Moth Mating: Modeling Female Pheromone Calling and Male Navigational Strategies to Optimize Reproductive Success
Abstract
:1. Introduction
2. Moth Mating Mechanisms
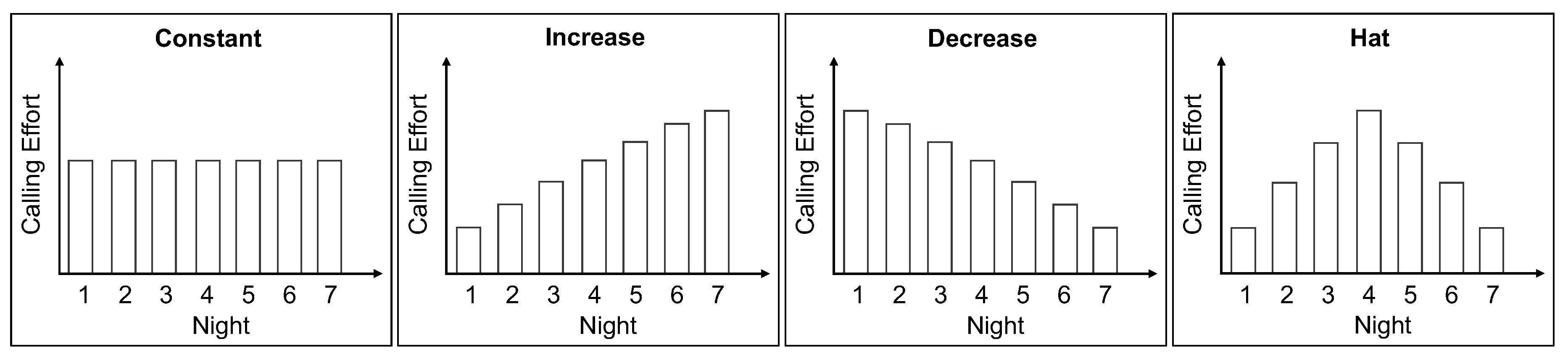
2.1. Female Calling Strategies
- calling duration: ranges from 0.5 to 8 h and
- emitted pheromone: ranges from 1 to 30 ng per night.
2.2. Male Navigational Strategies
- male flight speed: ranges from 0.5 to 5 m/s
- male turning rate: ranges from 3 to 4 turns/s
3. Male Random Flight Model
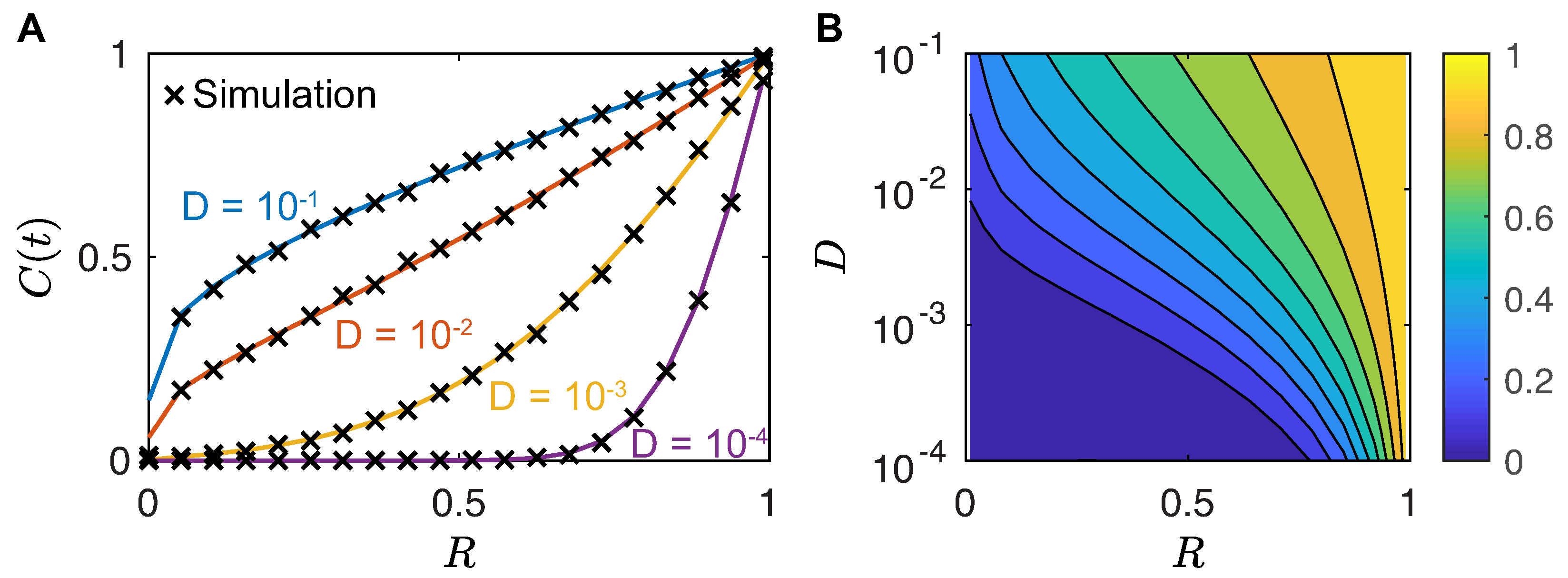
First Arrival and Mating Time
4. Numerical Results for the Male Random Flight Model
4.1. Single Female Calling Period
4.2. First Arrival Time
4.3. Multiple Female Calling Periods
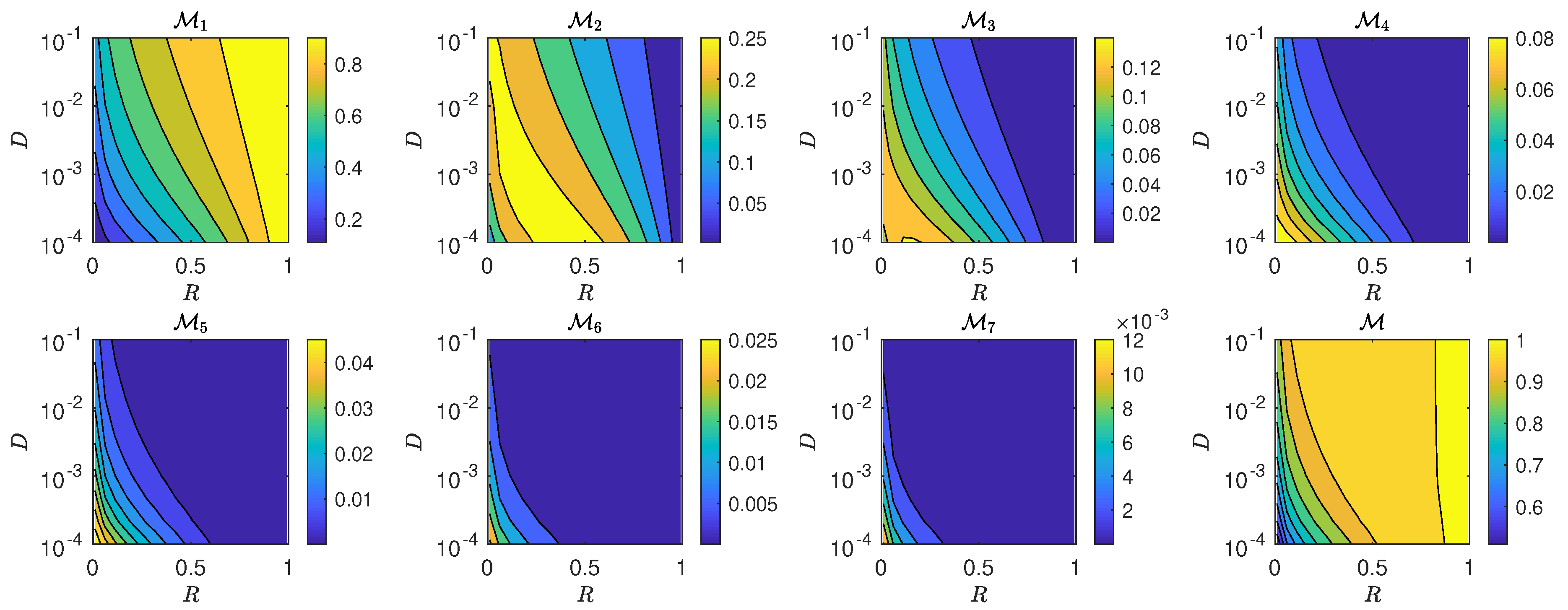
4.4. Night-by-Night Mating Probabilities
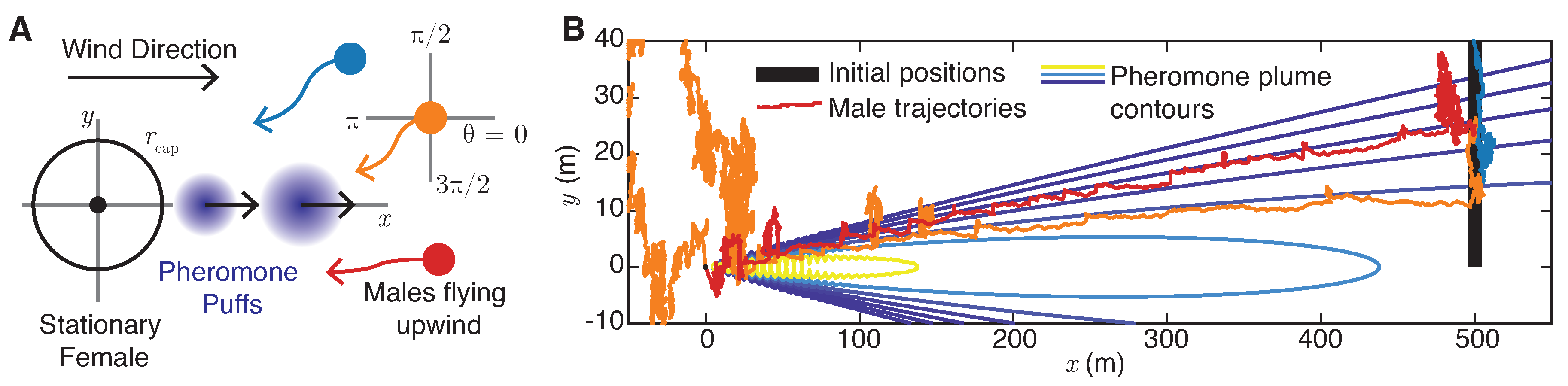
5. Plume Navigation Model
5.1. Male Navigation Algorithm
- Random flight: If the male moth does not detect pheromone () and has never detected pheromone at earlier times , the moth chooses a random direction drawn uniformly from . Note that this motion implies that, overall, the moth executes a random flight with effective diffusivity . For the experimentally measured male flight speeds reported in Section 2.2, to 5 m/s, and for s, this corresponds to to m2/s.
- Surging: Upon detection of pheromone signals for , the moth will align with the upwind direction of airflow () with a margin of error . The updated direction of moment will be selected uniformly from at each timestep.
- Casting: Upon loss of contact with the pheromone plume for , the moth will search for the plume perpendicular to the direction of airflow () with a margin of error . The updated direction of movement will be selected uniformly in or in at each timestep. If the male moth is unable to find the lost pheromone plume after casting for a given time , it returns to random flight mode. Otherwise, it returns to surging. Typical total casting times are on the order of 10 s (Martinez et al. [20]), so we choose s.
- Mating: Once the male moth is within a radius of a female, the two successfully mate.
5.2. Female-Generated Pheromone Plume Dynamics
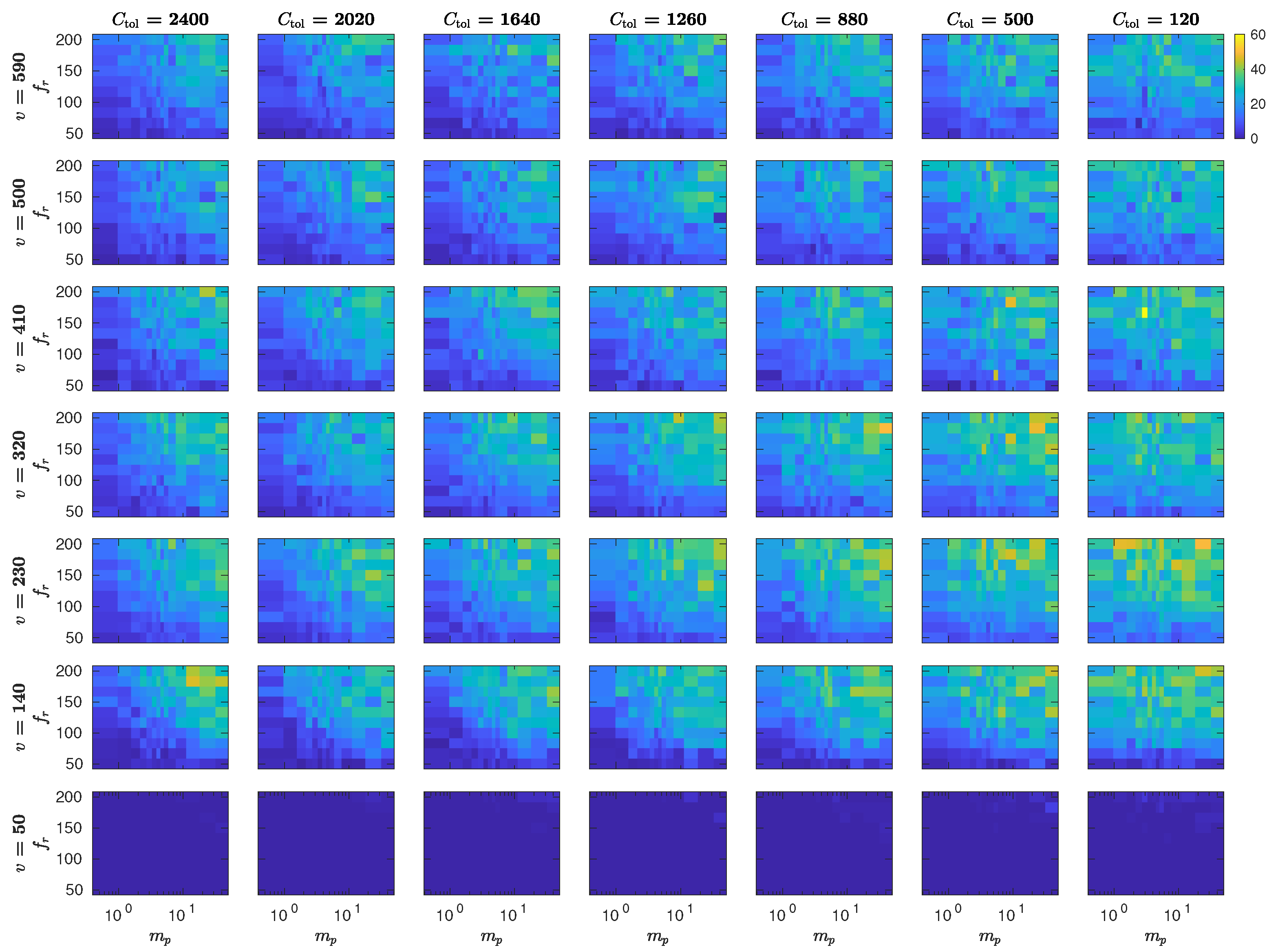
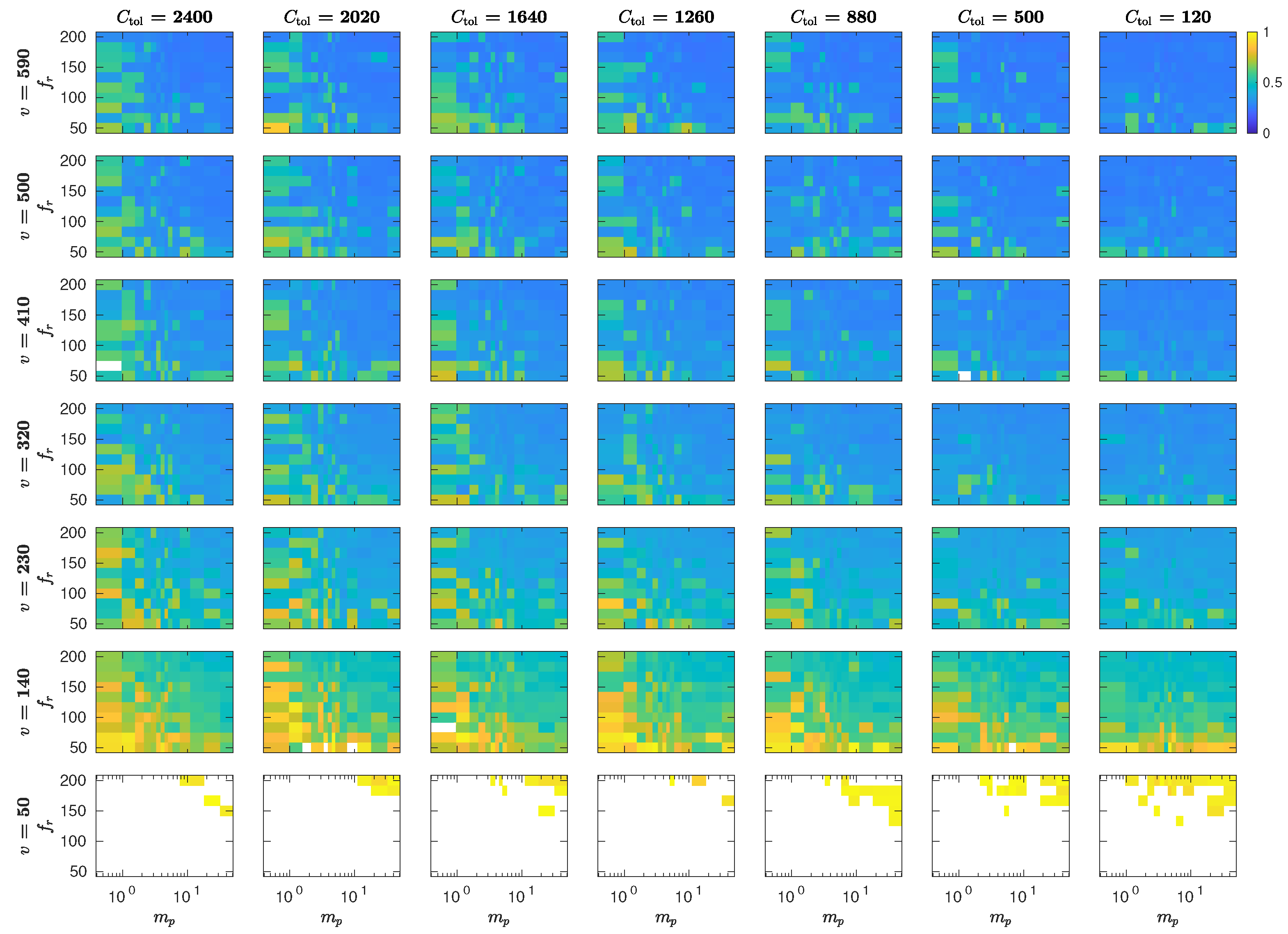
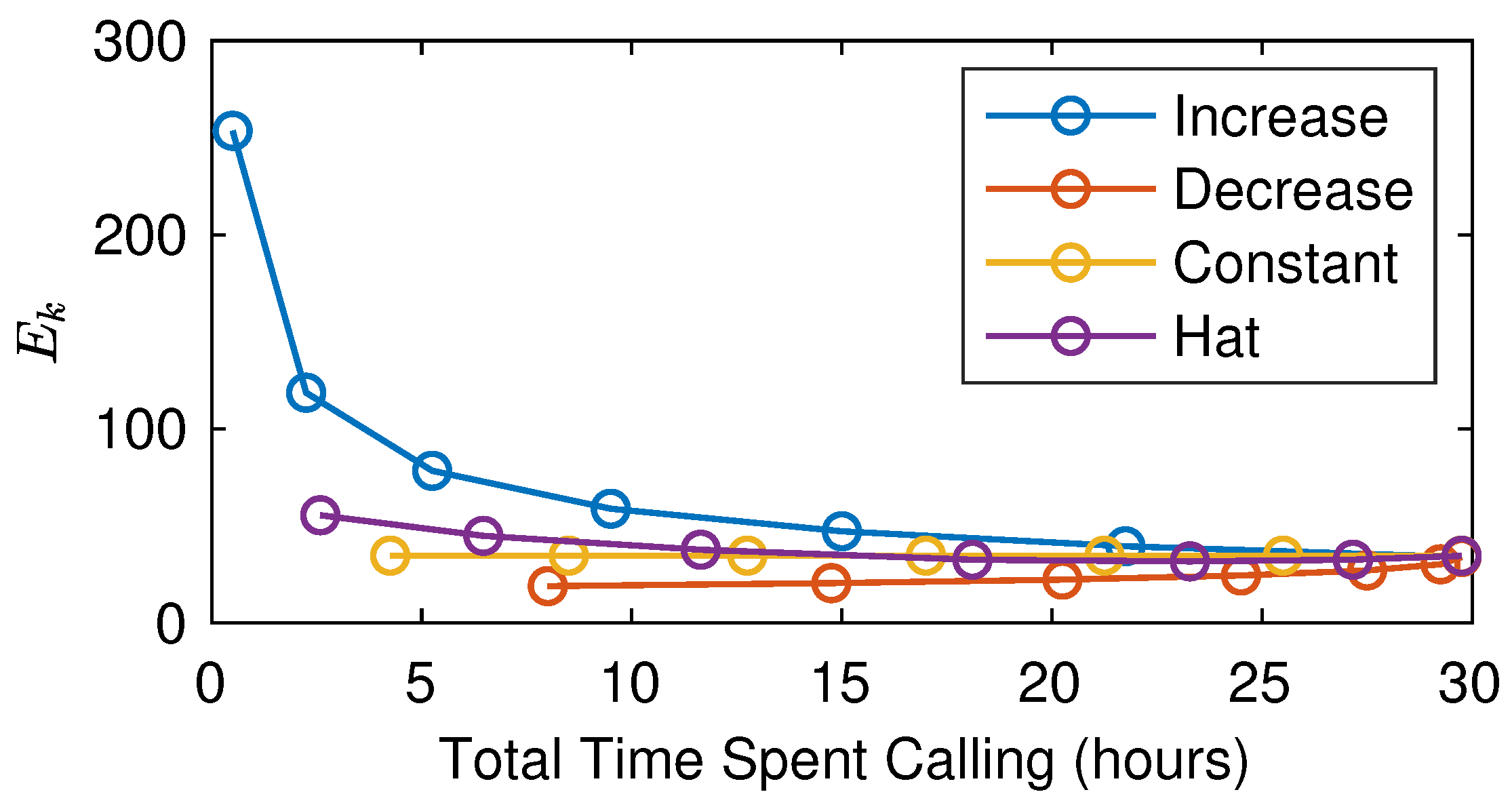
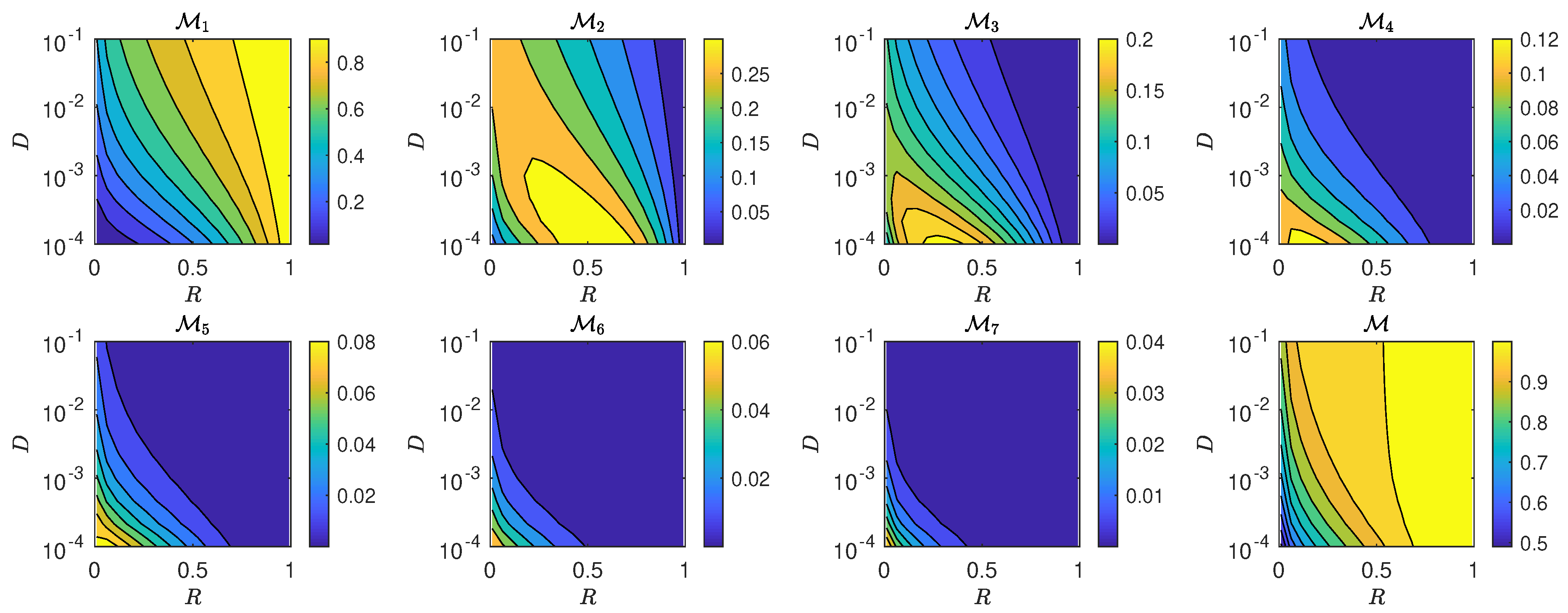
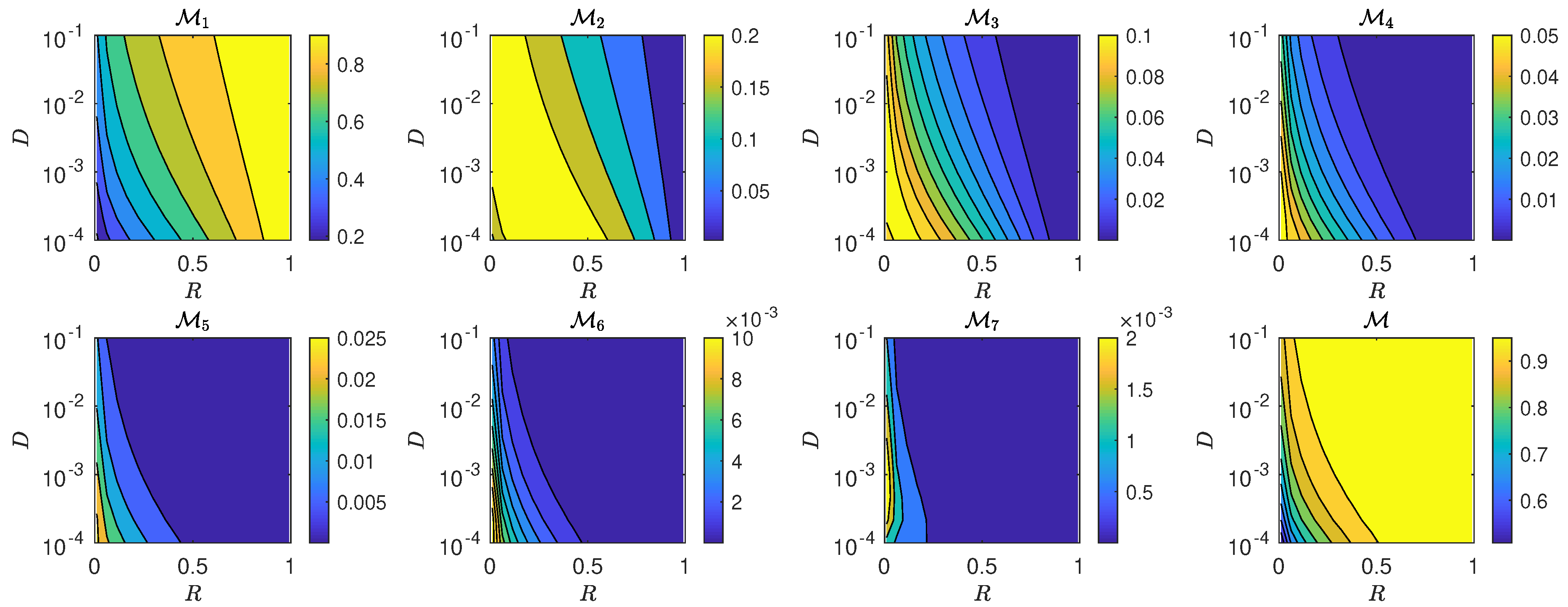
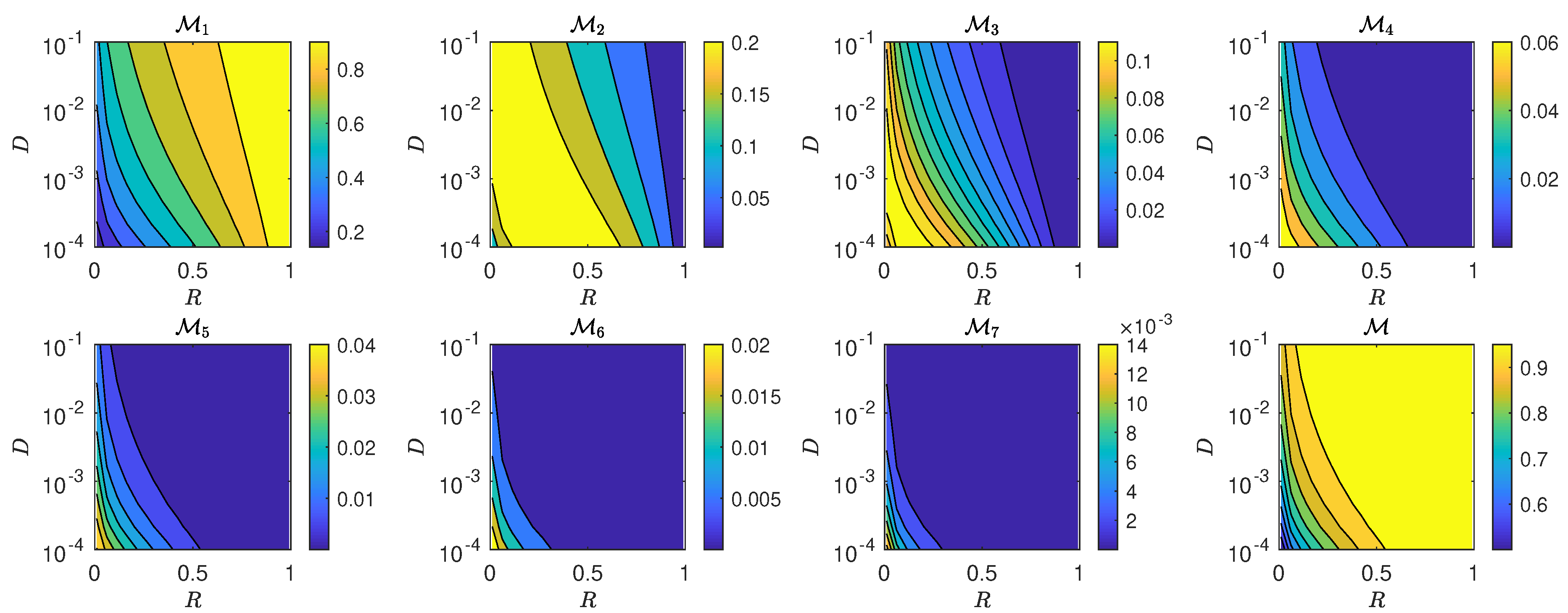
5.3. The Interplay of Calling Time and Pheromone Amount on Male Fitness
6. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Derivation of Arrival Time to Circular Plumes
Appendix B. Asymptotic of the Survival Probability at Short Times
Appendix C. MothPy Simulations
- female pheromone puff release rate:
- female pheromone puff molecular amount:
- male ground flight speed:
- male pheromone detection threshold:
References
- Lees, D.; Zilli, A. Moths: A Complete Guide to Biology and Behavior; Smithsonian Books: Washington, DC, USA, 2019. [Google Scholar]
- Naka, H. Female Sex Pheromones and Mating Behavior in Diurnal Moths: Implications for Conservation Biology. In Chemical Ecology of Insects: Applications and Associations with Plants and Microbes, 1st ed.; Tabata, J., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 170–196. [Google Scholar] [CrossRef]
- i Monteys, V.S.; Quero, C.; Santa-Cruz, M.; Rosell, G.; Guerrero, A. Sexual communication in day-flying Lepidoptera with special reference to castniids or ‘butterfly-moths’. Bull. Entomol. Res. 2016, 106, 421–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conner, W.E.; Eisner, T.; Vander Meer, R.K.; Guerrero, A.; Ghiringelli, D.; Meinwald, J. Sex attractant of an arctiid moth (Utetheisa Ornatrix): A Pulsed Chemical Signal. Behav. Ecol. Sociobiol. 1980, 7, 55–63. [Google Scholar] [CrossRef]
- Stengl, M. Pheromone transduction in moths. Front. Cell. Neurosci. 2010, 4, 133. [Google Scholar] [CrossRef] [Green Version]
- Futrelle, R. How molecules get to their detectors: The physics of diffusion of insect pheromones. Trends Neurosci. 1984, 7, 116–120. [Google Scholar] [CrossRef]
- Groot, A.T. Circadian rhythms of sexual activities in moths: A review. Front. Ecol. Evol. 2014, 2, 43. [Google Scholar] [CrossRef] [Green Version]
- Badeke, E.; Haverkamp, A.; Hansson, B.S.; Sachse, S. A challenge for a male noctuid moth? Discerning the female sex pheromone against the background of plant volatiles. Front. Physiol. 2016, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Leary, G.P.; Allen, J.E.; Bunger, P.L.; Luginbill, J.B.; Linn, C.E.; Macallister, I.E.; Kavanaugh, M.P.; Wanner, K.W. Single mutation to a sex pheromone receptor provides adaptive specificity between closely related moth species. Proc. Natl. Acad. Sci. USA 2012, 109, 14081–14086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenfield, M.D. Moth sex pheromones: An evolutionary perspective. Fla. Entomol. 1981, 64, 4–14. [Google Scholar] [CrossRef]
- Koshio, C.; Muraji, M.; Tatsuta, H.; Kudo, S.I. Sexual selection in a moth: Effect of symmetry on male mating success in the wild. Behav. Ecol. 2007, 18, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Niven, J.E.; Laughlin, S.B. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 2008, 211, 1792–1804. [Google Scholar] [CrossRef] [Green Version]
- Sane, S.P. Induced airflow in flying insects: I. A theoretical model of the induced flow. J. Exp. Biol. 2006, 209, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockl, A.; Heinze, S.; Charalabidis, A.; el Jundi, B.; Warrant, E.; Kelber, A. Differential investment in visual and olfactory brain areas reflects behavioural choices in hawk moths. Sci. Rep. 2016, 6, 26041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, S. How much air passes through a silkmoth’s antenna? J. Insect. Physiol. 1983, 29, 597–599, 601–602. [Google Scholar] [CrossRef]
- Wang, Q.; Shang, Y.; Hilton, D.S.; Inthavong, K.; Zhang, D.; Elgar, M.A. Antennal scales improve signal detection efficiency in moths. Proc. R. Soc. B 2018, 285, 20172832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umbers, K.D.L.; Symonds, M.R.E.; Kokko, H. The Mothematics of female pheromone signaling: Strategies for aging virgins. Am. Nat. 2015, 185, 417–432. [Google Scholar] [CrossRef]
- Harari, A.R.; Zahavi, T.; Thiéry, D. Fitness cost of pheromone production in signaling female moths. Evolution 2011, 65, 1572–1582. [Google Scholar] [CrossRef]
- Haupt, S.S.H.; Sakurai, T.; Namiki, S.; Kazawa, T.; Kanzaki, R. Olfactory Information Processing in Moths. In The Neurobiology of Olfaction; Menini, A., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; Chapter 3; pp. 71–112. [Google Scholar] [CrossRef]
- Martinez, D.; Chaffiol, A.; Voges, N.; Gu, Y.; Anton, S.; Rospars, J.P.; Lucas, P. Multiphasic on/off pheromone signalling in moths as neural correlates of a search strategy. PLoS ONE 2013, 8, e61220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bau, J.; Cardé, R.T. Modeling optimal strategies for finding a resource-linked, windborne odor plume: Theories, robotics, and biomimetic lessons from flying insects. Integr. Comp. Biol. 2015, 55, 461–477. [Google Scholar] [CrossRef] [Green Version]
- Grünbaum, D.; Willis, M.A. Spatial memory-based behaviors for locating sources of odor plumes. Mov. Ecol. 2015, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Baker, K.L.; Dickinson, M.; Findley, T.M.; Gire, D.H.; Louis, M.; Suver, M.P.; Verhagen, J.V.; Nagel, K.I.; Smear, M.C. Algorithms for olfactory search across species. J. Neurosci. 2018, 38, 9383–9389. [Google Scholar] [CrossRef] [Green Version]
- Loveless, J.; Webb, B. A neuromechanical model of larval chemotaxis. Integr. Comp. Biol. 2018, 58, 906–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, S.; Rutkowski, A.J.; Quinn, R.D.; Willis, M.A. Moth-inspired plume tracking strategies in three-dimensions. In Proceedings of the 2005 IEEE International Conference on Robotics and Automation, Barcelona, Spain, 18–22 April 2005; pp. 1669–1674. [Google Scholar]
- Ando, N.; Emoto, S.; Kanzaki, R. Insect-controlled robot: A mobile robot platform to evaluate the odor-tracking capability of an insect. J. Vis. Exp. 2016, 118, e54802. [Google Scholar] [CrossRef]
- Huang, Y.; Yen, J.; Kanso, E. Detection and tracking of chemical trails in bio-inspired sensory systems. Eur. J. Comput. Mech. 2017, 26, 98–114. [Google Scholar] [CrossRef]
- Liberzon, A.; Harrington, K.; Daniel, N.; Gurka, R.; Harari, A.; Zilman, G. Moth-inspired navigation algorithm in a turbulent odor plume from a pulsating source. PLoS ONE 2018, 6, e0198422. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Liu, H.; Ataei, A.; Munk, Y.; Daniel, T.; Paschalidis, I.C. Learning from animals: How to navigate complex terrains. PLoS Comput. Biol. 2020, 16, e1007452. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.S. Zigzagging and casting as a programmed response to wind-borne odour: A review. Physiol. Entomol. 1983, 8, 109–120. [Google Scholar] [CrossRef]
- Mafra-Neto, A.; Cardé, R.T. Fine-scale structure of pheromone plumes modulates upwind orientation of flying moths. Nature 1994, 369, 142–144. [Google Scholar] [CrossRef]
- Cardé, R.T.; Willis, M.A. Navigational strategies used by insects to find distant, wind-borne sources of odor. J. Chem. Ecol. 2008, 34, 854–866. [Google Scholar] [CrossRef]
- Benelli, N.; Gurka, R.; Golov, Y.; Harari, A.; Zilman, G.; Liberzon, A. Open source computational simulation for a moth-inspired navigation algorithm. bioRxiv 2019, 744912. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Q. Mechanisms of last male precedence in a moth: Sperm displacement at ejaculation and storage sites. Behav. Ecol. 2010, 21, 714–721. [Google Scholar] [CrossRef]
- Gao, K.; van Wijk, M.; Clement, Z.; Egas, M.; Groot, A.T. A life-history perspective on sexual selection in a polygamous species. BMC Evol. Biol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Goubault, M.; Burlaud, R. Do males choose their mates in the lekking moth Achroia Grisella? Influence Female Body Mass Male Reprod. Status Male Mate Choice. Insect Sci. 2018, 25, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.L.; Symonds, M.R.E.; Elgar, M.A. Sexual selection on receptor organ traits: Younger females attract males with longer antennae. Sci. Nat. 2017, 104, 44. [Google Scholar] [CrossRef] [PubMed]
- Murlis, J.; Elkinton, J.S.; Cardé, R.T. Odor plumes and how insects use them. Annu. Rev. Entomol. 1992, 37, 505–532. [Google Scholar] [CrossRef]
- Mankin, R.W.; Vick, K.W.; Mayer, M.S.; Coffelt, J.A.; Callahan, P.S. Models for dispersal of vapors in open and confined spaces: Applications to sex pheromone trapping in a warehouse. J. Chem. Ecol. 1980, 6, 929–950. [Google Scholar] [CrossRef]
- Kou, R.; Chow, Y.S. Calling behavior of the cotton bollworm, Heliothis Armigera (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1987, 80, 490–493. [Google Scholar] [CrossRef]
- Mazor, M.; Dunkelblum, E. Circadian rhythms of sexual behavior and pheromone titers of two closely related moth species Autographa Gamma Cornutiplusia Circumflexa. J. Chem. Ecol. 2005, 31, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Ming, Q.L.; Yan, Y.H.; Wang, C.Z. Mechanisms of premating isolation between Helicoverpa Armigera (Hübner) Helicoverpa Assulta (Guenée) (Lepidoptera: Noctuidae). J. Insect Physiol. 2007, 53, 170–178. [Google Scholar] [CrossRef]
- Giebultowicz, J.M.; Raina, A.K.; Uebel, E.C. Mated-like behaviour in senescent virgin females of gypsy moth, Lymantria Dispar. J. Insect Physiol. 1990, 36, 495–498. [Google Scholar] [CrossRef]
- Tang, J.D.; Charlton, R.E.; Cardé, R.T.; Yin, C.M. Diel periodicity and influence of age and mating on sex pheromone titer in gypsy moth, Lymantria Dispar (L.). J. Chem. Ecol. 1992, 18, 749–760. [Google Scholar] [CrossRef]
- Foster, S.P.; Howard, A.J.; Ayers, R.H. Age-related changes in reproductive characters of four species of tortricid moths. N. Z. J. Zool. 1995, 22, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Farrell, J.A.; Murlis, J.; Long, X.; Li, W.; Cardé, R.T. Filament-based atmospheric dispersion model to achieve short time-scale structure of odor plumes. Environ. Fluid Mech. 2002, 2, 143–169. [Google Scholar] [CrossRef]
- Girling, R.D.; Higbee, B.S.; Cardé, R.T. The plume also rises: Trajectories of pheromone plumes issuing from point sources in an orchard canopy at night. J. Chem. Ecol. 2013, 39, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Cardé, R.T.; Cardé, A.M.; Girling, R.D. Observations on the flight paths of the day-flying moth Virbia Lamae Periods Mate Locat. Do Males Have A Strategy Contacting Pheromone Plume? J. Anim. Ecol. 2012, 81, 268–276. [Google Scholar] [CrossRef]
- Elkinton, J.S.; Schal, C.; Onot, T.; Cardé, R.T. Pheromone puff trajectory and upwind flight of male gypsy moths in a forest. Physiol. Entomol. 1987, 12, 399–406. [Google Scholar] [CrossRef]
- Willis, M.A.; Ford, E.A.; Avondet, J.L. Odor tracking flight of male Manduca sexta moths along plumes of different cross-sectional area. J. Comp. Physiol. A 2013, 199, 1015–1036. [Google Scholar] [CrossRef]
- Belanger, J.H.; Arbas, E.A. Behavioral strategies underlying pheromone-modulated flight in moths: Lessons from simulation studies. J. Comp. Physiol. A 1998, 183, 345–360. [Google Scholar] [CrossRef]
- Kuenen, L.P.S.; Cardé, R.T. Effects of moth size on velocity and steering during upwind flight toward a sex pheromone source by Lymantria dispar (Lepidoptera: Lymantriidae). J. Insect Behav. 1993, 6, 177–193. [Google Scholar] [CrossRef]
- Gaydecki, P. Automated moth flight analysis in the vicinity of artificial light. Bull. Entomol. Res. 2019, 109, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M. Adult movements and resident ratios of the black-veined white, Aporia crataeyi, in a hilly region. Jpn. J Ecol. 1978, 28, 101–109. [Google Scholar]
- Kareiva, P.M. Local movement in herbivorous insects: Applying a passive diffusion model to mark-recapture field experiments. Oecologia 1983, 57, 322–327. [Google Scholar] [CrossRef] [PubMed]
- de Bruyne, M.; Baker, T.C. Odor detection in insects: Volatile codes. J. Chem. Ecol. 2008, 34, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.C.; Vogt, R.G. Measured behavioral latency in response to sex-pheromone loss in the large silk moth Antheraea Polyphemus. J. Exp. Biol. 1988, 137, 29–38. [Google Scholar] [PubMed]
- Angioy, A.M.; Desogus, A.; Barbarossa, I.T.; Anderson, P.; Hansson, B.S. Extreme sensitivity in an olfactory system. Chem. Senses 2003, 28, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabuchi, M.; Sakurai, T.; Mitsuno, H.; Namiki, S.; Minegishi, R.; Shiotsuki, T.; Uchino, K.; Sezutsu, H.; Tamura, T.; Haupt, S.S.; et al. Pheromone responsiveness threshold depends on temporal integration by antennal lobe projection neurons. Proc. Natl. Acad. Sci. USA 2013, 110, 15455–15460. [Google Scholar] [CrossRef] [Green Version]
- Nowinszky, L.; Mészáros, Z.; Puskás, J. The hourly distribution of moth species caught by a light trap. Appl. Ecol. Environ. Res. 2007, 5, 103–107. [Google Scholar] [CrossRef]
- Sabelis, M.W.; Schippers, P. Variable wind directions and anemotactic strategies of searching for an odour plume. Oecologia 1984, 63, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Dusenbery, D.B. Optimal search direction for an animal flying or swimming in a wind or current. J. Chem. Ecol. 1989, 15, 2511–2519. [Google Scholar] [CrossRef]
- Dusenbery, D.B. Upwind searching for an odor plume is sometimes optimal. J. Chem. Ecol. 1990, 16, 1971–1976. [Google Scholar] [CrossRef]
- Symonds, M.R.E.; Johnson, T.L.; Elgar, M.A. Pheromone production, male abundance, body size, and the evolution of elaborate antennae in moths. Ecol. Evol. 2012, 2, 227–246. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.; D’Orsogna, M. First passage problems in biology. In First-Passage Phenomena and Their Application; Metzler, R., Oshanin, G., Redner, S., Eds.; World Scientific: Singapore, 2014; pp. 306–345. [Google Scholar] [CrossRef]
- Wendel, J.G. Hitting spheres with Brownian motion. Ann. Probab. 1980, 8, 164–169. [Google Scholar] [CrossRef]
- Carslaw, H.S.; Jaeger, J.C. Some two-dimensional problems in conduction of heat with circular symmetry. Proc. Lond. Math. Soc. 1940, s2-46, 361–388. [Google Scholar] [CrossRef]
- Lawley, S.D. Distribution of extreme first passage times of diffusion. J. Math. Biol. 2020, 80, 2301–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardé, R.T. Odour plumes and odour-mediated flight in insects. In Ciba Foundation Symposium 200—Olfaction in Mosquito-Host Interactions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 54–70. [Google Scholar] [CrossRef]
- Arbas, E.A.; Willis, M.A.; Kanzaki, R. Organization of goal-oriented locomotion: Pheromone-modulated flight behavior of moths. In Proceedings of the Workshop on “Locomotion Control in Legged Invertebrates” on Biological Neural Networks in Invertebrate Neuroethology and Robotics; Academic Press Professional, Inc.: San Diego, CA, USA, 1993; pp. 159–198. [Google Scholar]
- Baker, T.C.; Vickers, N.J. Pheromone-mediated flight in moths. In Insect Pheromone Research: New Directions; Cardé, R.T., Minks, A.K., Eds.; Springer: Boston, MA, USA, 1997; pp. 248–264. [Google Scholar] [CrossRef]
- Willis, M.A.; Arbas, E.A. Active behavior and reflexive responses: Another perspective on odor-modulated locomotion. In Insect Pheromone Research: New Directions; Cardé, R.T., Minks, A.K., Eds.; Springer: Boston, MA, USA, 1997; pp. 304–319. [Google Scholar] [CrossRef]
- Witzgall, P. Modulation of pheromone-mediated flight in male moths. In Insect Pheromone Research: New Directions; Cardé, R.T., Minks, A.K., Eds.; Springer: Boston, MA, USA, 1997; pp. 265–274. [Google Scholar] [CrossRef]
- Kaissling, K.E. Pheromone-controlled anemotaxis in moths. In Orientation and Communication in Arthropods; Lehrer, M., Ed.; Birkhäuser: Basel, Switzerland, 1997; pp. 343–374. [Google Scholar] [CrossRef]
- Benelli, N.; Liberzon, A. Alexliberzonlab/Mothpy: First Release of Mothpy—The Moth-Inspired Navigator Flying in Pompy Simulator (Version 0.0.1). Zenodo, 7 May 2019. [Google Scholar] [CrossRef]
- Graham, M. InsectRobotics/pompy: Initial release (Version v0.1.1). Zenodo, 9 November 2018. [Google Scholar] [CrossRef]
- Petrovskii, S.; Petrovskaya, N.; Bearup, D. Multiscale approach to pest insect monitoring: Random walks, pattern formation, synchronization, and networks. Phys. Life Rev. 2014, 11, 467–525. [Google Scholar] [CrossRef]
- Bläßle, A.; Tyson, R. First capture success in two dimensions: The search for prey by a random walk predator in a comprehensive space of random walks. Ecol. Complex. 2016, 28, 24–35. [Google Scholar] [CrossRef]
- Su, J.W.; Wang, H.T.; Ge, F. Female moths of cotton bollworm (Lepidoptera: Noctuidae) captured by waterbasin traps baited with synthetic female sex pheromone. Insect Sci. 2006, 13, 293–299. [Google Scholar] [CrossRef]
- Othmer, H.G.; Dunbar, S.R.; Alt, W. Models of Dispersal in Biological Systems. J. Math. Biol. 1988, 26, 263–298. [Google Scholar] [CrossRef]
- Tcheslavskaia, K.; Brewster, C.C.; Sharov, A.A. Mating success of gypsy moth (Lepidoptera: Lymantriidae) females in southern Wisconsin. Gt. Lakes Entomol. 2002, 35, 1–7. [Google Scholar]
- Kárpáti, Z.; Tasin, M.; Cardé, R.T.; Dekker, T. Early quality assessment lessens pheromone specificity in a moth. Proc. Natl. Acad. Sci. USA 2013, 110, 7377–7382. [Google Scholar] [CrossRef] [Green Version]
- Walton, R.E.; Sayer, C.D.; Bennion, H.; Axmacher, J.C. Nocturnal pollinators strongly contribute to pollen transport of wild flowers in an agricultural landscape. Biol. Lett. 2020, 16, 20190877. [Google Scholar] [CrossRef]
- Cardé, R.T.; Minks, A.K. Control of moth pests by mating disruption: Successes and constraints. Annu. Rev. Entomol. 1995, 40, 559–585. [Google Scholar] [CrossRef]
- Barclay, H.J.; Judd, G.J.R. Models for mating disruption by means of pheromone for insect pest control. Res. Popul. Ecol. 1995, 37, 239–247. [Google Scholar] [CrossRef]
- Gordon, D.; Zahavi, T.; Anshelevich, L.; Harel, M.; Ovadia, S.; Dunkelblum, E.; Harari, A.R. Mating disruption of Lobesia botrana (Lepidoptera: Tortricidae): Effect of pheromone formulations and concentrations. J. Econ. Entomol. 2009, 98, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, A.; Sambado, P.; Royo, A.B.J.; Bagnoli, B.; Benelli, G. Lobesia Botrana Males Mainly Fly Dusk: Video Camera-Assisted Pheromone Traps and Implications for Mating Disruption. J. Pest Sci. 2018, 91, 1327–1334. [Google Scholar] [CrossRef]
- Olver, F.W.J.; Daalhuis, A.B.O.; Lozier, D.W.; Schneider, B.I.; Boisvert, R.F.; Clark, C.W.; Miller, B.R.; Saunders, B.V.; Cohl, H.S.; McClain, M.A. (Eds.) NIST Digital Library of Mathematical Functions. Release 1.0.26 of 2020-03-15. Available online: http://dlmf.nist.gov/ (accessed on 15 March 2012).





| Symbol | Representation | Value/Units |
|---|---|---|
| Position of moth | m | |
| First arrival and mating time | s | |
| Male Random Flight Model | ||
| Largest simulation time step | s | |
| Smallest simulation time step | s | |
| Total simulation time | 100 s | |
| N | Number of male moths | 1000 to 5000 |
| Mating radius | 0 to 1 m | |
| Initial position of male moth | 1 m | |
| Mating radius | 0 to 1 m | |
| Male initial position to mating radius ratio | dimensionless | |
| D | Male moth diffusion constant | to m2/s |
| Plume Navigation Model | ||
| Time step | 1 s | |
| N | Number of male moths | 10,000 |
| Center of wind-advected plume | m | |
| v | Speed of male moths | 0.5 to 2 m/s |
| Pheromone detection threshold | to pg/m2 | |
| Casting and surging margin of error | ||
| Maximum time spent casting | 10 s | |
| Pheromone amount per puff | 1 to 10 pg | |
| Puff release rate | 1 puff/s | |
| Puff growth parameter | 0.1 m/s | |
| U | Wind velocity | 5 m/s |
| Capture distance | 5 m |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepien, T.L.; Zmurchok, C.; Hengenius, J.B.; Caja Rivera, R.M.; D’Orsogna, M.R.; Lindsay, A.E. Moth Mating: Modeling Female Pheromone Calling and Male Navigational Strategies to Optimize Reproductive Success. Appl. Sci. 2020, 10, 6543. https://doi.org/10.3390/app10186543
Stepien TL, Zmurchok C, Hengenius JB, Caja Rivera RM, D’Orsogna MR, Lindsay AE. Moth Mating: Modeling Female Pheromone Calling and Male Navigational Strategies to Optimize Reproductive Success. Applied Sciences. 2020; 10(18):6543. https://doi.org/10.3390/app10186543
Chicago/Turabian StyleStepien, Tracy L., Cole Zmurchok, James B. Hengenius, Rocío Marilyn Caja Rivera, Maria R. D’Orsogna, and Alan E. Lindsay. 2020. "Moth Mating: Modeling Female Pheromone Calling and Male Navigational Strategies to Optimize Reproductive Success" Applied Sciences 10, no. 18: 6543. https://doi.org/10.3390/app10186543





