Catalytic Performance of SBA-15-Supported Poly (Styrenesulfonic Acid) in the Esterification of Acetic Acid with n-Heptanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Catalyst
2.3. Characterization Techniques
2.4. Catalyst Performance Test
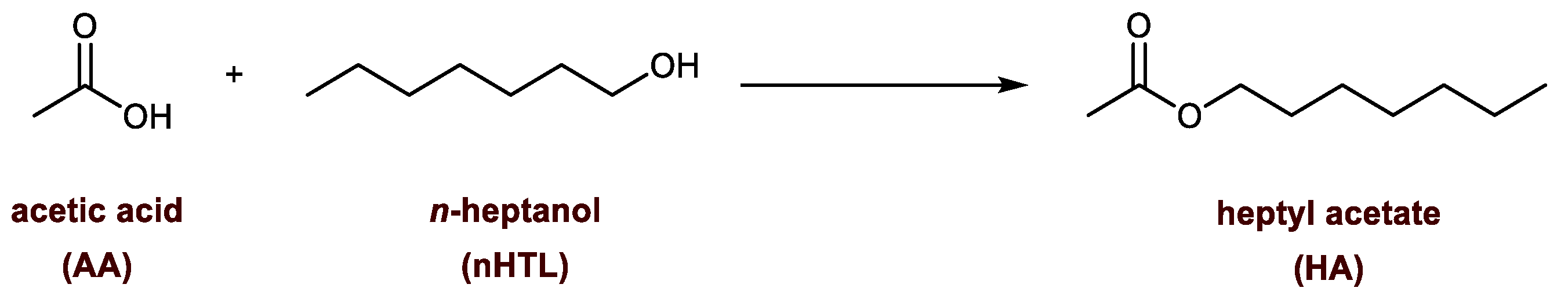
2.4.1. Esterification of AA
2.4.2. Calculations
2.5. Catalyst Reusability
3. Results
3.1. Characterization
3.1.1. TEM Analysis
3.1.2. FTIR Analysis
3.1.3. Thermal Analysis
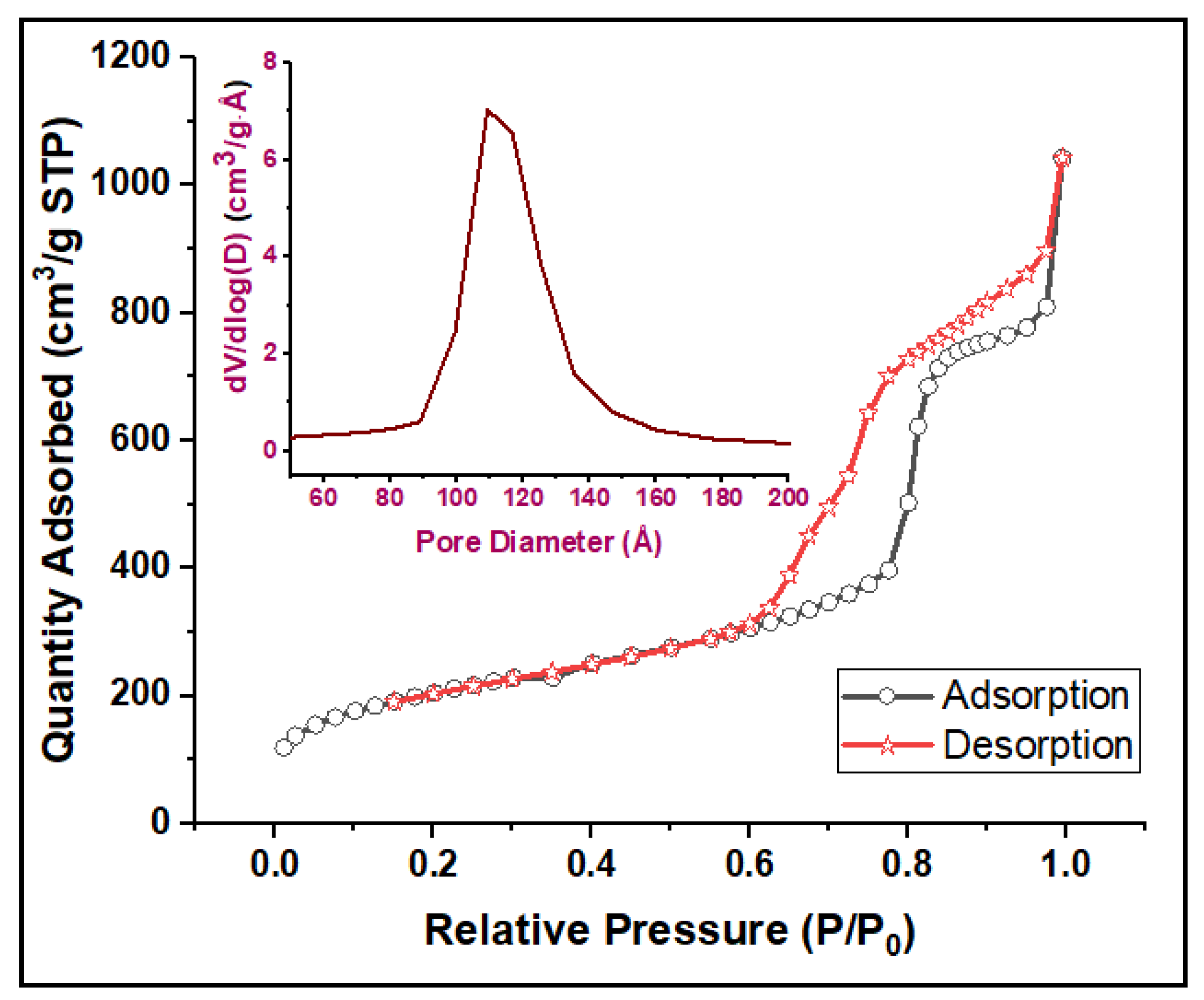
3.1.4. Nitrogen Adsorption Isotherms
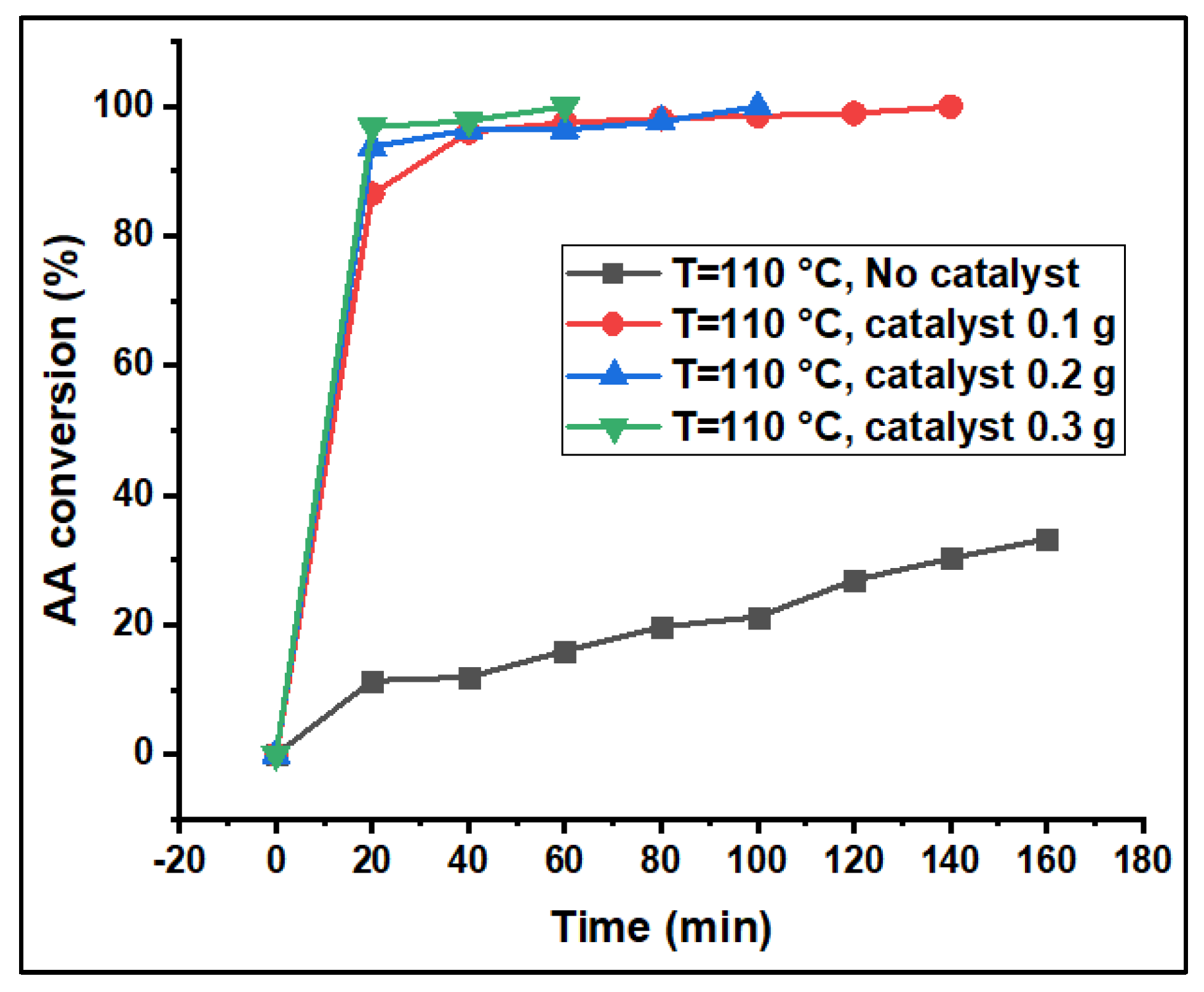
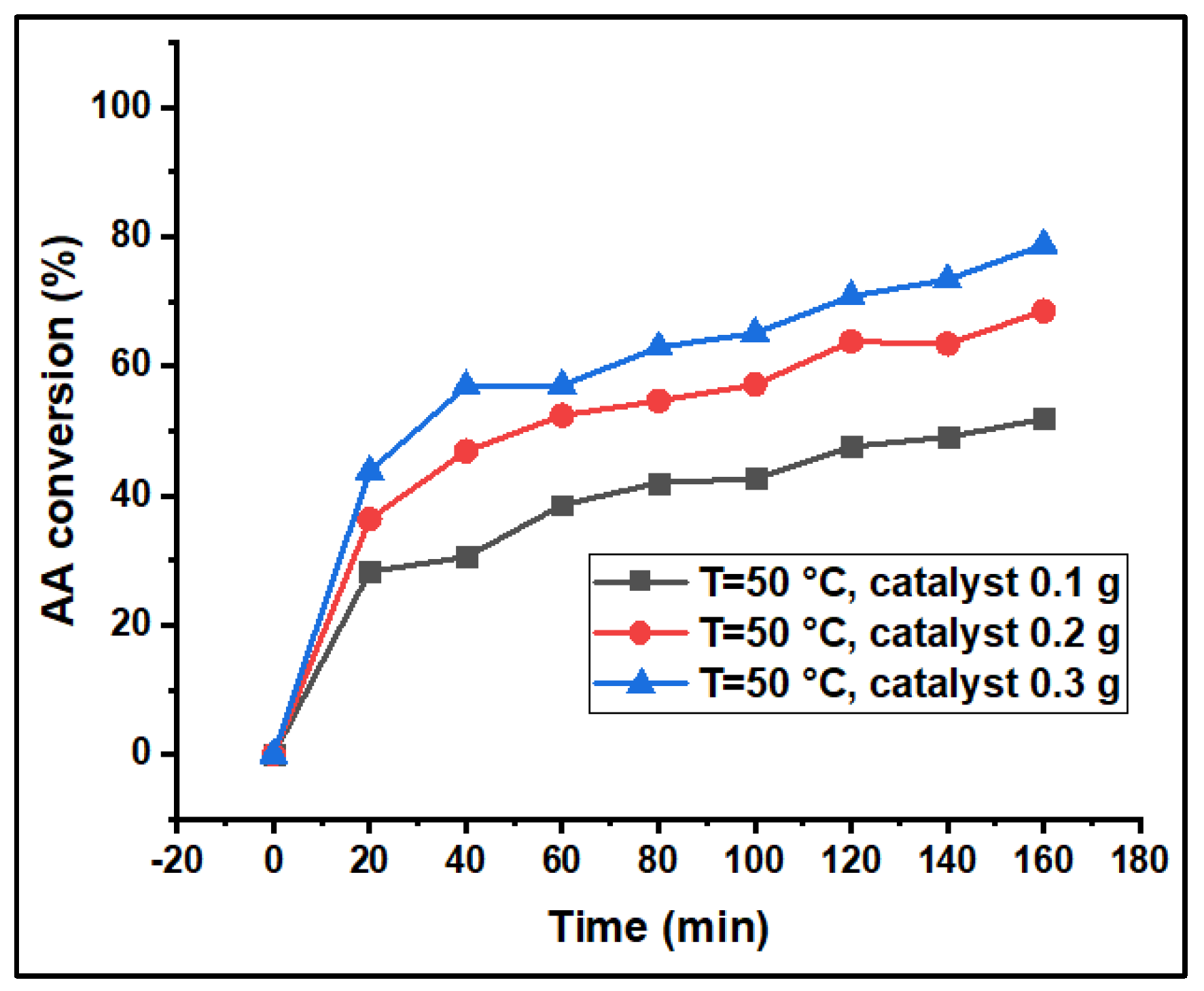
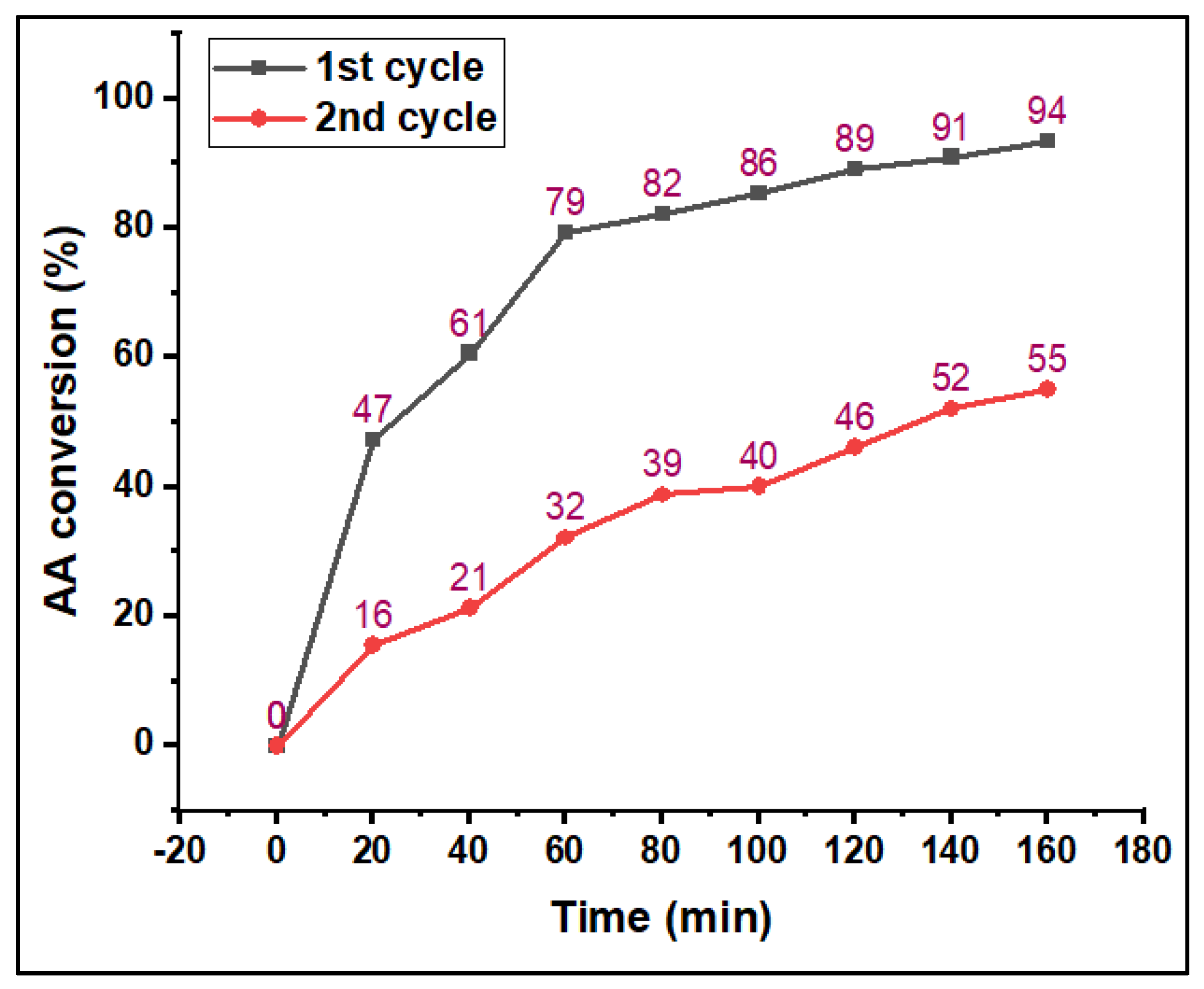
3.2. Catalytic Performance Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Long, W.; Jones, C.W. Hybrid sulfonic acid catalysts based on silica-supported poly (styrene sulfonic acid) brush materials and their application in ester hydrolysis. ACS Catal. 2011, 1, 674–681. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Wang, P.; Liu, J.; Yang, Q. An efficient solid acid catalyst: Poly-p-styrenesulfonic acid supported on SBA-15 via surface-initiated ATRP. Microporous Mesoporous Mater. 2009, 123, 228–233. [Google Scholar] [CrossRef]
- Dhainaut, J.; Dacquin, J.-P.; Lee, A.F.; Wilson, K. Hierarchical macroporous–mesoporous SBA-15 sulfonic acid catalysts for biodiesel synthesis. Green Chem. 2010, 12, 296–303. [Google Scholar] [CrossRef]
- Lourenço, J.P.; Macedo, M.I.; Fernandes, A. Sulfonic-functionalized SBA-15 as an active catalyst for the gas-phase dehydration of glycerol. Catal. Commun. 2012, 19, 105–109. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Lashgari, N.; Badiei, A. Sulfonic acid-functionalized mesoporous silica (SBA-Pr-SO3H) as solid acid catalyst in organic reactions. J. Mol. Catal. A Chem. 2015, 397, 166–191. [Google Scholar] [CrossRef]
- Melero, J.; Morales, G.; Iglesias, J.; Paniagua, M.; Hernández, B.; Penedo, S. Efficient conversion of levulinic acid into alkyl levulinates catalyzed by sulfonic mesostructured silicas. Appl. Catal. A Gen. 2013, 466, 116–122. [Google Scholar] [CrossRef]
- Pirez, C.; Caderon, J.-M.; Dacquin, J.-P.; Lee, A.F.; Wilson, K. Tunable KIT-6 mesoporous sulfonic acid catalysts for fatty acid esterification. ACS Catal. 2012, 2, 1607–1614. [Google Scholar] [CrossRef]
- Mbaraka, I.K.; Radu, D.R.; Lin, V.S.-Y.; Shanks, B.H. Organosulfonic acid-functionalized mesoporous silicas for the esterification of fatty acid. J. Catal. 2003, 219, 329–336. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef]
- Martin, A.; Morales, G.; Martinez, F.; Van Grieken, R.; Cao, L.; Kruk, M. Acid hybrid catalysts from poly (styrenesulfonic acid) grafted onto ultra-large-pore SBA-15 silica using atom transfer radical polymerization. J. Mater. Chem. 2010, 20, 8026–8035. [Google Scholar] [CrossRef]
- Liu, F.; Kong, W.; Qi, C.; Zhu, L.; Xiao, F.-S. Design and synthesis of mesoporous polymer-based solid acid catalysts with excellent hydrophobicity and extraordinary catalytic activity. ACS Catal. 2012, 2, 565–572. [Google Scholar] [CrossRef]
- Aboelhassan, M.M.; Peixoto, A.F.; Freire, C. Sulfonic acid functionalized silica nanoparticles as catalysts for the esterification of linoleic acid. New J. Chem. 2017, 41, 3595–3605. [Google Scholar] [CrossRef]
- Xue, Z.; Shang, H.; Xiong, C.; Lu, C.; An, G.; Zhang, Z.; Cui, C.; Xu, M. Synthesis of polyoxymethylene dimethyl ethers catalyzed by sulfonic acid-functionalized mesoporous SBA-15. RSC Adv. 2017, 7, 20300–20308. [Google Scholar] [CrossRef]
- Mendiratta, S.; Ali, A.A.A. Recent Advances in Functionalized Mesoporous Silica Frameworks for Efficient Desulfurization of Fuels. Nanomaterials 2020, 10, 1116. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yu, C.; Lei, J.; Zhang, Q.; Li, T.; Tu, B.; Zhou, W.; Zhao, D. Low-temperature strategy to synthesize highly ordered mesoporous silicas with very large pores. J. Am. Chem. Soc. 2005, 127, 10794–10795. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Kruk, M. Grafting of polymer brushes from nanopore surface via atom transfer radical polymerization with activators regenerated by electron transfer. Polym. Chem. 2010, 1, 97–101. [Google Scholar] [CrossRef]
- Moreno, J.; Sherrington, D. Well-Defined Mesostructured Organic− Inorganic Hybrid Materials via Atom Transfer Radical Grafting of Oligomethacrylates onto SBA-15 Pore Surfaces. Chem. Mater. 2008, 20, 4468–4474. [Google Scholar] [CrossRef]
- Kruk, M.; Hui, C.M. Synthesis and characterization of large-pore FDU-12 silica. Microporous Mesoporous Mater. 2008, 114, 64–73. [Google Scholar] [CrossRef]
- Yamamoto, E.; Mori, S.; Shimojima, A.; Wada, H.; Kuroda, K. Fabrication of colloidal crystals composed of pore-expanded mesoporous silica nanoparticles prepared by a controlled growth method. Nanoscale 2017, 9, 2464–2470. [Google Scholar] [CrossRef]
- Kruk, M.; Dufour, B.; Celer, E.B.; Kowalewski, T.; Jaroniec, M.; Matyjaszewski, K. Synthesis of mesoporous carbons using ordered and disordered mesoporous silica templates and polyacrylonitrile as carbon precursor. J. Phys. Chem. B 2005, 109, 9216–9225. [Google Scholar] [CrossRef]
- Savin, D.; Pyun, J.; Patterson, G.; Kowalewski, T.; Matyjaszewski, K.J. Synthesis and characterization of silica-graft-polystyrene hybrid nanoparticles: Effect of constraint on the glass-transition temperature of spherical polymer brushes. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2667–2676. [Google Scholar] [CrossRef]
- Ayoub, M.; Abdullah, A.Z. Instability of SBA-15 to strong base: Effects of LiOH impregnation on its surface characteristics and mesoporous structure. J. Appl. Sci. 2011, 11, 3510–3514. [Google Scholar] [CrossRef]
- Shawky, S.; Abo-AlHassan, A.; Lill, H.; Bald, D.; EL-Khamisy, S. Efficient Loading and Encapsulation of Anti-Tuberculosis Drugs using Multifunctional Mesoporous Silicate Nanoparticles Running Title: Mesoporous Silicate Nanoparticles as Smart Drug Delivery System. J. Nanosci. Curr. Res. 2016, 1, 1000103. [Google Scholar]
- Kokunešoski, M.; Gulicovski, J.; Matović, B.; Logar, M.; Milonjić, S.K.; Babić, B. Synthesis and surface characterization of ordered mesoporous silica SBA-15. Mater. Chem. Phys. 2010, 124, 1248–1252. [Google Scholar] [CrossRef]
- Lee, B.; Kim, Y.; Lee, H.; Yi, J. Synthesis of functionalized porous silicas via templating method as heavy metal ion adsorbents: The introduction of surface hydrophilicity onto the surface of adsorbents. Microporous Mesoporous Mater. 2001, 50, 77–90. [Google Scholar] [CrossRef]
- Dua’a, M.M.; Zughul, M.B.; Taha, M.O.; Hodali, H.A. Effect of particle morphology and pore size on the release kinetics of ephedrine from mesoporous MCM-41 materials. J. Porous Mater. 2012, 19, 825–833. [Google Scholar]
- Jin, Q.; Qu, F.; Jiang, J.; Dong, Y.; Guo, W.; Lin, H. A pH-sensitive controlled dual-drug release from meso-macroporous silica/multilayer-polyelectrolytes coated SBA-15 composites. J. Sol-Gel Sci. Technol. 2013, 66, 466–471. [Google Scholar] [CrossRef]
- Andrade, G.F.; Soares, D.C.F.; Almeida, R.K.d.S.; Sousa, E.M.B. Mesoporous silica SBA-16 functionalized with alkoxysilane groups: Preparation, characterization, and release profile study. J. Nanomater. 2012, 77, 186–204. [Google Scholar] [CrossRef]
- Poh, N.E.; Nur, H.; Muhid, M.N.M.; Hamdan, H. Sulphated AlMCM-41: Mesoporous solid Brønsted acid catalyst for dibenzoylation of biphenyl. Catal. Today 2006, 114, 257–262. [Google Scholar] [CrossRef]
- Anunziata, O.A.; Martínez, M.L.; Beltramone, A.R. Hydroxyapatite/MCM-41 and SBA-15 nano-composites: Preparation, characterization and applications. Materials 2009, 2, 1508–1519. [Google Scholar] [CrossRef]
- Xu, D.; Yang, L.; Wang, Y.; Wang, G.; Rensing, C.; Zheng, S. Proteins enriched in charged amino acids control the formation and stabilization of selenium nanoparticles in Comamonas testosteroni S44. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Umar, A.; Du, Y.; Wang, L.; Pei, M. Surface Modification of Bentonite with Polymer Brushes and Its Application as an Efficient Adsorbent for the Removal of Hazardous Dye Orange I. Nanomaterials 2020, 10, 1112. [Google Scholar] [CrossRef] [PubMed]
- Brijmohan, S.B.; Swier, S.; Weiss, R.; Shaw, M.T. Synthesis and characterization of cross-linked sulfonated polystyrene nanoparticles. Ind. Eng. Chem. Res. 2005, 44, 8039–8045. [Google Scholar] [CrossRef]
- Yang, J.C.; Jablonsky, M.J.; Mays, J.W. NMR and FT-IR studies of sulfonated styrene-based homopolymers and copolymers. Polymer 2002, 43, 5125–5132. [Google Scholar] [CrossRef]
- John, A.; Mahadeva, S.K.; Kim, J. The preparation, characterization and actuation behavior of polyaniline and cellulose blended electro-active paper. Smart Mater. Struct. 2010, 19, 045011. [Google Scholar] [CrossRef]





| Sample | TGA | DTG (°C) | DTA (°C) | Assignment | Residue (%) | |
|---|---|---|---|---|---|---|
| Temperature (°C) | Mass Loss (%) | |||||
| SBA-15-Br | 25–157 | 0.81 | 58 | - | Volatiles | 99.19 |
| 157–319 | 6.24 | 260 | (+) 249 | Initiator | 92.95 | |
| 319–700 | 3.4 | 517 | (+) 494 | Amine | 89.55 | |
| SBA-15-PSSA | 25–100 | 2.87 | 66 | - | Volatiles | 97.12 |
| 100–212 | 3.33 | 154 | - | Initiator-amine-based | 93.80 | |
| 212–365 | 2.53 | 363 | (+) 304 | 91.27 | ||
| 365–457 | 2.83 | 422 | (+) 442 | 88.44 | ||
| 458–617 | 13.81 | 545 | (+) 556 | Polymer-based | 74.63 | |
| Surface Area BET (m2/g) | Pore Size BJHads (A) | Pore Volume BJHads (cm3/g) | Acidity by TGA (mmol H+/g) |
|---|---|---|---|
| 726 | 107.61 | 1.52 | 0.70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, A.A.; Mrair, Y.M.; Alharthi, F.A.; Al-Odayni, A.-B. Catalytic Performance of SBA-15-Supported Poly (Styrenesulfonic Acid) in the Esterification of Acetic Acid with n-Heptanol. Appl. Sci. 2020, 10, 5835. https://doi.org/10.3390/app10175835
Alghamdi AA, Mrair YM, Alharthi FA, Al-Odayni A-B. Catalytic Performance of SBA-15-Supported Poly (Styrenesulfonic Acid) in the Esterification of Acetic Acid with n-Heptanol. Applied Sciences. 2020; 10(17):5835. https://doi.org/10.3390/app10175835
Chicago/Turabian StyleAlghamdi, Abdulaziz Ali, Yahya Musawi Mrair, Fahad A. Alharthi, and Abdel-Basit Al-Odayni. 2020. "Catalytic Performance of SBA-15-Supported Poly (Styrenesulfonic Acid) in the Esterification of Acetic Acid with n-Heptanol" Applied Sciences 10, no. 17: 5835. https://doi.org/10.3390/app10175835
APA StyleAlghamdi, A. A., Mrair, Y. M., Alharthi, F. A., & Al-Odayni, A.-B. (2020). Catalytic Performance of SBA-15-Supported Poly (Styrenesulfonic Acid) in the Esterification of Acetic Acid with n-Heptanol. Applied Sciences, 10(17), 5835. https://doi.org/10.3390/app10175835







