Recent Development of Morphology Controlled Conducting Polymer Nanomaterial-Based Biosensor
Abstract
1. Introduction
2. Polypyrrole-Based Electrodes
2.1. 0D Nanostructures
2.2. 1D Nanostructures
2.3. 2D Nanostructures
2.4. 3D Nanostructures
3. Polyaniline-Based Electrodes
3.1. 0D Nanostructures
3.2. 1D Nanostructures
3.3. 2D Nanostructures
3.4. 3D Nanostructures
4. Poly(3,4-ethylenedioxythiophene)-Based Electrodes
4.1. 0D Nanostructures
4.2. 1D Nanostructures
4.3. 2D Nanostructures
4.4. 3D Nanostructures
5. Others
6. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakajima, A. Application of Single-Electron Transistor to Biomolecule and Ion Sensors. Appl. Sci. 2016, 6, 94. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Fang, W.; Yang, L.; Hu, Q.; Wang, Z.; Sun, J.Z.; Tang, B.Z. Sugar-Based Aggregation-Induced Emission Luminogens: Design, Structures, and Applications. Chem. Rev. 2020, 120, 4534–4577. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Park, H.W.; Ma, G.; Lee, C.Y.; Kwon, T.; Han, J.H. Optimal Synthesis of Horizontally Aligned Single-Walled Carbon Nanotubes and Their Biofunctionalization for Biosensing Applications. J. Nanomater. 2016, 2016, 5140241. [Google Scholar] [CrossRef]
- Kim, K.; Seo, S. Flexible Pressure and Touch Sensor with Liquid Metal Droplet Based on Gallium Alloys. Mol. Cryst. Liq. Cryst. 2019, 685, 40–46. [Google Scholar] [CrossRef]
- Ye, H.; Kim, J.H.; Seo, S. Fabrication and Applications of Flexible Stacked Devices via Open-Hole Integration. Sci. Adv. Mater. 2017, 9, 1464–1467. [Google Scholar] [CrossRef]
- Yoon, H.; Jang, J. Conducting-Polymer Nanomaterials for High-Performance Sensor Applications: Issues and Challenges. Adv. Funct. Mater. 2009, 19, 1567–1576. [Google Scholar] [CrossRef]
- Islam, A.E.; Martineau, R.; Crasto, C.M.; Kim, H.; Rao, R.S.; Maruyama, B.; Kim, S.S.; Drummy, L.F. Graphene-Based Electrolyte-Gated Field-Effect Transistors for Potentiometrically Sensing Neuropeptide Y in Physiologically Relevant Environments. ACS Appl. Nano Mater. 2020, 3, 5088–5097. [Google Scholar] [CrossRef]
- Ban, D.K.; Liu, Y.; Wang, Z.; Ramachandran, S.; Sarkar, N.; Shi, Z.; Liu, W.; Karkisaval, A.G.; Martinez-Loran, E.; Zhang, F.; et al. Direct DNA Methylation Profiling with an Electric Biosensor. ACS Nano 2020, 14, 6743–6751. [Google Scholar] [CrossRef]
- Chung, Y.J.; Kim, J.; Park, C.B. Photonic Carbon Dots as an Emerging Nanoagent for Biomedical and Healthcare Applications. ACS Nano 2020, 14, 6470–6497. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K.H.; Lee, J.Y.; Kim, K.K.; Choa, Y.H.; Lim, J.H. Single-Walled Carbon Nanotube-Based Chemi-Capacitive Sensor for Hexane and Ammonia. Electron. Mater. Lett. 2019, 15, 712–719. [Google Scholar] [CrossRef]
- Chang, W.-S.; Li, P.; Kakade, S.; Xiong, Y.; Shang, H.; Zhang, Y.; Lee, G.U. Rapid and Sensitive Detection of Cardiac Troponin I Using a Force Enhanced Immunoassay with Nanoporous Membrane. Nanoscale 2020, 12, 12568–12577. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Lee, S.B.; Lee, D.K.; Smits, E.C.P.; Gelinck, G.H.; Cho, K.; Kim, J.J. Top-Split-Gate Ambipolar Organic Thin-Film Transistors. Adv. Electron. Mater. 2018, 4, 1700536. [Google Scholar] [CrossRef]
- Xue, Q.; Li, Z.; Wang, Q.; Pan, W.; Chang, Y.; Duan, X. Nanostrip Flexible Microwave Enzymatic Biosensor for Noninvasive Epidermal Glucose Sensing. Nanoscale Horiz. 2020, 5, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, J.; Liu, Y.; Li, P.; Liu, Y.; Chen, L.; Zhao, J. Multi-Praseodymium-and-Tungsten Bridging Octameric Tellurotungstate and Its 2D Honeycomb Composite Film for Detecting Estrogen. Nanoscale 2020, 12, 10842–10853. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Lee, J.U.; Kang, J.; Park, K.H.; Sim, S.J. Real-Time Monitoring of Distinct Binding Kinetics of Hot-Spot Mutant p53 Protein in Human Cancer Cells Using an Individual Nanorod-Based Plasmonic Biosensor. Sens. Actuator B Chem. 2020, 322, 128584. [Google Scholar] [CrossRef]
- Yoo, H.; Hong, S.; Moon, H.; On, S.; Ahn, H.; Lee, H.K.; Kim, S.; Hong, Y.K.; Kim, J.J. Chemical Doping Effects on CVD-Grown Multilayer MoSe2 Transistor. Adv. Electron. Mater. 2018, 4, 1700639. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Bai, W.; Li, Y. Electrogenerated Chemiluminescence Biosensing Method for DNA Hydroxymethylation Detection via Glycosylation and a New Multi-Functional ECL Signal Compound. Sens. Actuator B Chem. 2020, 322, 128582. [Google Scholar] [CrossRef]
- Jang, J. Conducting Polymer Nanomaterials and Their Applications. Adv. Polym. Sci. 2006, 199, 189–259. [Google Scholar]
- Kwon, O.S.; Song, H.S.; Park, T.H.; Jang, J. Conducting Nanomaterial Sensor Using Natural Receptors. Chem. Rev. 2019, 119, 36–93. [Google Scholar] [CrossRef]
- Yoo, H.; Ghittorelli, M.; Smits, E.C.P.; Gelinck, G.H.; Lee, H.K.; Torricelli, F.; Kim, J.J. Reconfigurable Complementary Logic Circuits with Ambipolar Organic Transistors. Sci. Rep. 2016, 6, 35585. [Google Scholar] [CrossRef]
- Roh, S.H.; Kim, J.; Park, W.I.; Kim, Y.D.; Lim, J.H. Fabrication and Characterization of Ge2Sb2Te5 Nanowire Arrays and PEDOT: PSS Hybrid Thermoelectric Composites. Korean J. Met. Mater. 2017, 55, 432–439. [Google Scholar]
- Yoo, H.; On, S.; Lee, S.B.; Cho, K.; Kim, J.J. Negative Transconductance Heterojunction Organic Transistors and their Application to Full-Swing Ternary Circuits. Adv. Mater. 2019, 31, 1808265. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, J.; Jänsch, D.; Hinkel, F.; Bunz, U.H.F. Immobilization Strategies for Organic Semiconducting Conjugated Polymers. Chem. Rev. 2018, 118, 5598–5689. [Google Scholar] [CrossRef] [PubMed]
- Das, G.S.; Shim, J.P.; Bhatnagar, A.; Tripathi, K.M.; Kim, T.Y. Biomass-Derived Carbon Quantum Dots for Visible-Light-Induced Photocatalysis and Label-Free Detection of Fe (iii) and Ascorbic Acid. Sci. Rep. 2019, 9, 15084. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Park, H.; Yoo, S.; On, S.; Seong, H.; Im, S.G.; Kim, J.J. Highly Stacked 3D Organic Integrated Circuits with via-Hole-Less Multilevel Metal Interconnects. Nat. Commun. 2019, 10, 2424. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, J.G.; Rincon, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical−Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef]
- Chia, P.-J.; Chua, L.-L.; Sivaramakrishnan, S.; Zhuo, J.-M.; Zhao, L.-H.; Sim, W.-S.; Eo, Y.-C.; Ho, P.K.-H. Injection-induced De-doping in a Conducting Polymer During Device Operation: Asymmetry in the Hole Injection and Extraction Rates. Adv. Mater. 2007, 19, 4202–4207. [Google Scholar] [CrossRef]
- Babeli, I.; Ruano, G.; Casanovas, J.; Ginebra, M.-P.; Garcıa-Torres, J.; Aleman, C. Conductive, Self-Healable and Reusable Poly(3,4-ethylenedioxythiophene)-Based Hydrogels for Highly Sensitive Pressure Arrays. J. Mater. Chem. C 2020, 8, 8654–8667. [Google Scholar] [CrossRef]
- Vaidya, S.; Veselska, O.; Zhadan, A.; Daniel, M.; Ledoux, G.; Fateeva, A.; Tsuruoka, T.; Demessence, A. Flexible and Luminescent Fibers of a 1D Au(I)–Thiophenolate Coordination Polymer and Formation of Gold Nanoparticle-Based Composite Materials for SERS. J. Mater. Chem. C 2020, 8, 8018–8026. [Google Scholar] [CrossRef]
- Teng, Y.; Wei, J.; Du, H.; Mojtaba, M.; Li, D. A Solar and Thermal Multi-Sensing Microfiber Supercapacitor with Intelligent Self-Conditioned Capacitance and Body Temperature Monitoring. J. Mater. Chem. A 2020, 8, 11695–11711. [Google Scholar] [CrossRef]
- Kim, D.; Jung, D.; Noh, J.K.; Han, J.H. Graphene Chemocapacitive Sensors for Dimethyl Methylphosphonate Detection. Sci. Adv. Mater. 2018, 10, 1268–1273. [Google Scholar] [CrossRef]
- Fernandez, F.D.M.; Khadka, R.; Yim, J.-H. Highly Porous, Soft, and Flexible Vapor-Phase Polymerized Polypyrrole–Styrene–Ethylene–Butylene–Styrene Hybrid Scaffold as Ammonia and Strain Sensor. RSC Adv. 2020, 10, 22533–22541. [Google Scholar] [CrossRef]
- Puiggalí-Jou, A.; Valle, L.J.D.; Alemań, C. Encapsulation and Storage of Therapeutic Fibrin-Homing Peptides using Conducting Polymer Nanoparticles for Programmed Release by Electrical Stimulation. ACS Biomater. Sci. Eng. 2020, 6, 2135–2145. [Google Scholar] [CrossRef]
- Chen, S.; Kang, E.S.H.; Chaharsoughi, M.S.; Stanishev, V.; Kühne, P.; Sun, H.; Wang, C.; Fahlman, M.; Fabiano, S.; Darakchieva, V.; et al. Conductive Polymer Nanoantennas for Dynamic Organic Plasmonics. Nat. Nanotechnol. 2020, 15, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Jun, J.; Lee, J.S.; Jang, J. A Highly Sensitive Wireless Nitrogen Dioxide Gas Sensor Based on an Organic Conductive Nanocomposite Paste. J. Mater. Chem. A 2019, 7, 8451–8459. [Google Scholar] [CrossRef]
- Rajesh, M.; Manikandan, R.; Kim, B.C.; Becuwe, M.; Yu, K.H.; Raj, J. Electrochemical Polymerization of Chloride Doped PEDOT Hierarchical Porous Nanostructure on Graphite as a Potential Electrode for High Performance Supercapacitor. Electrochim. Acta 2020, 354, 136669. [Google Scholar] [CrossRef]
- Yang, S.; Liu, D.; Meng, Q.B.; Wu, S.; Song, X.-M. Reduced Graphene Oxide-Supported Methylene Blue Nanocomposite as a Glucose Oxidase-Mimetic for Electrochemical Glucose Sensing. RSC Adv. 2018, 8, 32565–32573. [Google Scholar] [CrossRef]
- Gao, Z.; Siow, K.S.; Chan, H.S.O. Self-Assembled Conducting Polymer Monolayers of Poly(3-octylthiophene) on Gold Electrodes. Synth. Met. 1995, 75, 5–10. [Google Scholar] [CrossRef]
- Taheri, N.; Alizadeh, N. Vertically Grown Nanosheets Conductive Polypyrrole as a Sorbent for Nanomolar Detection of Salicylic Acid. J. Pharm. Biomed. Anal. 2020, 188, 113365. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.S.; Jang, J. Aptamer-Functionalized Three-Dimensional Carbon Nanowebs for Ultrasensitive and Free-Standing PDGF Biosensor. ACS Appl. Mater. Interfaces 2020, 12, 20882–20890. [Google Scholar] [CrossRef]
- Wan, A.M.-D.; Inal, S.; Williams, T.; Wang, K.; Leleux, P.; Estevez, L.; Giannelis, E.P.; Fischbach, C.; Malliaras, G.G.; Gourdon, D. 3D Conducting Polymer Platforms for Electrical Control of Protein Conformation and Cellular Functions. J. Mater. Chem. B 2015, 3, 5040–5048. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D Printing of Conducting Polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Oh, J.H.; Stucky, G.D. Fabrication of Ultrafine Conducting Polymer and Graphite Nanoparticles. Angew. Chem. Int. Ed. 2002, 41, 4016–4019. [Google Scholar] [CrossRef]
- Jang, J.; Yoon, H. Fabrication of Magnetic Carbon Nanotubes using a Metal-Impregnated Polymer Precursor. Adv. Mater. 2003, 15, 2088–2091. [Google Scholar] [CrossRef]
- Jang, J.; Lim, B. Facile Fabrication of Inorganic-Polymer Core–Shell Nanostructures by a One-Step Vapor Deposition Polymerization. Angew. Chem. Int. Ed. 2003, 42, 5600–5603. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bai, H.; Shi, G. Conducting Polymer Nanomaterials: Electrosynthesis and Applications. Chem. Soc. Rev. 2009, 38, 2397–2409. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bunimovich, Y.L.; Sui, G.; Savvas, S.; Wang, J.; Guo, Y.; Heath, J.R.; Tseng, H.-R. Electrochemical Fabrication of Conducting Polymer Nanowires in an Integrated Microfluidic System. Chem. Commun. 2006, 19, 3075–3077. [Google Scholar] [CrossRef]
- Yang, T.; Liu, Y.; Wang, H.; Duo, Y.; Zhang, B.; Ge, Y.; Zhang, H.; Chen, W. Recent Advances in 0D Nanostructure-Functionalized Low-Dimensional Nanomaterials for Chemiresistive Gas Sensors. J. Mater. Chem. C 2020, 8, 7272–7299. [Google Scholar] [CrossRef]
- Jang, J.; Oh, J.H. Facile Fabrication of Photochromic Dye-Conducting Polymer Core-Shell Nanomaterials and Their Photoluminescence. Adv. Mater. 2003, 15, 977–980. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, W.; Wang, C.; Wen, T.-C.; Wei, Y. One-Dimensional Conducting Polymer Nanocomposites: Synthesis, Properties and Applications. Prog. Polym. Sci. 2011, 36, 671–712. [Google Scholar] [CrossRef]
- He, H.-C.; Yan, A.-L.; Karapala, V.K.; Wang, S.-F.; Shen, M.-H.; Lin, Y.-L.; Chen, Y.-F.; Sugiyama, T.; Chen, J.-T. Laser-Assisted Nanowetting: Selective Fabrication of Polymer/Gold Nanorod Arrays Using Anodic Aluminum Oxide Templates. Macromol. Rapid Commun. 2020, 41, 2000035. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Qiu, H.; Dou, C.; Li, Y.; Pu, L.; Xu, J.; Shi, Y. Conducting Polymer Nanostructures: Template Synthesis and Applications in Energy Storage. Int. J. Mol. Sci. 2010, 11, 2636–2657. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Lu, Y. Water-Soluble Methyl Orange Fibrils as Versatile Templates for the Fabrication of Conducting Polymer Microtubules. Macromol. Rapid Commun. 2007, 28, 629–633. [Google Scholar] [CrossRef]
- Joo, S.; Kim, J.H.; Seo, S. Direct Fabrication of Electrochromic Devices with Complex Patterns on Three-Dimensional Substrates Using Polymeric Stencil Films. RSC Adv. 2017, 7, 43283–43288. [Google Scholar] [CrossRef]
- Nam, V.B.; Shin, J.; Yoon, Y.; Giang, T.T.; Kwon, J.; Suh, Y.D.; Yeo, J.; Hong, S.; Ko, S.H.; Lee, D. Highly Stable Ni-Based Flexible Transparent Conducting Panels Fabricated by Laser Digital Patterning. Adv. Funct. Mater. 2019, 29, 1806895. [Google Scholar] [CrossRef]
- Xia, L.; Wei, Z.; Wan, M. Conducting Polymer Nanostructures and Their Application in Biosensors. J. Colloid Interface Sci. 2010, 341, 1–11. [Google Scholar] [CrossRef]
- Long, Y.-Z.; Li, M.-M.; Gu, C.; Wan, M.; Duvail, J.-L.; Liu, Z.; Fan, Z. Recent Advances in Synthesis, Physical Properties and Applications of Conducting Polymer Nanotubes and Nanofibers. Prog. Polym. Sci. 2011, 36, 1415–1442. [Google Scholar] [CrossRef]
- Mulchandani, A.; Myung, N.V. Conducting Polymer Nanowires-Based Label-Free Biosensors. Curr. Opin. Biotechnol. 2011, 22, 502–508. [Google Scholar] [CrossRef]
- Jang, J.; Ha, J.; Kim, S. Fabrication of Polyaniline Nanoparticles Using Microemulsion Polymerization. Macromol. Res. 2007, 15, 154–159. [Google Scholar] [CrossRef]
- Zhou, F.; Chen, M.; Liu, W.; Liu, J.; Liu, Z.; Mu, Z. Fabrication of Positively Patterned Conducting Polymer Microstructures via One-Step Electrodeposition. Adv. Mater. 2003, 15, 1367–1370. [Google Scholar] [CrossRef]
- Jang, J.; Yoon, H. Multigram-Scale Fabrication of Monodisperse Conducting Polymer and Magnetic Carbon Nanoparticles. Small 2005, 1, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Martin, D.C. Microporous Conducting Polymers on Neural Microelectrode Arrays: II. Physical Characterization. Sens. Actuator A Phys. 2004, 113, 204–211. [Google Scholar] [CrossRef]
- Wan, M. A Template-Free Method towards Conducting Polymer Nanostructures. Adv. Mater. 2008, 20, 2926–2932. [Google Scholar] [CrossRef]
- Rahman, A.; Sanyal, M.K. Novel Switching Transition of Resistance Observed in Conducting Polymer Nanowires. Adv. Mater. 2007, 19, 3956–3960. [Google Scholar] [CrossRef]
- Seo, Y.D.; Jung, J.; Lee, H.; Yeo, J.; Hong, S.; Lee, P.; Lee, D. Nanowire Reinforced Nanoparticle Nanocomposite for Highly Flexible Transparent Electrodes: Borrowing Idea from Macrocomposites in Steel-Wire Reinforced Concrete. J. Mater. Chem. C 2017, 5, 791–798. [Google Scholar]
- Geethalakshmi, D.; Muthukumarasamy, N.; Balasundaraprabhu, R. CSA-Doped PANI Semiconductor Nanofilms: Synthesis and Characterization. J. Mater. Sci. Mater. Electron. 2015, 26, 7797–7803. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, B.; Ou, X.; Wang, C.; Peng, C.; Zhang, J. Synergistical Coupling Interconnected ZnS/SnS2 Nanoboxes with Polypyrrole-Derived N/S Dual-Doped Carbon for Boosting High-Performance Sodium Storage. Small 2019, 15, 1804861. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Yoon, H.; Jang, J. Highly Sensitive and Selective Chemiresistive Sensors Based on Multidimensional Polypyrrole Nanotubes. Chem. Commun. 2012, 48, 10526–10528. [Google Scholar] [CrossRef]
- Jang, J.; Ha, H. Fabrication of Hollow Polystyrene Nanospheres in Microemulsion Polymerization Using Triblock Copolymers. Langmuir 2002, 18, 5613–5618. [Google Scholar] [CrossRef]
- Jang, J.; Yoon, H. Facile Fabrication of Polypyrrole Nanotubes Using Reverse Microemulsion Polymerization. Chem. Commun. 2003, 6, 720–721. [Google Scholar] [CrossRef]
- Plausinaitis, D.; Sinkevicius, L.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Evaluation of Electrochemical Quartz Crystal Microbalance Based Sensor Modified by Uric Acid-Imprinted Polypyrrole. Talanta 2020, 220, 121414. [Google Scholar] [CrossRef]
- Colak, O.; Yasar, A.; Cete, S.; Arslan, F. Glucose Biosensor Based on the Immobilization of Glucose Oxidase on Electrochemically Synthesized Polypyrrole-Poly(vinyl sulphonate) Composite Film by Cross-Linking with Glutaraldehyde. Artif. Cells Blood Substit. Biotechnol. 2012, 40, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chu, Y.; Yang, L. Adjusting the Inner-Structure of Polypyrrole Nanoparticles through Microemulsion. Polymerization 2006, 98, 304–308. [Google Scholar] [CrossRef]
- Ovando-Medina, V.M.; Peralta, R.D.; Mendizábal, E.; Martínez-Gutiérrez, H.; Lara-Ceniceros, T.E.; Ledezma-Rodríguez, R. Synthesis of Polypyrrole Nanoparticles by Oil-in-Water Microemulsion Polymerization with Narrow Size Distribution. Colloid Polym. Sci. 2011, 289, 759–765. [Google Scholar] [CrossRef]
- Jang, J.; Oh, J.H. Novel Crystalline Supramolecular Assemblies of Amorphous Polypyrrole Nanoparticles through Surfactant Templating. Chem. Commun. 2002, 19, 2200–2201. [Google Scholar] [CrossRef]
- Zha, Z.; Yue, X.; Ren, Q.; Dai, Z. Uniform Polypyrrole Nanoparticles with High Photothermal Conversion Efficiency for Photothermal Ablation of Cancer Cells. Adv. Mater. 2013, 25, 777–782. [Google Scholar] [CrossRef]
- Men’shikova, A.Y.; Shabsel’s, B.M.; Evseeva, T.G. Synthesis of Polypyrrole Nanoparticles by Dispersion Polymerization. Russ. J. Appl. Chem. 2003, 76, 822–826. [Google Scholar]
- Hong, J.-Y.; Yoon, H.; Jang, J. Kinetic Study of the Formation of Polypyrrole Nanoparticles in Water-Soluble Polymer/Metal Cation Systems: A Light-Scattering Analysis. Small 2010, 6, 679–686. [Google Scholar] [CrossRef]
- Kwon, O.S.; Ahn, S.R.; Park, S.J.; Song, H.S.; Lee, S.H.; Lee, J.S.; Hong, J.-Y.; Lee, J.S.; You, S.A.; Yoon, H.; et al. Ultrasensitive and Selective Recognition of Peptide Hormone Using Close-Packed Arrays of hPTHR-Conjugated Polymer Nanoparticles. ACS Nano 2012, 6, 5549–5558. [Google Scholar] [CrossRef]
- Lee, J.S.; Oh, J.; Kim, S.G.; Jang, J. Highly Sensitive and Selective Field-Effect-Transistor NonEnzyme Dopamine Sensors Based on Pt/Conducting Polymer Hybrid Nanoparticles. Small 2015, 11, 2399–2406. [Google Scholar] [CrossRef]
- Oh, J.; Yang, H.; Jeong, G.E.; Moon, D.; Kwon, O.S.; Phyo, S.; Lee, J.; Song, H.S.; Park, T.H.; Jang, J. Ultrasensitive, Selective, and Highly Stable Bioelectronic Nose That Detects the Liquid and Gaseous Cadaverine. Anal. Chem. 2019, 91, 12181–12190. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Na, W.; Jang, J. One-Pot Synthesis of Multidimensional Conducting Polymer Nanotubes for Superior Performance Field Effect Transistor-Type Carcinoembryonic Antigen Biosensors. RSC Adv. 2016, 6, 14335–14343. [Google Scholar] [CrossRef]
- Ghanbari, K.; Bathaie, S.Z.; Mousavi, M.F. Electrochemically Fabricated Polypyrrole Nanofiber-Modified Electrode as a New Electrochemical DNA Biosensor. Biosens. Bioelectron. 2008, 23, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Kwon, O.S.; Lee, S.H.; Park, S.J.; Kim, U.-K.; Jang, J.; Park, T.H. Human Taste Receptor-Functionalized Field Effect Transistor as a Human-Like Nanobioelectronic Tongue. Nano Lett. 2013, 13, 172–178. [Google Scholar] [CrossRef]
- Na, W.; Park, J.W.; An, J.H.; Jang, J. Size-Controllable Ultrathin Carboxylated Polypyrrole Nanotube Transducer for Extremely Sensitive 17β-Estradiol FET-Type Biosensors. J. Mater. Chem. B 2016, 4, 5025–5034. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.S.; Shin, D.H.; Jang, J. Platinum Nanoparticles Immobilized on Polypyrrole Nanofibers for Non-Enzyme Oxalic Acid Sensor. J. Mater. Chem. B 2018, 6, 1272–1278. [Google Scholar] [CrossRef]
- Arora, K.; Chaubey, A.; Singhal, R.; Singh, R.P.; Pandey, M.K.; Samanta, S.B.; Malhotra, B.D.; Chand, S. Application of Electrochemically Prepared Polypyrrole–Polyvinyl Sulphonate Films to DNA Biosensor. Biosens. Bioelectron. 2006, 21, 1777–1783. [Google Scholar] [CrossRef]
- Ozer, B.O.; Cete, S. Development of a Novel Biosensor Based on a Polypyrrole–Dodecyl benzene Sulphonate (PPy–DBS) Film for the Determination of Amperometric Cholesterol. Artif. Cell Nanomed. Biotechnol. 2017, 45, 824–832. [Google Scholar] [CrossRef]
- Weng, B.; Morrin, A.; Shepherd, R.; Crowley, K.; Killard, A.J.; Innis, P.C.; Wallace, G.G. Wholly Printed Polypyrrole Nanoparticle-Based Biosensors on Flexible Substrate. J. Mater. Chem. B 2014, 2, 793–799. [Google Scholar] [CrossRef]
- Jeong, G.; Oh, J.; Jang, J. Fabrication of N-Doped Multidimensional Carbon Nanofibers for High Performance Cortisol Biosensors. Biosens. Bioelectron. 2019, 131, 30–36. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, W.; Cho, S.; Jun, J.; Cho, K.H.; Jang, J. Multidimensional Hybrid Conductive Nanoplate-Based Aptasensor for Platelet-Derived Growth Factor Detection. J. Mater. Chem. B 2016, 4, 4447–4454. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Shin, D.H.; Oh, J.; An, J.H.; Lee, J.S.; Jang, J. Multidimensional Conductive Nanofilm-Based Flexible Aptasensor for Ultrasensitive and Selective HBsAg Detection. ACS Appl. Mater. Interfaces 2018, 10, 28412–28419. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, S.G.; Jun, J.; Shin, D.H.; Jang, J. Aptamer-Functionalized Multidimensional Conducting-Polymer Nanoparticles for an Ultrasensitive and Selective Field-Effect-Transistor Endocrine-Disruptor Sensors. Adv. Funct. Mater. 2014, 24, 6145–6153. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.S.; Jun, J.; Jang, J. High-Sensitivity Hydrogen Gas Sensors Based on Pd-Decorated Nanoporous Poly(aniline-co-aniline-2-sulfonic acid):Poly(4-styrenesulfonic acid). J. Mater. Chem. A 2014, 2, 1955–1966. [Google Scholar] [CrossRef]
- Yang, L.; Yang, L.; Wu, S.; Wei, F.; Hu, Y.; Xu, X.; Zhang, L.; Sun, D. Three-Dimensional Conductive Organic Sulfonic Acid Co-Doped Bacterial Cellulose/Polyaniline Nanocomposite Films for Detection of Ammonia at Room Temperature. Sens. Actuator B Chem. 2020, 323, 128689. [Google Scholar] [CrossRef]
- Cochet, M.; Maser, W.K.; Benito, A.M.; Callejas, M.A.; Martínez, M.T.; Benoit, J.-M.; Schreiber, J.; Chauvet, O. Synthesis of a New Polyaniline/Nanotube Composite: “In-situ” Polymerisation and Charge Transfer Through Site-Selective Interaction. Chem. Commun. 2001, 16, 1450–1451. [Google Scholar] [CrossRef]
- He, L.; Cui, B.; Liu, J.; Song, Y.; Wang, M.; Peng, D.; Zhang, Z. Novel Electrochemical Biosensor Based on Core-Shell Nanostructured Composite of Hollow Carbon Spheres and Polyaniline for Sensitively Detecting Malathion. Sens. Actuator B Chem. 2018, 258, 813–821. [Google Scholar] [CrossRef]
- Salimian, R.; Shahrokhian, S.; Panahi, S. Enhanced Electrochemical Activity of a Hollow Carbon Sphere/Polyaniline-Based Electrochemical Biosensor for HBV DNA Marker Detection. ACS Biomater. Sci. Eng. 2019, 5, 2587–2594. [Google Scholar] [CrossRef]
- Al-Sagur, H.; Komathi, S.; Karakaş, H.; Atilla, D.; Gürek, A.G.; Basova, T.; Farmilo, N.; Hassan, A.K. A Glucose Biosensor Based on Novel Lutetium Bis-Phthalocyanine Incorporated Silica-Polyaniline Conducting Nanobeads. Biosens. Bioelectron. 2018, 102, 637–645. [Google Scholar] [CrossRef]
- Lou, F.; Lu, Z.; Hu, F.; Li, C.M. A 3D Bio-Platform Constructed by Glucose Oxidase Adsorbed on Au Nanoparticles Assembled Polyaniline Nanowires to Sensitively Detect Glucose by Electrochemiluminescence. J. Electroanal. Chem. 2017, 787, 125–131. [Google Scholar] [CrossRef]
- Hui, N.; Sun, X.; Niu, S.; Luo, X. PEGylated Polyaniline Nanofibers: Antifouling and Conducting Biomaterial for Electrochemical DNA Sensing. ACS Appl. Mater. Interfaces 2017, 9, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Asmatulu, R.; Veisi, Z.; Uddin, M.N.; Mahapatro, A. Highly Sensitive and Reliable Electrospun Polyaniline Nanofiber Based Biosensor as a Robust Platform for COX-2 Enzyme Detections. Fibers Polym. 2019, 20, 966–974. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, R.; Guo, H.; Wei, Y.; Yang, W. A Facile Horseradish Peroxidase Electrochemical Biosensor with Surface Molecular Imprinting Based on Polyaniline Nanotubes. J. Electroanal. Chem. 2018, 817, 184–194. [Google Scholar] [CrossRef]
- Soni, A.; Pandey, C.M.; Solanki, S.; Kotnala, R.K.; Sumana, G. Electrochemical Genosensor Based on Template Assisted Synthesized Polyaniline Nanotubes for Chronic Myelogenous Leukemia Detection. Talanta 2018, 187, 379–389. [Google Scholar] [CrossRef]
- Oh, W.-K.; Kim, S.; Shin, K.-H.; Jang, Y.; Choi, M.; Jang, J. Inkjet-Printed Polyaniline Patterns for Exocytosed Molecule Detection from Live Cells. Talanta 2013, 105, 333–339. [Google Scholar] [CrossRef]
- Kailasa, S.; Geeta, B.; Jayarambabu, N.; Reddy, R.K.K.; Sharma, S.; Rao, K.V. Conductive Polyaniline Nanosheets (CPANINS) for a Non-Enzymatic Glucose Sensor. Mater. Lett. 2019, 245, 118–121. [Google Scholar] [CrossRef]
- Kailasa, S.; Rani, B.G.; Reddy, M.S.B.; Jayarambabu, N.; Munindra, P.; Sharma, S.; Rao, K.V. NiO Nanoparticles-Decorated Conductive Polyaniline Nanosheets for Amperometric Glucose Biosensor. Mater. Chem. Phys. 2020, 242, 122524. [Google Scholar] [CrossRef]
- Liu, P.; Huang, J.; Sanchez, D.V.P.; Schwartzman, D.; Lee, S.H.; Yun, M. High Yield Two-Dimensional (2-D) Polyaniline Layer and Its Application in Detection of B-type Natriuretic Peptide in Human Serum. Sens. Actuator B Chem. 2016, 230, 184–190. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, Y.; Lee, S.H.; Yun, M. Two-Dimensional Polyaniline Nanostructure to the Development of Microfluidic Integrated Flexible Biosensors for Biomarker Detection. Biomed. Microdevices 2016, 18, 113. [Google Scholar] [CrossRef]
- Xu, L.-H.; Li, J.-J.; Zeng, H.-B.; Zhang, X.-J.; Cosnier, S.; Marks, R.S.; Shan, D. ATMP-Induced Three-Dimensional Conductive Polymer Hydrogel Scaffold for a Novel Enhanced Solid-State Electrochemiluminescence Biosensor. Biosens. Bioelectron. 2019, 143, 111601. [Google Scholar] [CrossRef]
- Qing, M.; Chen, S.L.; Han, L.; Yang, Y.Z.; Luo, H.Q.; Li, N.B. Three–Dimensional Donor–Acceptor–Type Photoactive Material/Conducting Polyaniline Hydrogel Complex for Sensitive Photocathodic Enzymatic Bioanalysis. Biosens. Bioelectron. 2020, 158, 112179. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A Nanostructured Conductive Hydrogels-Based Biosensor Platform for Human Metabolite Detection. Nano Lett. 2015, 15, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Pandey, C.M.; Pandey, M.K.; Sumana, G. Highly Efficient Polyaniline-MoS2 Hybrid Nanostructures Based Biosensor for Cancer Biomarker Detection. Anal. Chim. Acta 2019, 1055, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, G.B.V.S.; Sharma, A.; Solanki, P.R.; Avasthi, D.K. Mesoporous Polyaniline Nanofiber Decorated Graphene Micro-Flowers for Enzyme-Less Cholesterol Biosensors. Nanotechnology 2016, 27, 345101. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Gleason, K.K. Enhanced Optical Property with Tunable Band Gap of Cross-linked PEDOT Copolymers via Oxidative Chemical Vapor Deposition. Adv. Funct. Mater. 2015, 25, 85–93. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.K.; Son, Y. Glucose Biosensor Constructed from Capped Conducting Microtubules of PEDOT. Sens. Actuator B Chem. 2008, 133, 244–250. [Google Scholar] [CrossRef]
- Jiang, F.; Yue, R.; Du, Y.; Xu, J.; Yang, P. A One-Pot ‘Green’ Synthesis of Pd-Decorated PEDOT Nanospheres for Nonenzymatic Hydrogen Peroxide Sensing. Biosens. Bioelectron. 2013, 44, 127–131. [Google Scholar] [CrossRef]
- Liu, Y.; Turner, A.P.F.; Zhao, M.; Mak, W.C. Processable Enzyme-Hybrid Conductive Polymer Composites for Electrochemical Biosensing. Biosens. Bioelectron. 2018, 100, 374–381. [Google Scholar] [CrossRef]
- Kim, S.; Oh, W.-K.; Jeong, Y.S.; Jang, J. Dual-Functional Poly(3,4-ethylenedioxy thiophene)/MnO2 Nanoellipsoids for Enhancement of Neurite Outgrowth and Exocytosed Biomolecule Sensing in PC12 Cells. Adv. Funct. Mater. 2013, 23, 1947–1956. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Chen, L.-C.; Ho, K.-C. Synthesis of Redox Polymer Nanobeads and Nanocomposites for Glucose Biosensors. ACS Appl. Mater. Interfaces 2013, 5, 7852–7861. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, X.; Fan, X.; Yang, R.; Wu, T.; Zhang, C. Application of Conducting Micelles Self-Assembled from Commercial Poly(3,4-ethylenedioxythiophene):Poly(styrene sulfonate) and Chitosan for Electrochemical Biosensor. Colloid Polym. Sci. 2018, 296, 495–502. [Google Scholar] [CrossRef]
- Park, S.J.; Song, H.S.; Kwon, O.S.; Chung, J.H.; Lee, S.H.; An, J.H.; Ahn, S.R.; Lee, J.E.; Yoon, H.; Park, T.H.; et al. Human Dopamine Receptor Nanovesicles for Gate-Potential Modulators in High-Performance Field-Effect Transistor Biosensors. Sci. Rep. 2015, 4, 4342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, M.; Yadavalli, V.K. Silk Fibroin-Sheathed Conducting Polymer Wires as Organic Connectors for Biosensors. Biosensors 2019, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tang, N.; Zhang, M.; Xue, Q.; Duan, X. Highly Sensitive Protein Detection Using Conductive Polymer Nanowires Fabricated by Nanoscale Soft Lithography. In Proceedings of the 2018 IEEE Sensors, New Delhi, India, 28–31 October 2018; pp. 1–3. [Google Scholar]
- Çetin, M.Z.; Camurlu, P. An Amperometric Glucose Biosensor Based on PEDOT Nanofibers. RSC Adv. 2018, 8, 19724–19731. [Google Scholar] [CrossRef]
- Chen, Y.; Gai, P.; Jin, L.; Zhu, D.; Tian, D.; Abdel-Halim, E.S.; Zhang, J.; Zhu, J.-J. Fabrication of PEDOT Nanowhiskers for Electrical Connection of the Hemoglobin Active Center for H2O2 Electrochemical Biosensing. J. Mater. Chem. B 2013, 1, 3451–3457. [Google Scholar] [CrossRef]
- David, M.; Barsana, M.M.; Bretta, C.M.A.; Florescu, M. Improved Glucose Label-Free Biosensor with Layer-by-Layer Architecture and Conducting Polymer Poly(3,4-ethylenedioxythiophene). Sens. Actuator B Chem. 2018, 255, 3227–3234. [Google Scholar] [CrossRef]
- Kim, M.; Iezzi, R., Jr.; Shim, B.S.; Martin, D.C. Impedimetric Biosensors for Detecting Vascular Endothelial Growth Factor (VEGF) Based on Poly(3,4-ethylene dioxythiophene) (PEDOT)/Gold Nanoparticle (Au NP) Composites. Front. Chem. 2019, 7, 234. [Google Scholar] [CrossRef]
- Scotto, J.; Piccinini, E.; Bilderling, C.V.; Coria-Oriundoc, L.L.; Battaglini, F.; Knoll, W.; Marmisolle, W.A.; Azzaroni, O. Flexible Conducting Platforms Based on PEDOT and Graphite Nanosheets for Electrochemical Biosensing Applications. Appl. Surf. Sci. 2020, 525, 146440. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Hui, N. A Low Fouling Electrochemical Biosensor Based on the Zwitterionic Polypeptide Doped Conducting Polymer PEDOT for Breast Cancer Marker BRCA1 Detection. Bioelectrochemistry 2020, 136, 107595. [Google Scholar] [CrossRef]
- Jia, H.; Xu, J.; Lu, L.; Yu, Y.; Zuo, Y.; Tian, Q.; Li, P. Three-Dimensional Au Nanoparticles/ Nano-Poly(3,4-ethylene dioxythiophene)-Graphene Aerogel Nanocomposite: A High-Performance Electrochemical Immunosensing Platform for Prostate Specific Antigen Detection. Sens. Actuator B Chem. 2018, 260, 990–997. [Google Scholar] [CrossRef]
- Meng, L.; Turner, A.P.F.; Mak, W.C. Tunable 3D Nanofibrous and Bio-Functionalised PEDOT Network Explored as a Conducting Polymer-Based Biosensor. Biosens. Bioelectron. 2020, 159, 112181. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, S.H.; Yang, H.; Park, C.S.; Lee, C.-S.; Kwon, O.S.; Park, T.H.; Jang, J. Human Dopamine Receptor-Conjugated Multidimensional Conducting Polymer Nanofiber Membrane for Dopamine Detection. ACS Appl. Mater. Interfaces 2016, 8, 28897–28903. [Google Scholar] [CrossRef] [PubMed]
- Soylemez, S.; Kaya, H.Z.; Udum, Y.A.; Toppare, L. A Multipurpose Conjugated Polymer: Electrochromic Device and Biosensor Construction for Glucose Detection. Org. Electron. 2019, 65, 327–333. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.; Li, B. A Colorimetric Aptamer Biosensor Based on Cationic Polythiophene Derivative as Peroxidase Mimetics for the Ultrasensitive Detection of Thrombin. Talanta 2017, 175, 224–228. [Google Scholar] [CrossRef]
- Voccia, D.; Sosnowska, M.; Bettazzi, F.; Roscigno, G.; Fratini, E.; Franciscis, V.D.; Condorelli, G.; Chitta, R.; D’Souza, F.; Kutner, W.; et al. Direct Determination of Small RNAs Using a Biotinylated Polythiophene Impedimetric Genosensor. Biosens. Bioelectron. 2017, 87, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
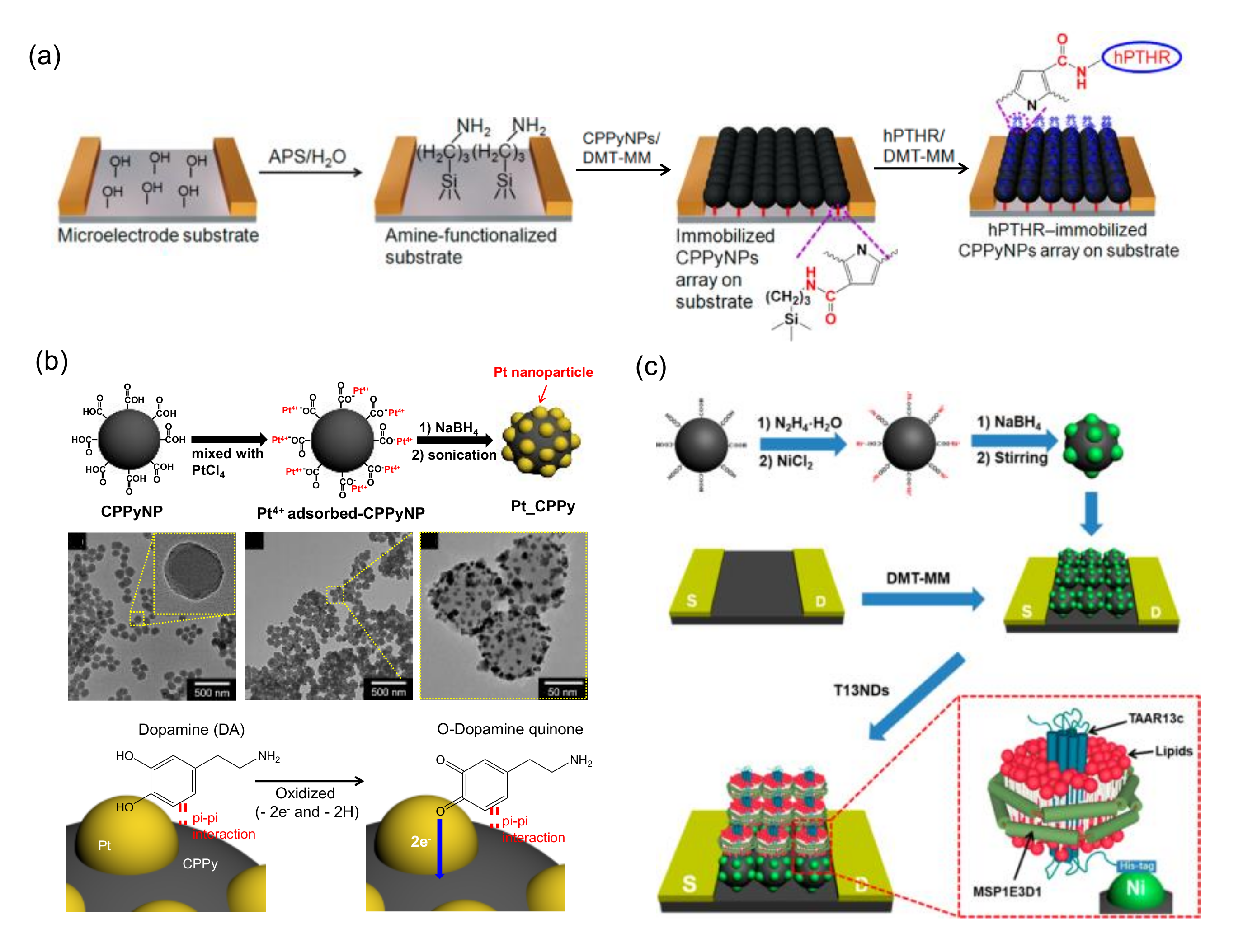










| Nanostructure | Receptor | Analyte | Mechanism | LOD 1 | Response Time | Working Temperature | Linearity | Cycle Stability | Reference |
|---|---|---|---|---|---|---|---|---|---|
| CPPyNP 2 | hPTHR | hTPH 3 | FET 4 | 48 fM | <10 s | 25 °C | 48 fM–480 pM | 2 weeks | [79] |
| Pt_CPPyNP | - | Dopamine | FET | 100 fM | <10 s | 25 °C | 0.1 pM–1 nM | 4 weeks | [80] |
| Ni-CPPyNP | TAAR13c 5-embedded nanodisk | Cadaverine | FET | 100 aM | <5 s | 25 °C | 0.1 fM–100 µM | 5 weeks | [81] |
| CPPyNT | hTAS2R38 6 | PTC 7 PROP 8 | FET | 1 fM 10 fM | <5 s | 25 °C | 1 fM–1 µM | 1 week | [84] |
| CPPyNT | 17β-estradiol binding aptamer | 17β-estradio | FET | 1 fM | <10 s | 25 °C | 1 fM–1 nM | 4 weeks | [85] |
| Pt_CPPyNF | - | Oxalic acid | FET | 100 fM | <10 s | 25 °C | 10 fM–100 pM | 8 weeks | [86] |
| PPy-PVS 9 film | CT-dsDNA 10 | 2-aminoantharcene o-chlorophenol | Amperometric | 0.01 ppm 0.1 ppm | <30 s | 25 °C | 0.01–20 ppm 0.1–30 ppm | - | [87] |
| PPy-DBS 11 film | ChOx 12 | Cholesterol | Amperometric | 0.11 µM | - | 25 °C | 0.11 µM–1.9 mM | 30 days | [88] |
| PPy-PVA | HRP 13 GoD 14 | H2O2 Glucose | Amperometric | 10 µM 1 mM | <10 s | 25 °C | 10 µM–10 mM 1–5 mM | - | [89] |
| 3D PPyNF | F4P1A3 15 | Cortisol | FET | 100 aM | <5 s | 25 °C | 100 aM–10 nM | 30 days | [90] |
| 3D CPPy plate-based film | PDGF-B binding aptamer | PDGF-BB 16 | FET | 1.78 fM | <10 s | 25 °C | 1.78 fM–17.8 pM | 4 weeks | [91] |
| 3D PPy film | HBsAg-binding aptamer | HBsAg 17 | FET | 10 aM | <10 s | 25 °C | 10 aM–0.1 µM | 500 cycles | [92] |
| Urchin-like CPPyNP | BPA binding aptamer | BPA 18 | FET | 1 fM | <10 s | 25 °C | 1 fM–10 pM | 4 weeks | [93] |
| Nanostructure | Receptor | Analyte | Mechanism | LOD 1 | Response Time | Working Temperature | Linearity | Cycle Stability | Reference |
|---|---|---|---|---|---|---|---|---|---|
| HCS@PANI 2 | AChE 3 | Malation | Electrochemical | 2.15 pM | - | 25 °C | 1.0 ng mL−1–10 µg mL−1 | - | [97] |
| HCS-PANI 4 | Thiolated-probe DNA | Hepatitis B Virus | Electrochemical | 3.62 fM | - | 25 °C | 10 fM–1 nM | - | [98] |
| SiO2 (LuPc2) PANI (PVIA)-CNB 5 | GOx | Glucose | Electrochemical | 0.1 mM | <2 s | 25 °C | 1–16 mM | 45 days | [99] |
| Au/PANI NW | GOx | Glucose | ECL 6 | 0.05 µM | - | 25 °C | 0.1–100 µM | 40 cycles | [100] |
| PEGlated PANI NF | Aptamer | BRCA1 7 | Electrochemical | 3.8 fM | - | 25 °C | 0.01 pM–1 nM | - | [101] |
| Electrospun PANI NFs | COX-2 polyclonal antibody | COX-2 enzyme 8 | Electrochemical | 0.01 pg mL−1 | - | 25 °C | 0.01 pg mL−1–1 µg mL−1 | - | [102] |
| PANI NTs | HRP 9 | H2O2 | Electrochemical | 8.1 fM | <200 s | 25 °C | 0.01–90 µM | - | [103] |
| PANI NTs | Aptamer | CML 10 | Electrochemical | 0.1 fM | - | 25 °C | 0.1 fM–1 µM | 40 days | [104] |
| 2D PANI | RGD 11 | Dopamine | Electrical | 2 nM | - | 25 °C | 2 nM–1 µM | 48 h | [105] |
| PANI nanosheet | - | Glucose | Electrochemical | 0.043 µM | <1 s | 25 °C | 1 µM–1 mM | - | [106] |
| NiO/PANI nanosheets | - | Glucose | Electrochemical | 0.06 µM | <10 s | 25 °C | 1 µM–1 mM | 60 days | [107] |
| 2D PANI layer | Antibody | BNT 12 | FET 13 | 50 pg mL−1 | - | 25 °C | 50–1000 pg mL−1 | - | [108] |
| 2D PANI | Antibody | BNT | FET | 100 pg mL−1 | - | 25 °C | 100–1000 pg mL−1 | - | [109] |
| 3D PANI hydrogel/Ag/ABEI | Xanthine oxidase | Xanthine | ECL | 9.6 nM | - | 25 °C | 0.01–200 µM | - | [110] |
| 3D PANI hydrogel/PtB7-Th | Xanthine oxidase | Guanine | PEC 14 | 0.02 µM | <2 s | 25 °C | 0.1–80 µM | 2 days | [111] |
| 3D PANI hydrogel/Pt | Uricase | Uric acid | Electrochemical | 1 µM | <3 s | 25 °C | 0.07–1 mM | - | [112] |
| 3D PANI-MoS2 nanoflower | Aptamer | CML | Electrochemical | 3 aM | - | 25 °C | 0.01 fM–1 µM | - | [113] |
| PANI/graphene microflower | - | Cholesterol | Electrochemical | 1.93 mg dL−1 | - | 25 °C | 1.93–464.04 mg mL−1 | - | [114] |
| Nanostructure | Receptor | Analyte | Mechanism | LOD 1 | Response Time | Working Temperature | Linearity | Cycle Stability | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Pt/PEDOT nanosphere | - | H2O2 | Electrochemical | 2.84 µM | <10 s | 25 °C | 2.5 µM–mM | 3 weeks | [117] |
| Pt/PEDOT microsphere | GOx | Glucose | Electrochemical | 1.15 µM | <10 s | 25 °C | 0.1–10 mM | 12 days | [118] |
| PEDOT/MnO2 nanoellipsod | - | Catechol amine | Electrical | 0.25 mM | <20 s | 25 °C | 0.25–25 mM | - | [119] |
| BPEI-FC 2/PEDOT:PSS nanobead | GOx | Glucose | Electrochemical | 2.4 mM | <20 s | 25 °C | 0.5–5 mM | - | [120] |
| PEDOT:PSS/CS micelle | HRP | H2O2 | Electrochemical | 30 pM | - | 25 °C | 0.1 nM–10 µM | 35 days | [121] |
| PEDOT NT | hDRD1 3 | Dopamine | FET 4 | 10 fM | <1 s | 25 °C | 10 pM–10 nM | - | [122] |
| PEDOT:PSS-silk fibroin core-sheath wires | - | Ascorbic acid | Electrical | 1.14 µM | <20 s | 25 °C | 1.14–800 µM | 4 weeks | [123] |
| PEDOT:PSS | PLL 5-g-OEG 6-Biotin | Streptavidin | Electrochemical | 1 fg mL−1 | - | 25°C | 1–1000 fg mL−1 | - | [124] |
| PEDOT NF | GOx | Glucose | Electrochemical | 2.9 µM | <3 s | 25 °C | 2.9 µM–25 mM | 25 cycles | [125] |
| PEDOT nanowhisker | Au-Hb 7 | H2O2 | Electrochemical | 0.6 µM | <20 s | 25 °C | 1 µM–1.1 mM | 60 days | [126] |
| PEDOT:PSS-based LBL film | GOx | Glucose | Electrochemical | 41 µM | <20 s | 25 °C | 0.1–1.4 mM | - | [127] |
| PEDOT/Au | Antibody | VEGF 8 | Electrochemical | 0.5 pg mL−1 | - | 25 °C | 1–20 pg mL−1 | - | [128] |
| Au-PEDOT-Graphene Aerogel | Antibody | PSA 9 | Electrochemical | 0.03 pg mL−1 | - | 25 °C | 0.1 pg mL−1–50 ng mL−1 | - | [131] |
| 3D PEDOT NF network | LDH 10 | Lactate | Electrochemical | 0.05 mM | <10 s | 25 °C | 0.05–1.8 mM | - | [132] |
| 3D PEDOT NF membrane | hDRD1 | Dopamine | FET | 100 fM | <2 s | 25 °C | 0.1–100 pM | - | [133] |
| Nanostructure | Receptor | Analyte | Mechanism | LOD 1 | Response Time | Working Temperature | Linearity | Cycle Stability | Reference |
|---|---|---|---|---|---|---|---|---|---|
| PTTzFr 2 film | GOx 3 | Glucose | Electrochromic | 12.8 µM | <10 s | 25 °C | 12.8–500 µM | - | [134] |
| PMNT 4 film | Aptamer | Thrombin | Colorimetric | 4 pM | - | 25 °C | 0.01–0.1 nM | - | [135] |
| PT-based LBL 5 film | Biotin | miR-221 | Electrochemical | 0.7 pM | - | 25 °C | 0.7–100 pM | - | [136] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Lee, J.S. Recent Development of Morphology Controlled Conducting Polymer Nanomaterial-Based Biosensor. Appl. Sci. 2020, 10, 5889. https://doi.org/10.3390/app10175889
Cho S, Lee JS. Recent Development of Morphology Controlled Conducting Polymer Nanomaterial-Based Biosensor. Applied Sciences. 2020; 10(17):5889. https://doi.org/10.3390/app10175889
Chicago/Turabian StyleCho, Sunghun, and Jun Seop Lee. 2020. "Recent Development of Morphology Controlled Conducting Polymer Nanomaterial-Based Biosensor" Applied Sciences 10, no. 17: 5889. https://doi.org/10.3390/app10175889
APA StyleCho, S., & Lee, J. S. (2020). Recent Development of Morphology Controlled Conducting Polymer Nanomaterial-Based Biosensor. Applied Sciences, 10(17), 5889. https://doi.org/10.3390/app10175889






