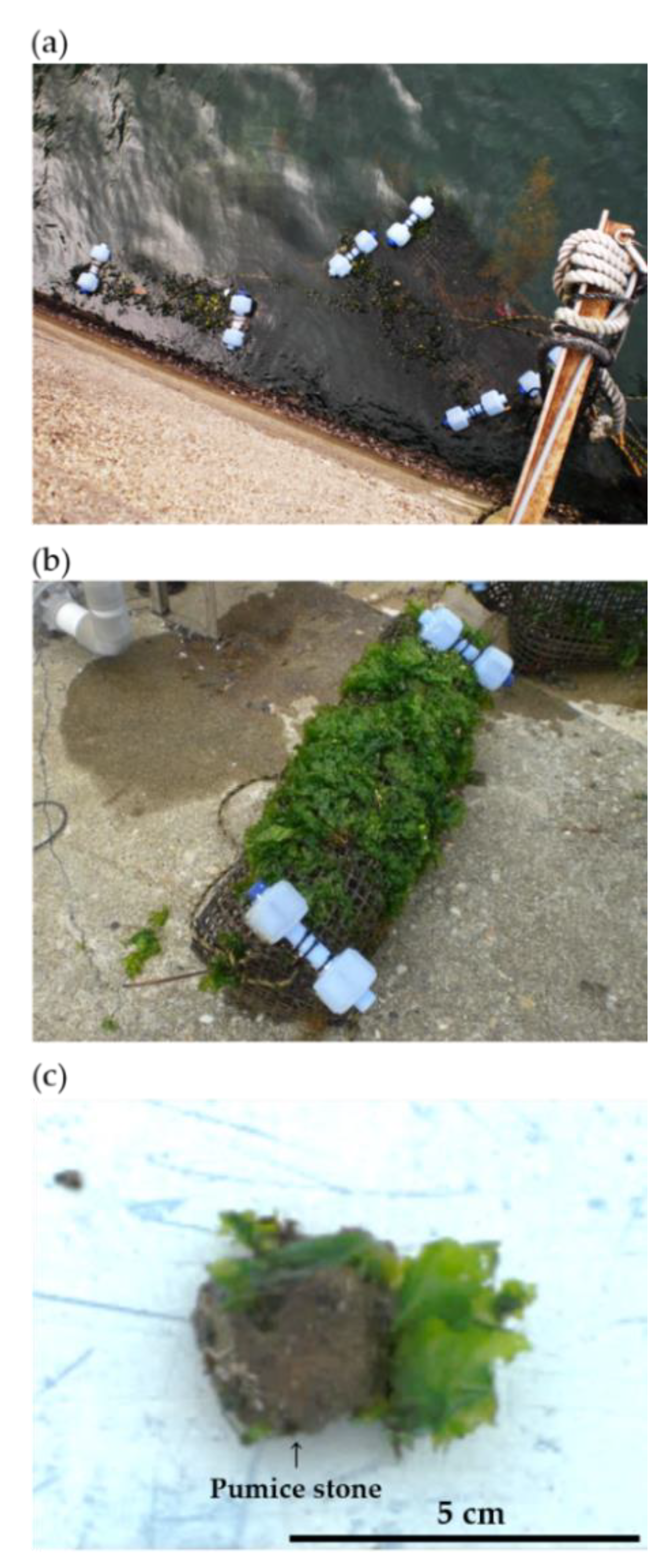
2.1. Installation of Artificial Ulva Bed
2.2. Sampling
2.3. Sample Processing and Bacterial Culturing
2.4. Targeted Harmful Algal Bloom (HAB) Species
2.5. Co-Culture Experiment Using Bacterial Isolates
2.6. 16S rRNA Gene Sequencing for Identification
3. Results
3.1. Culturable and Total Bacterial Enumeration
3.2. Density of Growth-Limiting Bacteria
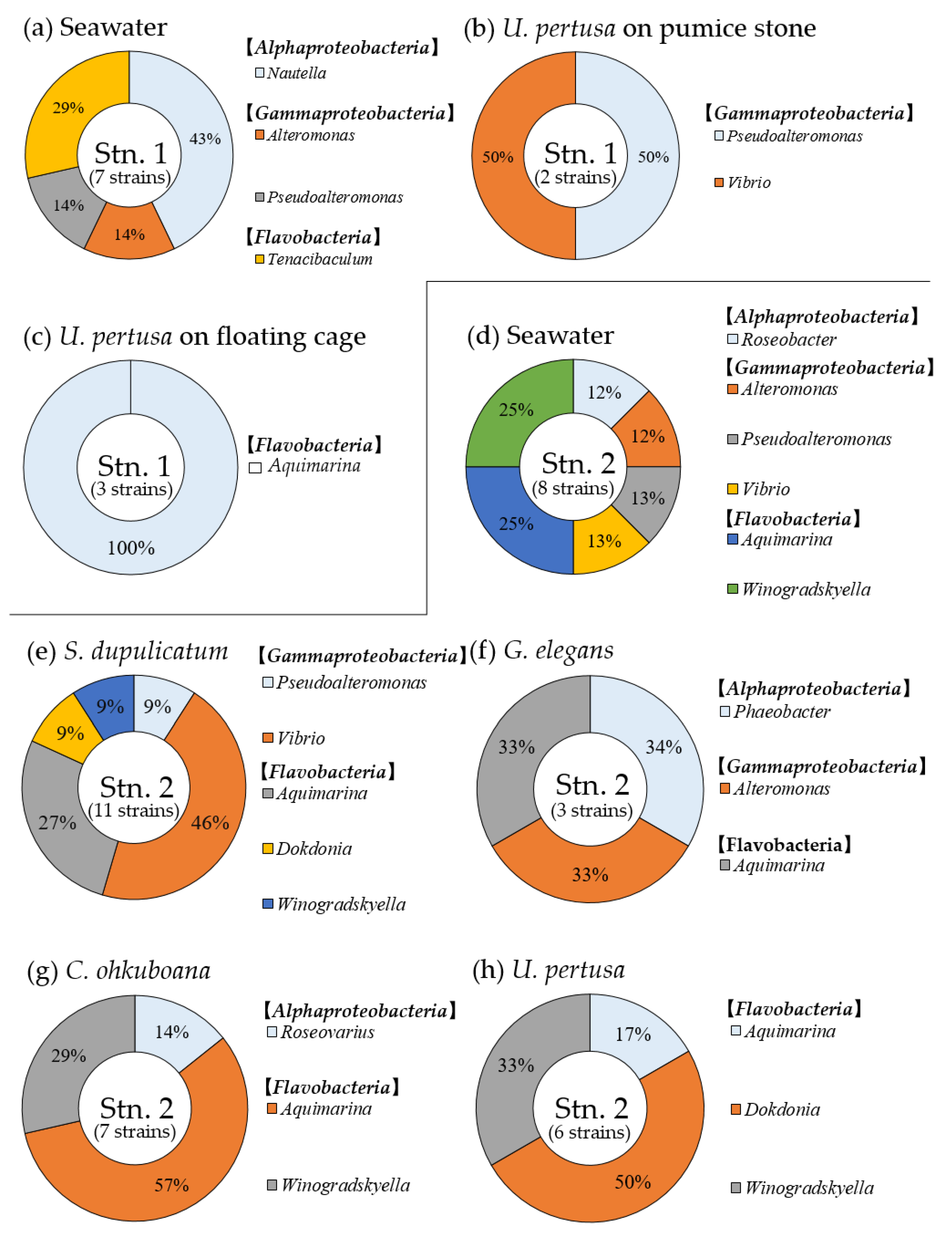
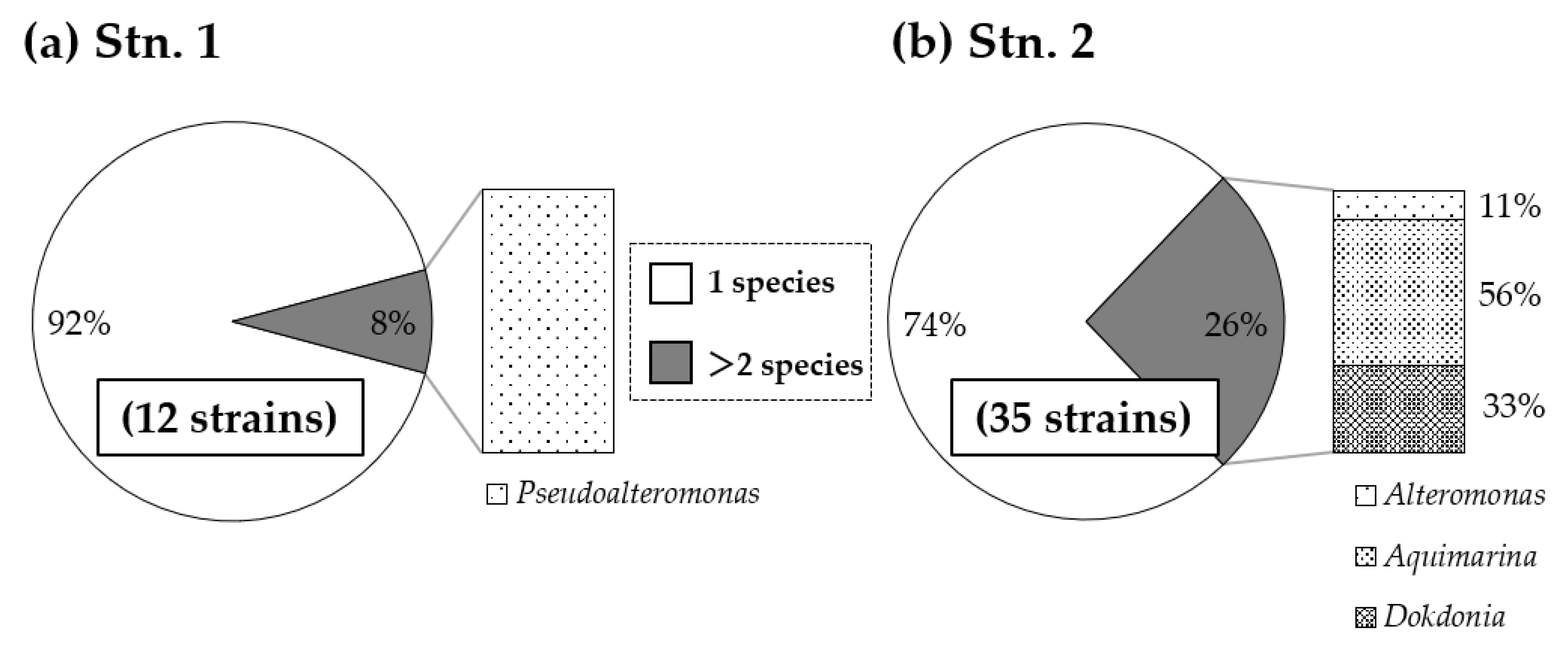
3.3. Composition of Growth-Limiting Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trainer, V.L.; Eberhart, B.-T.L.; Wekell, J.C.; Adams, N.G.; Hanson, L.; Cox, F.; Dowell, J. Paralytic shellfish toxins in Puget Sound, Washington State. J. Shellfish Res. 2003, 22, 213–223. [Google Scholar]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef] [Green Version]
- Imai, I.; Yamaguchi, M. Life cycle, physiology, ecology and red tide occurrences of the fish-killing raphidophyte Chattonella. Harmful Algae 2012, 14, 46–70. [Google Scholar] [CrossRef]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef] [Green Version]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.O.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [Green Version]
- Gobler, C.J.; Doherty, O.M.; Hattenrath-Lehmann, T.K.; Griffith, A.W.; Kang, Y.; Litaker, R.W. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proc. Natl. Acad. Sci. USA 2017, 114, 4975–4980. [Google Scholar] [CrossRef] [Green Version]
- Gobler, C.J. Climate change and harmful algal blooms: Insights and perspective. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef]
- Seger, A.; Park, T.G.; Hallegraeff, G. Assessment of the efficacy of clay flocculation in Korean fish farm waters: Cochlodinium cell removal and mitigation of ichthyotoxicity. Harmful Algae 2017, 61, 46–55. [Google Scholar] [CrossRef]
- Archambault, M.-C.; Bricelj, V.M.; Grant, J.; Anderson, D.M. Effects of clay, used to control harmful algal blooms, on juvenile Mercenaria mercenaria. J. Shellfish Res. 2002, 21, 395–396. [Google Scholar]
- Shumway, S.E.; Frank, D.M.; Ewart, L.M.; Ward, J.E. Effect of yellow loess on clearance rate in seven species of benthic, filter-feeding invertebrates. Aquac. Res. 2003, 34, 1391–1402. [Google Scholar] [CrossRef]
- Sengco, M.R.; Anderson, D.M. Controlling harmful algal blooms through clay flocculation. J. Eukaryot. Microbiol. 2004, 51, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.S.; Lee, C.K.; Park, Y.T.; Lee, Y. Effect of yellow clay on respiration and phytoplankton uptake of bivalves. Fish. Sci. 2008, 74, 120–127. [Google Scholar] [CrossRef]
- Shirota, A. Red tide problem and countermeasures. Int. J. Aquat. Fish. Technol. 1989, 1, 195–223. [Google Scholar]
- Matsuyama, Y.; Miyamoto, M.; Kotani, Y. Grazing impacts of the heterotrophic dinoflagellate Polykrikos kofoidii on a bloom of Gymnodinium catenatum. Aquat. Microb. Ecol. 1999, 17, 91–98. [Google Scholar] [CrossRef]
- Sakata, T. Control of harmful microalgae by microorganisms. In Mechanisms, Prediction, and Mitigation of Harmful Algal Blooms in Japan; Ishida, Y., Honjo, T., Fukuyo, Y., Imai, I., Eds.; The Japan Fisheries Resource Conservation Association: Tokyo, Japan, 2000; pp. 215–235. (In Japanese) [Google Scholar]
- Jeong, H.J.; Kim, J.S.; Yoo, Y.D.; Kim, S.T.; Kim, T.H.; Park, M.G.; Lee, C.H.; Seong, K.A.; Rang, N.S.; Shim, J.H. Feeding by the heterotrophic dinoflagellate Oxyrrhis marina on the red-tide raphidophyte Heterosigma akashiwo: A potential biological method to control red tides using mass-cultured grazers. J. Eukaryot. Microbiol. 2003, 50, 274–282. [Google Scholar] [CrossRef]
- Brussaard, C.P.D. Viral control of phytoplankton populations—A review. J. Eukaryot. Microbiol. 2004, 51, 125–138. [Google Scholar] [CrossRef]
- Park, M.G.; Yih, W.; Coats, D.W. Parasites and phytoplankton, with special emphasis on dinoflagellate infections. J. Eukaryot. Microbiol. 2004, 51, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Salomon, P.S.; Imai, I. Pathogens of harmful microalgae. In Ecology of Harmful Algae, Ecological Studies Vol 189; Granéli, E., Turner, J.T., Eds.; Springer: Berlin, Germany, 2006; pp. 271–282. [Google Scholar]
- Imai, I.; Kakumu, A.; Ohara, S.; Yuki, T.; Koike, K.; Hagiwara, E.; Ogawa, K.; Yoneyama, H. Feasibility studies on sediment perturbation as control strategies for Chattonella red tides. Bull. Fish. Sci. Hokkaido Univ. 2017, 67, 57–66. [Google Scholar] [CrossRef]
- Imai, I.; Kim, M.C.; Nagasaki, K.; Itakura, S.; Ishida, Y. Relationships between dynamics of red tide-causing raphidophycean flagellates and algicidal micro-organisms in the coastal sea of Japan. Phycol. Res. 1998, 46, 139–146. [Google Scholar] [CrossRef]
- Imai, I.; Sunahara, T.; Nishikawa, T.; Hori, Y.; Kondo, R.; Hiroishi, S. Fluctuations of the red tide flagellates Chattonella spp. (Raphidophyceae) and the algicidal bacterium Cytophaga sp. in the Seto Inland Sea, Japan. Mar. Biol. 2001, 138, 1043–1049. [Google Scholar] [CrossRef]
- Lovejoy, C.; Bowman, J.P.; Hallegraeff, G.M. Algicidal effects of a novel marine Pseudoalteromonas isolate (class proteobacteria gamma subdivision) on harmful algal bloom species of the genera Chattonella, Gymnodinium and Heterosigma. Appl. Environ. Microbiol. 1998, 64, 2806–2813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doucette, G.J.; Kodama, M.; Franca, S.; Gallacher, S. Bacterial interactions with harmful algal bloom species: Bloom ecology, toxigenesis and cytology. In Physiological Ecology of Harmful Algal Blooms, NATO ASI Series; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer: Berlin, Germany, 1998; Volume G41, pp. 619–647. [Google Scholar]
- Doucette, G.J.; McGovern, E.R.; Babinchak, J.A. Algicidal bacteria active against Gymnodinium breve (Dinophyceae). I. Bacterial isolation and characterization of killing activity. J. Phycol. 1999, 35, 1447–1454. [Google Scholar] [CrossRef]
- Skerratt, J.H.; Bowman, J.P.; Hallegraeff, G.; James, S.; Nichols, P.D. Algicidal bacteria associated with blooms of a toxic dinoflagellate in a temperate Australian estuary. Mar. Ecol. Prog. Ser. 2002, 244, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mayali, X.; Azam, F. Algicidal bacteria in the sea and their impact on algal blooms. J. Eukaryot. Microbiol. 2004, 51, 139–144. [Google Scholar] [CrossRef]
- Park, J.H.; Yoshinaga, I.; Nishikawa, T.; Imai, I. Algicidal bacteria in particle-associated form and in free-living form during a diatom bloom in the Seto Inland Sea, Japan. Aquat. Microb. Ecol. 2010, 60, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Paul, C.; Pohnert, G. Interactions of the algicidal bacterium Kordia algicida with diatoms: Regulated protease excretion for specific algal lysis. PLoS ONE 2011, 6, e21032. [Google Scholar] [CrossRef]
- Pokrzywinski, K.L.; Place, A.R.; Warner, M.E.; Coyne, K.J. Investigation of the algicidal exudate produced by Shewanella sp. IRI-160 and its effect on dinoflagellates. Harmful Algae 2012, 19, 23–29. [Google Scholar] [CrossRef]
- Inaba, N.; Trainer, V.L.; Nagai, S.; Kojima, S.; Sakami, T.; Takagi, S.; Imai, I. Dynamics of seagrass bed microbial communities used to control artificial Chattonella blooms: A microcosm study. Harmful Algae 2019, 84, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Sanders, N.J.; Gotelli, N.J.; Heller, N.E.; Gordon, D.M. Community disassembly by an invasive species. Proc. Natl. Acad. Sci. USA 2003, 100, 2474–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secord, D. Biological control of marine invasive species: Cautionary tales and land-based lessons. Biol. Invasions 2003, 5, 117–131. [Google Scholar] [CrossRef]
- Imai, I.; Fujimaru, D.; Nishigaki, T. Co-culture of fish with macroalgae and associated bacteria: A possible mitigation strategy for noxious red tides in enclosed coastal sea. Fish. Sci. 2002, 68 (Suppl. S1), 493–496. [Google Scholar] [CrossRef] [Green Version]
- Imai, I.; Yamamoto, T.; Ishii, K.I.; Yamamoto, K. Promising prevention strategies for harmful red tides by seagrass beds as enormous sources of algicidal bacteria. In Proceedings of the 5th World Fisheries Congress, Tokyo, Japan, 13 December 2009. 6C_0995_133. [Google Scholar]
- Onishi, Y.; Mohri, Y.; Tuji, A.; Ohgi, K.; Yamaguchi, A.; Imai, I. The seagrass Zostera marina harbors growth-inhibiting bacteria against the toxic dinoflagellate Alexandrium tamarense. Fish. Sci. 2014, 80, 353–362. [Google Scholar] [CrossRef]
- Inaba, N.; Trainer, V.L.; Onishi, Y.; Ishii, K.; Wyllie-Echeverria, S.; Imai, I. Algicidal and growth-inhibiting bacteria associated with seagrass and macroalgae beds in Puget Sounds, WA, USA. Harmful Algae 2017, 62, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Sakami, T.; Sakamoto, S.; Takagi, S.; Inaba, N.; Imai, I. Distribution of three algicidal Alteromonas sp. strains in seagrass beds and surrounding areas in the Seto Inland Sea, Japan. Fish. Sci. 2017, 83, 113–121. [Google Scholar] [CrossRef]
- Green, E.P.; Short, F.T. World Atlas of Seagrasses; University of California Press: Berkeley, CA, USA, 2003; p. 324. [Google Scholar]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; p. 551. [Google Scholar] [CrossRef]
- Yamada, Y.; Saito, E. On Some Culture Experiments with the Swarmers of Certain Species belonging to the Ulvaceae; Scientific Papers of the Institute of Algological Research; Faculty of Science, Hokkaido Imperial University: Hokkaido, Japan, 1938; Volume 2, pp. 35–51. [Google Scholar]
- Mori, S.; Hidaka, K.; Ushirokawa, T. Aratana Kaiso No Saibyokishitsu No Kento. Bull. Fukuoka Fish. Mar. Technol. Res. Cent. 2017, 27, 19–26. (In Japanese) [Google Scholar]
- Yoshinaga, I.; Kawai, T.; Ishida, Y. Analysis of algicidal ranges of the bacteria killing the marine dinoflagellates Gymnodinium mikimotoi isolated from Tanabe Bay, Wakayama Pref., Japan. Fish. Sci. 1997, 63, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Inaba, N.; Watanabe, T.; Sakami, T.; Nishi, H.; Tahara, Y.; Imai, I. Temporal and spatial distribution of algicidal and growth-inhibiting bacteria in the coastal sea of southwest Japan. J. Plankton Res. 2014, 36, 388–397. [Google Scholar] [CrossRef] [Green Version]
- Porter, K.G.; Feig, Y.S. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980, 25, 943–948. [Google Scholar] [CrossRef]
- Chen, L.C.M.; Edelstein, T.; McLachlan, J. Bonnemaisonia hamifera Hariot in nature and in culture. J. Phycol. 1969, 5, 211–220. [Google Scholar] [CrossRef]
- Imai, I.; Itakura, S.; Matsuyama, Y.; Yamaguchi, M. Selenium requirement for growth of a novel red tide flagellate Chattonella verruculosa (Raphidophyceae) in culture. Fish. Sci. 1996, 62, 834–835. [Google Scholar] [CrossRef] [Green Version]
- Holt, J.G.; Krieg, N.R. Enrichment and isolation. In Methods for General and Molecular Bacteriology; Gerhardt, P., Murray, R.G.E., Hood, W.A., Krieg, N.R., Eds.; ASM Press: Washington, DC, USA, 1994; pp. 179–215. [Google Scholar]
- Delong, E.F. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 1992, 89, 5685–5689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; Jon Wiley & Sons: Chichester, UK, 1991; pp. 115–176. [Google Scholar]
- Imai, I. Interactions between harmful algae and algicidal bacteria associated with seaweeds and seagrasses. In Marine Protists; Ohtsuka, S., Suzuki, N., Horiguchi, T., Eds.; Springer: Tokyo, Japan, 2015; pp. 597–619. [Google Scholar]
- Middelboe, A.L.; Sand-Jensen, K. Long-term changes in macroalgal communities in a Danish estuary. Phycologia 2000, 39, 245–257. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L., Jr.; Hughes, A.R.; et al. Accelerating loss of seagrass across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2019, 106, 12377–12381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filbee-Dexter, K.; Wernberg, T. Rise of turfs: A new battlefront for globally declining kelp forests. Bioscience 2018, 68, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Kemp, W.M.; Boynton, W.R.; Adolf, J.E.; Boesch, D.F.; Boicourt, W.C.; Brush, G.; Cornwell, J.C.; Fisher, T.R.; Glibert, P.M.; Hagy, J.D.; et al. Eutrophication of Chesapeake Bay: Historical trends and ecological interactions. Mar. Ecol. Prog. Ser. 2005, 303, 1–29. [Google Scholar] [CrossRef]
- Abdenadher, M.; Hamza, A.; Fekih, W.; Hannachi, I.; Bellaaj, A.Z.; Bradai, M.N.; Aleya, L. Factors determining the dynamics of toxic blooms of Alexandrium minutum during a 10-year study along the shallow southwestern Mediterranean coasts. Estuar. Coast. Shelf. Sci. 2012, 106, 102–111. [Google Scholar] [CrossRef]
- Tanaka, T.; Furukawa, K.; Kuwae, T.; Imai, I. Report of the 32nd Joint symposium of Laiaison council of Academic societies on coastal environment “Road of Zostera bed restoration in Japanese coastal zones” -Past processes and the future prospects. Nippon Suisan Gakkaishi 2017, 83, 1042–1053. [Google Scholar] [CrossRef] [Green Version]
- Tamburello, L.; Papa, L.; Guarnieri, G.; Basconi, L.; Zampardi, S.; Scipione, M.B.; Terlizzi, A.; Zupo, V.; Fraschetti, S. Are we ready for scaling up restoration actions? An insight from Mediterranean macroalgal canopies. PLoS ONE 2019, 14, e0224477. [Google Scholar] [CrossRef]
- Anderson, C.R.; Sellner, K.G.; Anderson, D.M. Bloom prevention and control. In Harmful Algal Blooms (HABs) and Desalination: A Guide to Impacts, Monitoring, and Management; Anderson, D.M., Boerlage, S.F.E., Dixon, M.B., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2017; pp. 205–222. [Google Scholar]
- Pedersen, M.F.; Borum, J. Nutrient control of algal growth in estuarine waters: Nutrient limitation and the importance of nitrogen requirements and nitrogen storage among phytoplankton and species of macroalgae. Mar. Ecol. Prog. Ser. 1996, 142, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, M.F.; Borum, J.; Fotel, F.L. Phosphorus dynamics and limitation of fast- and slow-growing temperate seaweeds in Oslofjord, Norway. Mar. Ecol. Prog. Ser. 2010, 399, 103–115. [Google Scholar] [CrossRef]
- Hirata, H.; Kohirata, E.; Guo, F.; Xu, B.T.; Danakusumah, E. Culture of the sterile Ulva sp. (Chlorophyceae) in a mariculture farm. Suisan Zoshoku 1993, 41, 541–545. [Google Scholar]
- Neori, A.; Krom, M.D.; Ellner, S.P.; Boyd, C.E.; Popper, D.; Rabinovitch, R.; Davison, P.J.; Dvir, O.; Zuber, D.; Ucko, M.; et al. Seaweed biofilters as regulators of water quality in integrated fish-seaweed culture units. Aquaculture 1996, 141, 183–199. [Google Scholar] [CrossRef]
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.P.; Halling, C.; Shpigel, M.; Yarish, C. Integrated aquaculture: Rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Z.; Song, X.; Tang, X.; Zhang, S. Effects of macroalgae Ulva pertusa (Chlorophyta) and Gracilaria lemaneiformis (Rhodophyta) on growth of four species of bloom-forming dinoflagellates. Aquat. Bot. 2007, 86, 139–147. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Gobler, C.J. The green macroalga, Ulva lactuca, inhibits the growth of seven common harmful algal bloom species via allelopathy. Harmful Algae 2011, 10, 480–488. [Google Scholar] [CrossRef]
- Bolton, J.J.; Robertson-Andersson, D.V.; Shuuluka, D.; Kandjengo, L. Growing Ulva (Chlorophyta) in integrated systems as a commercial crop for abalone feed in South Africa: A SWOT analysis. J. Appl. Phycol. 2008, 21, 575–583. [Google Scholar] [CrossRef]
- Kawashima, Y.; Akasaki, T.; Matsumoto, Y.; Yamazaki, Y.; Shimada, S. Species identification of imported and Japanese commercial green algal products based on phylogenetic analyses using the nrITS2 and 5S rDNA spacer regions. Fish. Sci. 2013, 79, 521–529. [Google Scholar] [CrossRef]
- Msuya, F.E.; Kyewalyanga, M.S.; Salum, D. The performance of the seaweed Ulva reticulata as a biofilter in a low-tech, low-cost, gravity generated water regime in Zanzibar, Tanzania. Aquaculture 2006, 254, 284–292. [Google Scholar] [CrossRef]
- Duggins, D.O.; Eckman, J.E.; Sewell, A.T. Ecology of understory kelp environments. II. Effects of kelps on recruitment of benthic invertebrates. J. Exp. Mar. Biol. Ecol. 1990, 143, 27–45. [Google Scholar] [CrossRef]
- Leclerc, J.C.; Riera, P.; Leroux, C.; Lévêque, L.; Davoult, D. Temporal variation in organic matter supply in kelp forests: Linking structure to trophic functioning. Mar. Ecol. Prog. Ser. 2013, 494, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Campbell, I.; Macleod, A.; Sahlmann, C.; Neves, L.; Funderud, J.; Øverland, M.; Hughes, A.D.; Stanley, M.S. The environmental risks associated with the development of seaweed farming in Europe—prioritizing key knowledge gaps. Front. Mar. Sci. 2019, 6, 107. [Google Scholar] [CrossRef]
- Smith, D.C.; Simon, M.; Alldredge, A.L.; Azam, F. Intense hydrolytic enzyme activity on marine aggregates and implications for rapid particle dissolution. Nature 1992, 359, 139–142. [Google Scholar] [CrossRef]
- Bidle, K.D.; Azam, F. Accelerated dissolution of diatom silica by marine bacterial assemblages. Nature 1999, 397, 508–512. [Google Scholar] [CrossRef]
- Teeling, H.; Fuchs, B.; Becher, D.; Klockow, C.; Gardebrecht, A.; Bennke, C.; Kassabgy, M.; Huang, S.; Mann, A.; Waldmann, J.; et al. Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science 2012, 336, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Kirchman, D. The ecology of Cytophaga-Flavobacteria in aquatic environments. FEMS Microbiol. Ecol. 2002, 39, 91–100. [Google Scholar] [CrossRef]
- Matsuo, Y.; Imagawa, H.; Nishizawa, M.; Shizuri, Y. Isolation of an algal morphogenesis inducer from a marine bacterium. Science 2005, 307, 1598. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.; Joint, I.; Callow, M.E. Effect of marine bacterial isolates on the growth and morphology of axenic plantlets of the green alga Ulva linza. Microb. Ecol. 2006, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Callow, M.E.; Joint, I.; Callow, J.A. Specificity in the settlement-modifying response of bacterial biofilms towards zoospores of the marine alga Enteromorpha. Environ. Microbiol. 2003, 5, 338–349. [Google Scholar] [CrossRef]
- Matsuo, Y.; Suzuki, M.; Kasai, H.; Shizuri, Y.; Harayama, S. Isolation and phylogenetic characterization of bacteria capable of inducing differentiation in the green alga Monostroma oxyspermum. Environ. Microbiol. 2003, 5, 25–35. [Google Scholar] [CrossRef]
- Zozaya-Valdes, E.; Egan, S.; Thomas, T. A comprehensive analysis of the microbial communities of healthy and diseased marine macroalgae and the detection of known and potential bacterial pathogens. Front. Microbiol. 2015, 6, 146. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Lu, G.; Zheng, Y.; Xie, W.; Li, S.; Hu, Z. Aquimarina agarilytica sp. nov., agarolytic species isolated from a red alga. Int. J. Syst. Evol. Microbiol. 2012, 62, 869–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beleneva, I.A.; Zhukova, N.V. Bacterial communities of some brown and red algae from Peter the Great Bay, the Sea of Japan. Microbiology 2006, 75, 348–357. [Google Scholar] [CrossRef]
- Crump, B.C.; Koch, E.K. Attached bacterial populations shared by four species of aquatic angiosperms. Appl. Environ. Microbiol. 2008, 74, 5948–5957. [Google Scholar] [CrossRef] [PubMed] [Green Version]


 ) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
 ) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
 ) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
 ) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.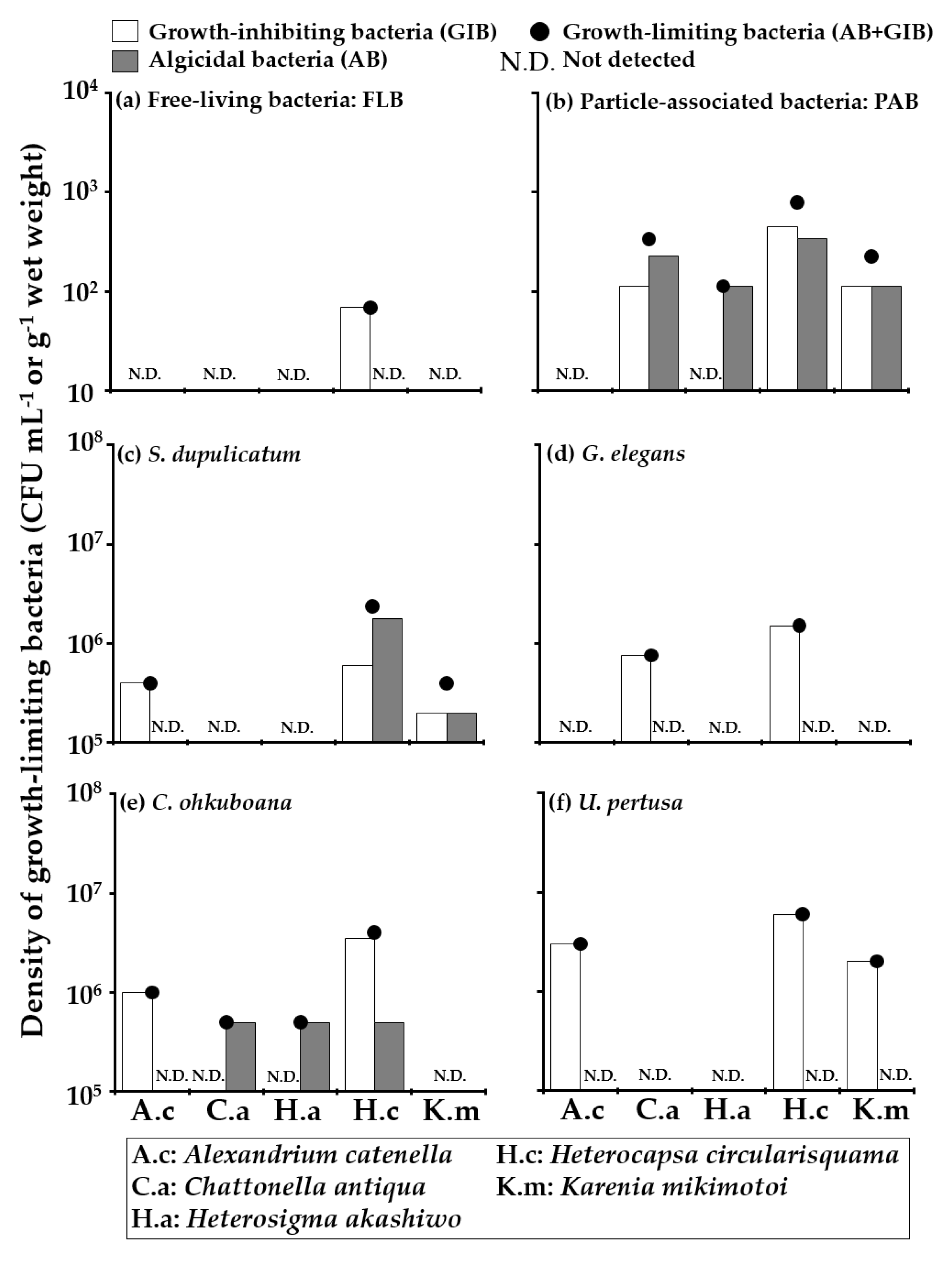
| Date | Station | Sample Name | Sample Type | Culturable Bacterial Density (CFU mL−1 or g−1 Wet Weight) | Total Bacterial Density (Cells mL−1 or g−1 Wet Weight) |
|---|---|---|---|---|---|
| 2017/9/7 | Stn. 1 | Seawater | FLB | 1.7 × 103 | 3.1 × 105 |
| PAB | 1.0 × 103 | 2.6 × 106 | |||
| Ulva pertusa on pumice stone | Biofilm | 2.4 × 108 | 2.7 × 109 | ||
| U. pertusa on floating cage | Biofilm | 1.9 × 107 | 1.7 × 109 | ||
| 2017/9/8 | Stn. 2 | Seawater | FLB | 2.3 × 103 | 2.8 × 105 |
| PAB | 1.8 × 103 | 3.9 × 105 | |||
| Sargassum dupulicatum | Biofilm | 7.9 × 106 | 3.9 × 108 | ||
| Gelidium elegans | Biofilm | 1.5 × 107 | 6.0 × 108 | ||
| U. pertusa | Biofilm | 4.0 × 107 | 1.2 × 109 | ||
| Cladophora ohkuboana | Biofilm | 2.0 × 107 | 9.9 × 108 |
| Date | Station | Sample Name | Sample Type | The Density of GLB against Five Different HAB Species (CFU mL−1 or g−1 Wet Weight) | ||||
|---|---|---|---|---|---|---|---|---|
| Alexandrium catenella | Chattonella antiqua | Heterosigma akashiwo | Heterocapsa circularisquama | Karenia mikimotoi | ||||
| 2017/9/7 | Stn. 1 | Seawater | FLB | ― | 3.5 × 102 | ― | ― | ― |
| PAB | ― | 1.9 × 102 | ― | 1.9 × 102 | 93 | |||
| Ulva pertusa on pumice stone | Biofilm | ― | 5.9 × 106 | ― | 5.9 × 106 | ― | ||
| U. pertusa on floating cage | Biofilm | ― | ― | ― | 1.5 × 106 | ― | ||
| 2017/9/8 | Stn. 2 | Seawater | FLB | ― | ― | ― | 69 | ― |
| PAB | ― | 3.4 × 102 | 1.1 × 102 | 7.9 × 102 | 2.3 × 102 | |||
| Sargassum dupulicatum | Biofilm | 4.0 × 105 | ― | ― | 2.4 × 106 | 4.0 × 105 | ||
| Gelidium elegans | Biofilm | ― | 7.6 × 105 | ― | 1.5 × 106 | ― | ||
| Cladophora ohkuboana | Biofilm | 1.0 × 106 | 5.0 × 105 | 5.0 × 105 | 4.0 × 106 | ― | ||
| U. pertusa | Biofilm | 3.0 × 106 | ― | ― | 6.0 × 106 | 2.0 × 106 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inaba, N.; Kodama, I.; Nagai, S.; Shiraishi, T.; Matsuno, K.; Yamaguchi, A.; Imai, I. Distribution of Harmful Algal Growth-Limiting Bacteria on Artificially Introduced Ulva and Natural Macroalgal Beds. Appl. Sci. 2020, 10, 5658. https://doi.org/10.3390/app10165658
Inaba N, Kodama I, Nagai S, Shiraishi T, Matsuno K, Yamaguchi A, Imai I. Distribution of Harmful Algal Growth-Limiting Bacteria on Artificially Introduced Ulva and Natural Macroalgal Beds. Applied Sciences. 2020; 10(16):5658. https://doi.org/10.3390/app10165658
Chicago/Turabian StyleInaba, Nobuharu, Isamu Kodama, Satoshi Nagai, Tomotaka Shiraishi, Kohei Matsuno, Atsushi Yamaguchi, and Ichiro Imai. 2020. "Distribution of Harmful Algal Growth-Limiting Bacteria on Artificially Introduced Ulva and Natural Macroalgal Beds" Applied Sciences 10, no. 16: 5658. https://doi.org/10.3390/app10165658






