Biomechanical Analysis of Allograft Spacer Failure as a Function of Cortical-Cancellous Ratio in Anterior Cervical Discectomy/Fusion: Allograft Spacer Alone Model
Abstract
1. Introduction
2. Materials and Methods
2.1. FEM of an Intact Cervical Spine
2.2. Allograft Spacer Model
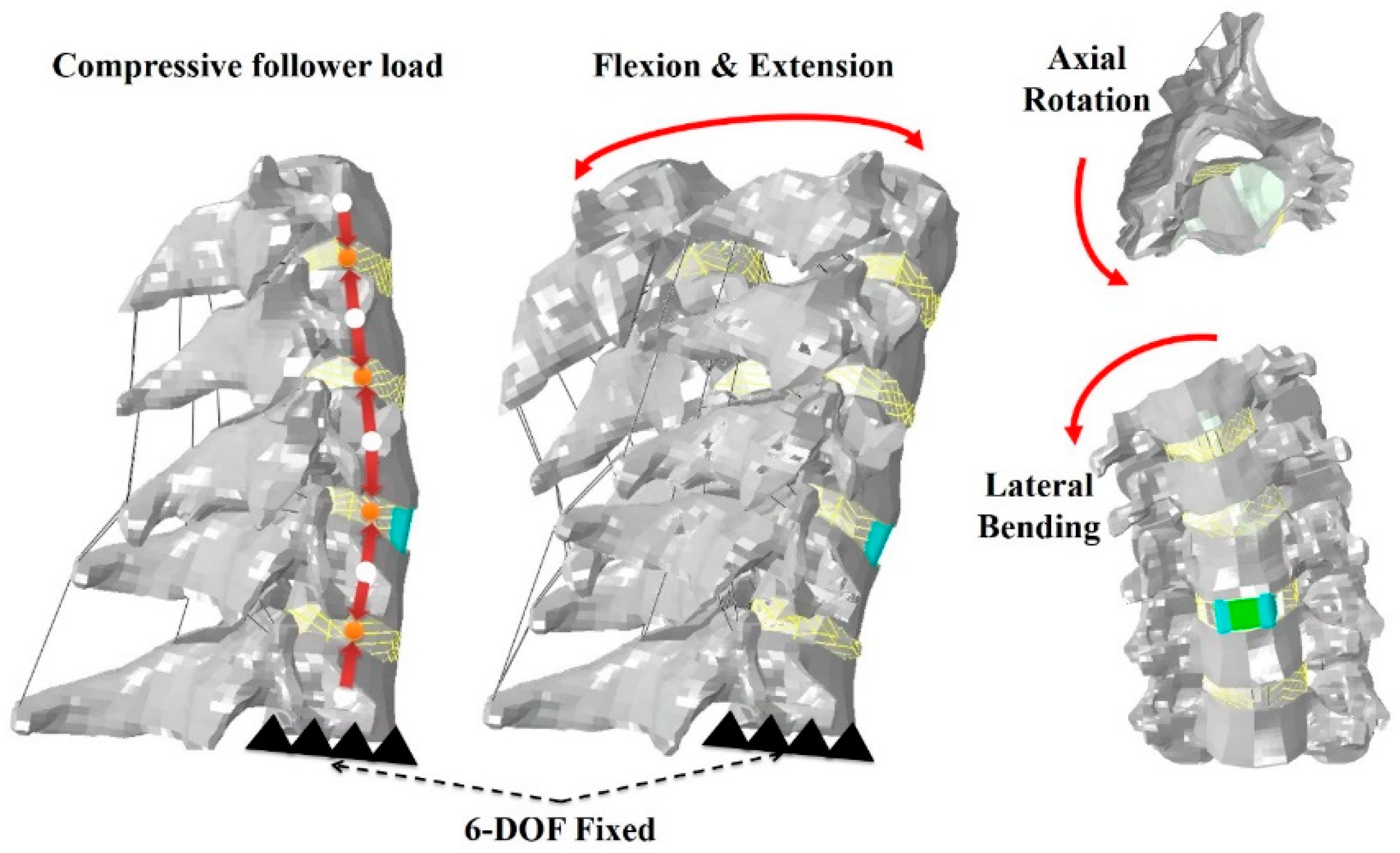
2.3. Loading and Boundary Conditions
3. Results
3.1. Range of Motion
3.2. Stress Analysis of Cervical Spacers with Different Cortico-Cancellous Ratios
3.3. Stress Analysis of Endplates of Involved Lower Cervical Segments
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, C.-H.; Chung, C.-K.; Kim, C.H.; Kwon, J.-W. Health Care Burden of Spinal Diseases in the Republic of Korea: Analysis of a Nationwide Database From 2012 Through 2016. Neurospine 2018, 15, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Kotkansalo, A.; Leinonen, V.; Korajoki, M.; Salmenkivi, J.; Korhonen, K.; Malmivaara, A. Surgery for degenerative cervical spine disease in Finland, 1999–2015. Acta Neurochir. 2019, 161, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Yu, C.H.; Chang, B.-S.; Yeom, J.S.; Lee, J.H.; Lee, C.-K. Subsidence and Nonunion after Anterior Cervical Interbody Fusion Using a Stand-Alone Polyetheretherketone (PEEK) Cage. Clin. Orthop. Surg. 2011, 3, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Pandita, N.; Gupta, S.; Raina, P.; Srivastava, A.; Hakak, A.Y.; Singh, O.; Darokhan, M.A.-U.-D.; Butt, M.F. Neurological Recovery Pattern in Cervical Spondylotic Myelopathy after Anterior Surgery: A Prospective Study with Literature Review. Asian Spine J. 2019, 13, 423–431. [Google Scholar] [CrossRef]
- Viswanathan, V.K.; Manoharan, S.R. To Plate or Not to Plate after a Single- or Two-Level Anterior Cervical Discectomy: Fusion with Cage-Plate Construct or Stand-Alone Cage. Asian Spine J. 2017, 11, 1–3. [Google Scholar] [CrossRef][Green Version]
- Schmieder, K.; Wolzik-Grossmann, M.; Pechlivanis, I.; Engelhardt, M.; Scholz, M.; Harders, A. Subsidence of the Wing titanium cage after anterior cervical interbody fusion: 2-year follow-up study. J. Neurosurg. Spine 2006, 4, 447–453. [Google Scholar] [CrossRef]
- Čabraja, M.; Oezdemir, S.; Koeppen, D.; Kroppenstedt, S. Anterior cervical discectomy and fusion: Comparison of titanium and polyetheretherketone cages. BMC Musculoskelet. Disord. 2012, 13, 172. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Lu, X.; Yang, L.; Yang, H.; Yuan, W.; Chen, D. Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: A prospective, randomized, control study with over 7-year follow-up. Eur. Spine J. 2013, 22, 1539–1546. [Google Scholar] [CrossRef]
- Chau, A.M.T.; Mobbs, R.J. Bone graft substitutes in anterior cervical discectomy and fusion. Eur. Spine J. 2009, 18, 449–464. [Google Scholar] [CrossRef]
- Ryu, S.I.; Lim, J.T.; Kim, S.M.; Paterno, J.; Willenberg, R.; Kim, D.H. Comparison of the biomechanical stability of dense cancellous allograft with tricortical iliac autograft and fibular allograft for cervical interbody fusion. Eur. Spine J. 2006, 15, 1339–1345. [Google Scholar] [CrossRef][Green Version]
- Lee, J.C.; Jang, H.-D.; Ahn, J.; Choi, S.-W.; Kang, D.; Shin, B.-J. Comparison of Cortical Ring Allograft and Plate Fixation with Autologous Iliac Bone Graft for Anterior Cervical Discectomy and Fusion. Asian Spine J. 2019, 13, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ordway, N.R.; Rim, B.C.; Tan, R.; Hickman, R.; Fayyazi, A.H. Anterior cervical interbody constructs: Effect of a repetitive compressive force on the endplate. J. Orthop. Res. 2011, 30, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Kim, D.H.; Park, K.D.; Park, J.H.; Yoo, N.K.; Cho, K.H.; Kim, S.H. Clinical Outcomes and Finite Element Method Results of Anterior Cervical Discectomy and Fusion Using H-Beam Shaped Allospacer: A Comparison with Rim-Shaped Allospacer. Nerve 2019, 5, 49–54. [Google Scholar] [CrossRef]
- Jung, T.-G.; Woo, S.-H.; Park, K.-M.; Jang, J.-W.; Han, D.-W.; Lee, S.J. Biomechanical behavior of two different cervical total disc replacement designs in relation of concavity of articular surfaces: ProDisc-C® vs. Prestige-LP®. Int. J. Precis. Eng. Manuf. 2013, 14, 819–824. [Google Scholar] [CrossRef]
- Kwon, J.-W.; Bang, S.H.; Park, T.H.; Lee, S.-J.; Lee, H.-M.; Lee, S.-B.; Lee, B.H.; Moon, S.-H. Biomechanical comparison of cervical discectomy/fusion model using allograft spacers between anterior and posterior fixation methods (lateral mass and pedicle screw). Clin. Biomech. 2020, 73, 226–233. [Google Scholar] [CrossRef]
- Galbusera, F.; Bellini, C.M.; Raimondi, M.T.; Fornari, M.; Assietti, R. Cervical spine biomechanics following implantation of a disc prosthesis. Med. Eng. Phys. 2008, 30, 1127–1133. [Google Scholar] [CrossRef]
- Ritzel, H.; Amling, M.; Pösl, M.; Hahn, M.; Delling, G. The Thickness of Human Vertebral Cortical Bone and its Changes in Aging and Osteoporosis: A Histomorphometric Analysis of the Complete Spinal Column from Thirty-Seven Autopsy Specimens. J. Bone Miner. Res. 1997, 12, 89–95. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Teo, E.C.; Ng, H.W.; Lee, V.S.; Lee, P.V.S. Finite element analysis of moment-rotation relationships for human cervical spine. J. Biomech. 2006, 39, 189–193. [Google Scholar] [CrossRef]
- Ha, S.K. Finite element modeling of multi-level cervical spinal segments (C3–C6) and biomechanical analysis of an elastomer-type prosthetic disc. Med. Eng. Phys. 2006, 28, 534–541. [Google Scholar] [CrossRef]
- Kim, J.-D.; Kim, N.-S.; Hong, C.-S.; Oh, C.-Y. Design optimization of a xenogeneic bone plate and screws using the Taguchi and finite element methods. Int. J. Precis. Eng. Manuf. 2011, 12, 1119–1124. [Google Scholar] [CrossRef]
- Whyne, C.M.; Hu, S.S.; Klisch, S.; Lotz, J.C. Effect of the Pedicle and Posterior Arch on Vertebral Body Strength Predictions in Finite Element Modeling. Spine 1998, 23, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Faizan, A.; Goel, V.K.; Garfin, S.R.; Bono, C.M.; Serhan, H.; Biyani, A.; Elgafy, H.; Krishna, M.; Friesem, T. Do design variations in the artificial disc influence cervical spine biomechanics? A finite element investigation. Eur. Spine J. 2009, 21, 653–662. [Google Scholar]
- Harrison, D.E.; Harrison, D.D.; Cailliet, R.; Troyanovich, S.J.; Janik, T.J.; Holland, B. Cobb Method or Harrison Posterior Tangent Method: Which to choose for lateral cervical radiographic analysis. Spine 2000, 25, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Wang, F.; Wang, N.; Li, X.; Wang, Q. 3-D finite element analysis of the influence of synovial condition in sacroiliac joint on the load transmission in human pelvic system. Med. Eng. Phys. 2014, 36, 745–753. [Google Scholar] [CrossRef]
- Black, J.; Hastings, G. Handbook of Biomaterial Properties; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Smith, G.W.; Robinson, R.A. The Treatment of Certain Cervical-Spine Disorders by Anterior Removal of the Intervertebral Disc and Interbody Fusion. J. Bone Jt. Surg. 1958, 40, 607–624. [Google Scholar] [CrossRef]
- Sis, H.L.; Mannen, E.M.; Wong, B.M.; Cadel, E.S.; Bouxsein, M.L.; Anderson, D.E.; Friis, E.A. Effect of follower load on motion and stiffness of the human thoracic spine with intact rib cage. J. Biomech. 2016, 49, 3252–3259. [Google Scholar] [CrossRef]
- Panjabi, M.M. Hybrid multidirectional test method to evaluate spinal adjacent-level effects. Clin. Biomech. 2007, 22, 257–265. [Google Scholar] [CrossRef]
- Panjabi, M.M.; Crisco, J.J.; Vasavada, A.; Oda, T.; Cholewicki, J.; Nibu, K.; Shin, E. Mechanical Properties of the Human Cervical Spine as Shown by Three-Dimensional Load–Displacement Curves. Spine 2001, 26, 2692–2700. [Google Scholar] [CrossRef]
- Ivancic, P.C. Biomechanics of Sports-Induced Axial-Compression Injuries of the Neck. J. Athl. Train. 2012, 47, 489–497. [Google Scholar] [CrossRef]
- Kwon, J.-W.; Bang, S.-H.; Kwon, Y.-W.; Cho, J.-Y.; Park, T.-H.; Lee, S.-J.; Lee, H.-M.; Moon, S.-H.; Lee, B.-H. Biomechanical comparison of the angle of inserted screws and the length of anterior cervical plate systems with allograft spacers. Clin. Biomech. 2020, 76, 105021. [Google Scholar] [CrossRef]
- Kao, T.H.; Wu, C.H.; Chou, Y.C.; Chen, H.T.; Chen, W.H.; Tsou, H.K. Risk factors for subsidence in anterior cervical fusion with stand-alone polyetheretherketone (PEEK) cages: A review of 82 cases and 182 levels. Arch. Orthop. Trauma Surg. 2014, 134, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.K.; Faizan, A.; Palepu, V.; Bhattacharya, S. Parameters that effect spine biomechanics following cervical disc replacement. Eur. Spine J. 2012, 21, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, Y.B.; Park, S.W. Risk factors for postoperative subsidence of single-level anterior cervical discectomy and fusion: The significance of the preoperative cervical alignment. Spine (Phila Pa 1976) 2014, 39, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- De Leo–Vargas, R.A.; Muñoz–Romero, I.; Mondragón–Soto, M.G.; Martínez–Anda, J.J. Locking Stand-Alone Cage Constructs for the Treatment of Cervical Spine Degenerative Disease. Asian Spine J. 2019, 13, 630. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Karikari, I.O.; Jain, D.; Owens, T.R.; Gottfried, O.; Hodges, T.R.; Nimjee, S.M.; Bagley, C.A. Impact of subsidence on clinical outcomes and radiographic fusion rates in anterior cervical discectomy and fusion: A systematic review. J. Spinal Disord. Tech. 2014, 27, 1–10. [Google Scholar] [CrossRef]
- Cheng, C.C.; Ordway, N.R.; Zhang, X.; Lu, Y.M.; Fang, H.; Fayyazi, A.H. Loss of cervical endplate integrity following minimal surface preparation. Spine (Phila Pa 1976) 2007, 32, 1852–1855. [Google Scholar] [CrossRef]
- Chiang, M.-F.; Teng, J.-M.; Huang, C.-H.; Cheng, C.-K.; Chen, C.-S.; Chang, T.-K.; Chao, S.-H. Finite element analysis of cage subsidence in cervicalinterbody fusion. J. Med. Biol. Eng. 2004, 24, 201–208. [Google Scholar]
- Liu, N.; Lu, T.; Wang, Y.; Sun, Z.; Li, J.; He, X. Effects of new cage profiles on the improvement in biomechanical performance of multilevel anterior cervical Corpectomy and fusion: A finite element analysis. World Neurosurg. 2019, 129, e87–e96. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Guo, X.; Cai, Z.; Liu, H.; Xue, Y. Biomechanical Effect of Different Graft Heights on Adjacent Segment and Graft Segment Following C4/C5 Anterior Cervical Discectomy and Fusion: A Finite Element Analysis. Med. Sci. Monit. Int. Med J. Exp. Clin. Res. 2019, 25, 4169. [Google Scholar] [CrossRef]
- Wang, J.; Qian, Z.; Ren, L. Biomechanical Comparison of Optimal Shapes for the Cervical Intervertebral Fusion Cage for C5–C6 Cervical Fusion Using the Anterior Cervical Plate and Cage (ACPC) Fixation System: A Finite Element Analysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 8379. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, W.M.; Kim, Y.H.; Jahng, T.-A. A biomechanical analysis of an artificial disc with a shock-absorbing core property by using whole-cervical spine finite element analysis. Spine 2016, 41, E893–E901. [Google Scholar] [CrossRef]
- Lee, S.-H.; Im, Y.-J.; Kim, K.-T.; Kim, Y.-H.; Park, W.-M.; Kim, K. Comparison of cervical spine biomechanics after fixed-and mobile-core artificial disc replacement: A finite element analysis. Spine 2011, 36, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Chuang, S.-Y.; Chiang, C.-J.; Tsuang, Y.-H.; Chen, W.-P. Finite element analysis of cervical spine with different constrained types of total disc replacement. J. Mech. Med. Biol. 2014, 14, 1450038. [Google Scholar] [CrossRef]
- Kim, Y.H.; Khuyagbaatar, B.; Kim, K. Recent advances in finite element modeling of the human cervical spine. J. Mech. Sci. Technol. 2018, 32, 1–10. [Google Scholar] [CrossRef]
- Kim, M.K.; Kwak, D.S.; Park, C.K.; Park, S.H.; Oh, S.M.; Lee, S.W.; Han, S.H. Quantitative anatomy of the endplate of the middle and lower cervical vertebrae in Koreans. Spine (Phila Pa 1976) 2007, 32, E376–E381. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Yin, P.; Khan, K.; Tsai, T.Y.; Li, J.S.; Hai, Y.; Tang, P.; Li, G. Differences of the Morphology of Subaxial Cervical Spine Endplates between Chinese and White Men and Women. Biomed. Res. Int. 2018, 2018, 2854175. [Google Scholar] [CrossRef] [PubMed]



| Component Name | Young’s Modulus (MPa) | Poisson’s Ratio | Ref. |
|---|---|---|---|
| Cortical bone | 12,000 | 0.3 | [22] |
| Cancellous bone | 100 | 0.29 | [18] |
| Posterior element | 3.500 | 0.29 | [16] |
| End plate | 500 | 0.4 | [19] |
| Annulus matrix | 4.2 | 0.45 | [19] |
| Annulus Fibers | 500 | Cross-sectional Area 0.1 (mm2) | [20] |
| Nucleus pulposus | 1.0 | 0.499 (Incompressible) | [19] |
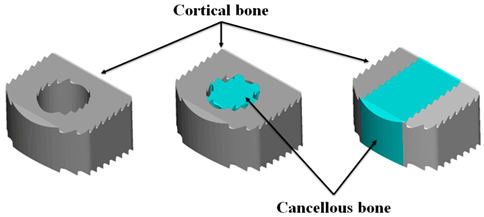
| Cortical only | Cortico-Cancellous | Cortical lateral Walls with a Cancellous Center Bone | ||||
|---|---|---|---|---|---|---|
| Cortical | Cancellous | Cortical | Cancellous | Cortical | Cancellous | |
| 11 mm | 1 | 0 | 1 | 0.32 | 0.46 | 0.54 |
| 12 mm | 1 | 0 | 1 | 0.28 | 0.47 | 0.53 |
| 14 mm | 1 | 0 | 1 | 0.23 | 0.47 | 0.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.-W.; Lee, H.-M.; Park, T.-H.; Lee, S.J.; Kwon, Y.-W.; Moon, S.-H.; Lee, B.H. Biomechanical Analysis of Allograft Spacer Failure as a Function of Cortical-Cancellous Ratio in Anterior Cervical Discectomy/Fusion: Allograft Spacer Alone Model. Appl. Sci. 2020, 10, 6413. https://doi.org/10.3390/app10186413
Kwon J-W, Lee H-M, Park T-H, Lee SJ, Kwon Y-W, Moon S-H, Lee BH. Biomechanical Analysis of Allograft Spacer Failure as a Function of Cortical-Cancellous Ratio in Anterior Cervical Discectomy/Fusion: Allograft Spacer Alone Model. Applied Sciences. 2020; 10(18):6413. https://doi.org/10.3390/app10186413
Chicago/Turabian StyleKwon, Ji-Won, Hwan-Mo Lee, Tae-Hyun Park, Sung Jae Lee, Young-Woo Kwon, Seong-Hwan Moon, and Byung Ho Lee. 2020. "Biomechanical Analysis of Allograft Spacer Failure as a Function of Cortical-Cancellous Ratio in Anterior Cervical Discectomy/Fusion: Allograft Spacer Alone Model" Applied Sciences 10, no. 18: 6413. https://doi.org/10.3390/app10186413
APA StyleKwon, J.-W., Lee, H.-M., Park, T.-H., Lee, S. J., Kwon, Y.-W., Moon, S.-H., & Lee, B. H. (2020). Biomechanical Analysis of Allograft Spacer Failure as a Function of Cortical-Cancellous Ratio in Anterior Cervical Discectomy/Fusion: Allograft Spacer Alone Model. Applied Sciences, 10(18), 6413. https://doi.org/10.3390/app10186413





