Immediate Effects of an Inverted Body Position on Energy Expenditure and Blood Lactate Removal after Intense Running
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Subjects
2.3. Procedures
2.4. Measurements
2.5. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramírez-Campillo, R.; Henríquez-Olguín, C.; Burgos, C.; Andrade, D.C. Effect of progressive volume-based overload during plyometric training on explosive and endurance performance in young soccer players. J. Strength Cond. Res. 2015, 29, 1884–1893. [Google Scholar] [CrossRef]
- Fry, A.; Mullinger, K.J.; O’Neill, G.C.; Mullinger, K.J.; Brookers, M.J. The effect of physical fatigue on oscillatory dynamics of the sensorimotor cortex. Acta Physiol. 2017, 220, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Duclos, M.; Gleespn, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D.; Raglin, J.; Rietjens, G.; Steinacker, J.; et al. Prevention, diagnosis and treatment of the overtraining syndrome. Eur. J. Appl. Physiol. 2006, 6, 1–14. [Google Scholar] [CrossRef]
- Halson, S.; Jeukendrup, A.E. Does overtraining exist? An analysis of overreaching and overtraining research. Sports Med. 2004, 34, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Thiel, C.; Vogt, L.; Bürklein, M.; Rosenhagen, A. Functional overreaching during preparation training of elite tennis professionals. J. Hum. Kinet. 2011, 28, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Hausswirth, C.; Mujika, I. Recovery for Performance in Sport; Human Kinetics: Champain, France, 2012. [Google Scholar]
- Belcastro, A.N.; Bonen, A. Lactic acid removal rates during controlled and uncontrolled recovery exercise. J. Appl. Physiol. 1975, 39, 932–936. [Google Scholar] [CrossRef]
- Dupont, G.; Moalla, W.; Guinhouya, C.; Ahmadi, S. Passive versus active recovery during high-intensity intermittent exercises. Med. Sci. Sports Exerc. 2004, 36, 302–308. [Google Scholar] [CrossRef]
- Menzies, P.; Menzies, C.; Mclntyre, L.; Paterson, P. Blood lactate clearance during active recovery after an intense running bout depends on the intensity of the active recovery. J. Sports Sci. 2010, 28, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Mota, M.R.; Dantas, R.A.E.; Oliveira-Silva, I.; Mahalhaes Sales, M.; da Costa Sotero, R.; Espiodola Mota Venancio, P.; Teixeira Junior, J.; Nobre Chaves, S.; de Lima, F.D. Effect of self-paced active recovery and passive recovery on blood lactate removal following a 200 m freestyle swimming trial. Open Access J. Sports Med. 2017, 8, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Soares, A.H.; Oliveira, T.P.; Cavalcante, B.R.; Farah, B.Q.; Lima, A.; Cucato, G.G.; Cardoso, C.G.; Ritti-Dias, R.M. Effects of active recovery on autonomic and haemodynamic responses after aerobic exercise. Clin. Physiol. Funct. Imaging 2017, 37, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Fairchild, T.J.; Armstrong, A.A.; Rao, A.; Hawk, L. Glycogen synthesis in muscle fibers during active recovery from intense exercise. Med. Sci. Sports Exerc. 2003, 35, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Baquet, G.; Dupont, G.; Gamelin, F.-X.; Aucountier, J.; Berthoin, S. Active versus passive recovery in high-intensity intermittent exercises in children: An exploratory study. Pediatr. Exerc. Sci. 2019, 31, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Toubekis, A.G.; Douda, H.T.; Tokmakidis, S.P. Influence of different rest intervals during active or passive recovery on repeated sprint swimming performance. Eur. J. Appl. Physiol. 2005, 93, 694–700. [Google Scholar] [CrossRef]
- Monedero, J.; Donne, B. Effect of recovery interventions on lactate removal and subsequent performance. Int. J. Sports Med. 2000, 21, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Draper, N.; Bird, E.L.; Coleman, I.; Hodgson, C. Effects of active recovery on lactate concentration, heart rate and RPE in climbing. J. Sports Sci. Med. 2006, 5, 97–105. [Google Scholar] [PubMed]
- Gladden, L.B. Muscle as a consumer of lactate. Med. Sci. Sport Exer. 2000, 32, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Starkey, C. Therapeutic Modalities; FA Davis Company: Philadelphia, PA, USA, 2013. [Google Scholar]
- Maestrini, D. Genesis of the so-called insufficient contractions of the heart in decompensation. Policlin. Prat. 1951, 58, 257–268. [Google Scholar]
- Brooks, G.A.; Fahey, T.D.; Baldwin, K.M. Exercise Physiology: Human Bioenergenetics and Its Applications; McGraw Hill: New York, NY, USA, 2005; pp. 293–308. [Google Scholar]
- McInnis, N.H.; Journeay, W.S.; Jay, O.; Lelair, E.; Kenny, G.P. 15° Head-down tilt attenuates the postexercise reduction in cutaneous vascular conductance and sweating and decreases esophageal temperature recovery time. J. Appl. Physiol. 2006, 101, 840–847. [Google Scholar] [CrossRef]
- Journeay, W.S.; Jay, O.; McInnis, N.H.; Lelair, E.; Kenny, G.P. Postexercise heat loss and hemodynamic responses during head-down tilt are similar between genders. Med. Sci. Sports Exerc. 2007, 39, 1308–1804. [Google Scholar] [CrossRef]
- Siamwala, J.H.; Lee, P.C.; Macias, B.R.; Hargens, A.R. Lower-body negative pressure restores leg bone microvascular flow to supine levels during head-down tilt. J. Appl. Physiol. 2015, 119, 101–109. [Google Scholar] [CrossRef]
- Karvonen, J.; Vuorimaa, T. Heart rate and exercise intensity during sports activities. Sports Med. 1988, 5, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; McAuliffe, J.; Johnson, M.J.; Button, D.C. Seated inversion adversely affects vigilance tasks and suppresses heart rate and blood pressure. Occup. Ergon. 2013, 11, 153–163. [Google Scholar] [CrossRef]
- Hart, S.; Drevets, K.; Alford, M.; Salacinski, A.; Hunt, B.E. A method-comparison study regarding the validity and reliability of the Lactate Plus analyzer. BMJ Open 2013, 3, e001899. [Google Scholar] [CrossRef]
- Shahid, A.; Wilkinson, K.; Marcu, S.; Shapiro, C.M. Visual analogue scale to evaluate fatigue severity (VAS-F.). In STOP, THAT and One Hundred Other Sleep Scales; Spinger: New York, NY, USA, 2011; pp. 399–402. [Google Scholar]
- Wolfe, F. Fatigue assessments in rheumatoid arthritis: Comparative performance of visual analog scales and longer fatigue questionnaires in 7760 patients. J. Rheumatol. 2004, 31, 1896–1902. [Google Scholar] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of seletcing and reporting intraclass correlation coefficients for reliability research. J. Chripract. Med. 2016, 15, 155–163. [Google Scholar]
- Ali Rasooli, S.; Koushkie Jahromi, M.; Asadmanesh, A.; Salesi, M. Influence of massage, active and passive recovery on swimming performance and blood lactate. J. Sport Med. Phys. Fit. 2012, 52, 122–127. [Google Scholar]
- Ouergui, I.; Hammouda, O.; Chtourou, H.; Gmada, N.; Franchini, E. Effects of recovery type after a kickboxing match on blood lactate and performance in anaerobic tests. Asian J. Sports Med. 2014, 5, 99–107. [Google Scholar]
- Warren, C.D.; Szymanski, D.J.; Landers, M.R. Effects of three recovery protocols on range of motion, heart rate, rating of perceived exertion, and blood lactate in baseball pitchers during a simulated game. J. Strength Cond. Res. 2015, 29, 3016–3025. [Google Scholar] [CrossRef]
- Goto, K.; Morishima, T. Compression garment promotes muscular strength recovery after resistance exercise. Med. Sci. Sports Exerc. 2014, 46, 2265–2270. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Flanagan, S.D.; Comstock, B.A.; Fragala, M.; Earp, J.; Dunn-Lewis, C.; Ho, J.; Thomas, G.; Solomon-Hill, G.; Penwell, Z.; et al. Effects of a whole body compression garment on markers of recovery after a heavy resistance workout in men and women. J. Strength Cond. Res. 2010, 24, 804–814. [Google Scholar] [CrossRef]
- Dawson, L.G.; Dawson, K.A.; Tiidus, P.M. Evaluating the influence of massage on leg strength, swelling, and pain following a half-marathon. J. Sport Scimed. 2004, 3, 37–43. [Google Scholar]
- Knechtle, B.; Vinzent, T.; Kirby, S.; Knechtle, P. The recovery phase following a triple iron triathlon. J. Hum. Kinet. 2009, 21, 65–74. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Contreras, B. The pump: Potential mechanisms and applications for enhancing hypertrophic adaptations. Strength Cond. J. 2014, 36, 21–25. [Google Scholar] [CrossRef]
- Schoenfeld, B.J. The mecahnisms of muscle hypertrophy and their application to resistance training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef] [Green Version]
- Fellmann, N.; Bedu, M.; Giry, J.; Pharmakis-amadieu, M.; Bezou, M.; Barlet, J.; Coudert, J. Hormonal, fluid, and electrolyte changes during a 72-h recovery from a 24-h endurance run. Int. J. Sports Med. 1989, 10, 406–412. [Google Scholar] [CrossRef]
- Engström, E.; Ottosson, E.; Wohlfart, B.; Grundstorm, N.; Wisen, A. Comparison of heart rate measured by Polar RS400 and ECG, validity and repeatability. Adv. Physiother. 2012, 14, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Besson, H.; Brage, S.; Jakes, R.W.; Ekelund, U.; Wareham, N. Estimating physical activity energy expenditure, sedentary time, and physical activity intensity by self-report in adults. Am. J. Clin. Nutr. 2010, 91, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Irwin, M.L.; Ainsworth, B.E.; Conway, J.M. Estimation of energy expenditure from physical activity measures: Determinants of accuracy. Obes. Res. 2001, 9, 517–525. [Google Scholar] [CrossRef]
- Haskvitz, E.M.; Hanten, W.P. Blood pressure response to inversion traction. Phys. Ther. 1986, 66, 1361–1364. [Google Scholar] [CrossRef]
- LeMarr, J.D.; Golding, L.A.; Crehan, K.D. Cardiorespiratory responses to inversion. Physician Sportsmed. 1983, 11, 51–57. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Verheyden, B.; Aubert, A.E.; Fagard, R.H. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J. Hum. Hypertens. 2010, 24, 175–182. [Google Scholar] [CrossRef]


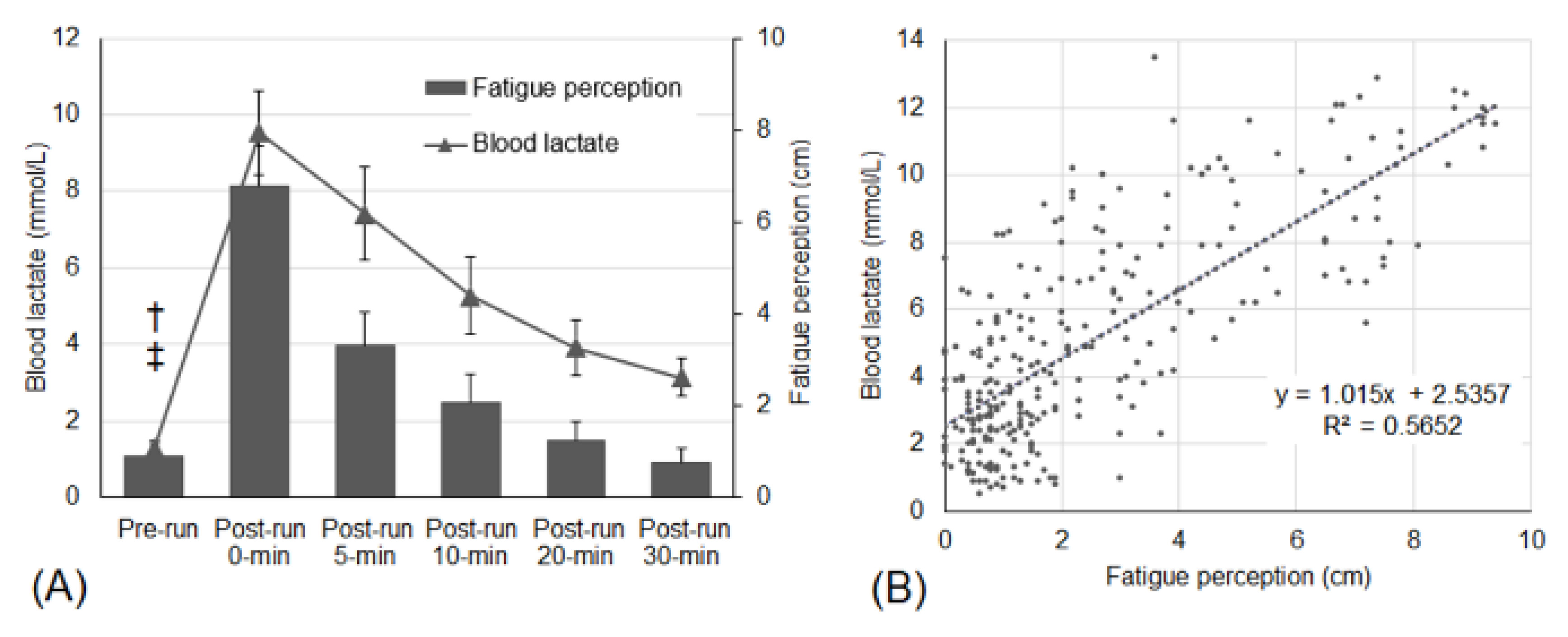
| Mean (95% CIs) | Condition | Pre- Run | Post-Run 0 Min | Post-Run 5 Min | Post-Run 10 Min | Post-Run 20 Min | Post-Run 30 Min |
|---|---|---|---|---|---|---|---|
| Blood lactate (mmol/L) ICC: 0.75 | IBP | 1.3 (0.2) | 9.8 (1.1) | 6.7 (1.2) | 5.4 (1.1) | 4.2 (0.8) | 3.3 (0.5) |
| Active | 1.3 (0.2) | 9.4 (1.0) | 6.9 (1.1) | 4.4 (0.8) | 3.3 (0.6) | 2.7 (0.4) | |
| Passive | 1.3 (0.3) | 9.3 (1.2) | 8.6 † (1.2) | 6.0 ‡ (0.9) | 4.3 (0.7) | 3.5 (0.5) | |
| Heart rate (bpm) ICC: 0.91 | IBP | 72.3 (6.2) | 185.0 (2.0) | 95.9 (5.7) | 110.2 (7.6) | 95.3 (6.9) | 90.1 (5.1) |
| Active | 73.8 (5.2) | 184.9 (1.5) | 123.3 # (5.3) | 117.9 (8.2) | 96.3 (4.8) | 91.1 (5.0) | |
| Passive | 72.8 (5.8) | 185.5 (1.1) | 102.9 (5.5) | 114.0 (7.3) | 94.2 (5.4) | 90.9 (4.9) | |
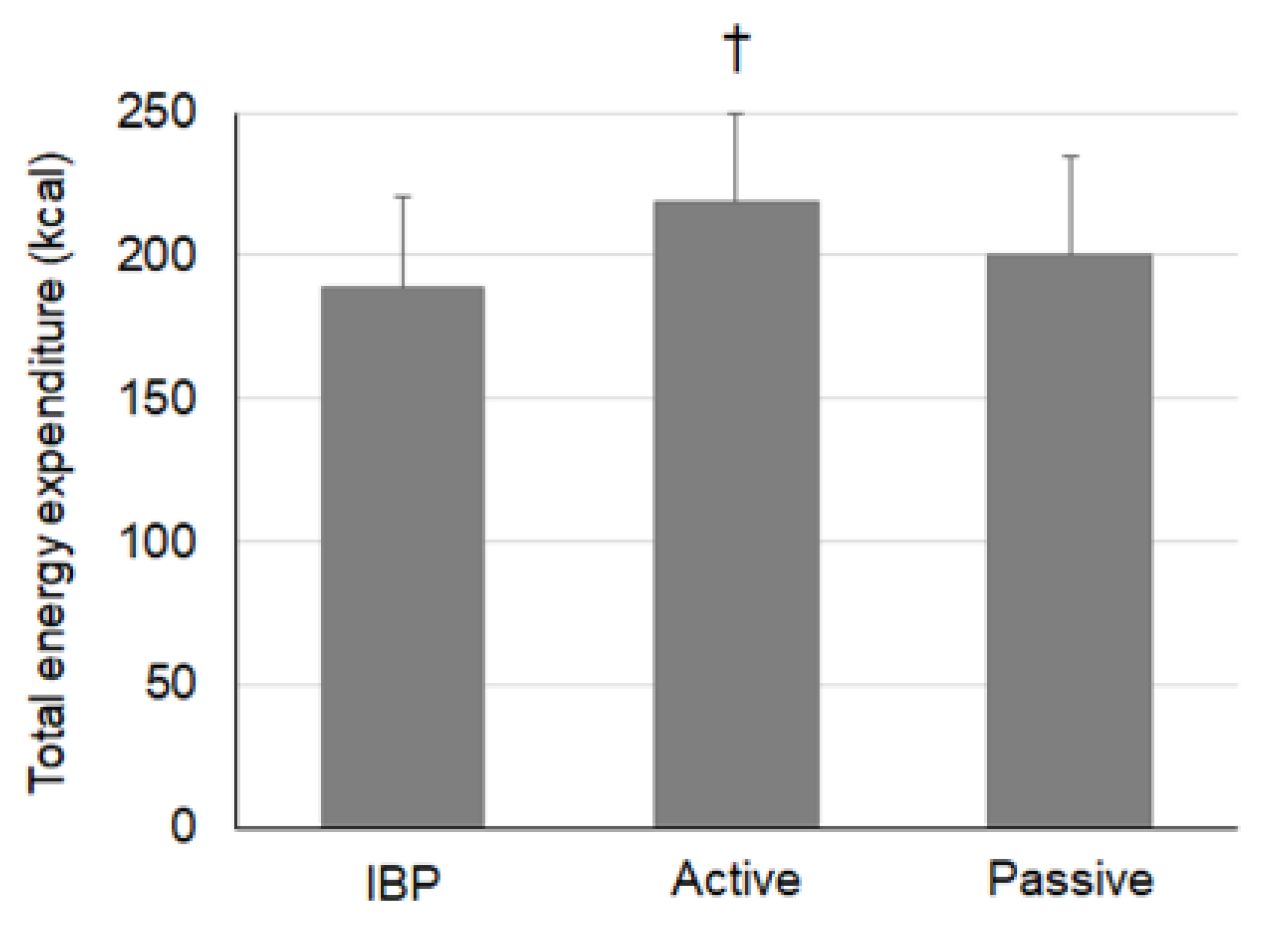
| Energy expenditure (kcal) ICC: N/A | IBP | - | 74.5 (10.9) | 20.5 (4.6) | 29.9 (7.1) | 33.2 (7.9) | 30.6 (6.8) |
| Active | - | 76.3 (12.5) | 38.8 (5.1) | 34.1 (6.2) | 37.3 (8.1) | 31.9 (7.1) | |
| Passive | - | 75.7 (12.0) | 24.7 (5.5) | 31.9 (6.5) | 36.0 (8.0) | 31.9 (7.3) | |
| Fatigue perception (cm) ICC: 0.76 | IBP | 1.1 (0.4) | 6.8 (0.8) | 3.4 (0.9) | 2.1 (0.5) | 1.3 (0.4) | 0.8 (0.2) |
| Active | 0.7 (0.2) | 6.6 (0.9) | 3.3 (0.7) | 1.9 (0.5) | 1.2 (0.4) | 0.7 (0.2) | |
| Passive | 0.9 (0.3) | 7.0 (0.9) | 3.3 (0.6) | 2.2 (0.8) | 1.2 (0.4) | 0.8 (0.4) |
| Mean (95% CIs) | Condition | Pre- Run | Post-Run 0 Min | Post-Run 5 Min | Post-Run 10 Min | Post-Run 20 Min | Post-Run 30 Min |
|---|---|---|---|---|---|---|---|
| Left thigh (cm) ICC: 0.99 | IBP | 54.4 (1.2) | 54.7 (1.2) | 54.5 (1.2) | 54.4 (1.2) | 54.4 (1.2) | 54.4 (1.2) |
| Active | 54.2 (1.4) | 54.6 (1.4) | 54.4 (1.4) | 54.3 (1.4) | 54.3 (1.4) | 54.3 (1.4) | |
| Passive | 54.4 (1.4) | 54.7 (1.4) | 54.7 (1.5) | 54.5 (1.4) | 54.4 (1.4) | 54.4 (1.4) | |
| Right thigh (cm) ICC: 0.99 | IBP | 54.6 (1.1) | 54.9 (1.2) | 54.6 (1.2) | 54.6 (1.2) | 54.6 (1.1) | 54.5 (1.1) |
| Active | 54.4 (1.3) | 55.0 (1.4) | 54.7 (1.2) | 54.6 (1.3) | 54.5 (1.3) | 54.5 (1.3) | |
| Passive | 54.5 (1.3) | 54.9 (1.3) | 54.8 (1.3) | 54.8 (1.3) | 54.7 (1.3) | 54.6 (1.3) | |
| Left calf (cm) ICC: 0.99 | IBP | 36.3 (0.9) | 36.9 (1.0) | 36.6 (0.9) | 36.5 (0.9) | 36.4 (0.9) | 36.4 (0.9) |
| Active | 36.4 (1.1) | 37.0 (1.1) | 36.9 (1.1) | 36.7 (1.1) | 36.6 (1.1) | 36.6 (1.1) | |
| Passive | 36.4 (1.0) | 37.0 (1.0) | 36.9 (1.0) | 36.7 (1.0) | 36.7 (1.0) | 36.6 (1.0) | |
| Right calf (cm) ICC: 0.99 | IBP | 36.5 (0.9) | 37.2 (0.9) | 36.9 (0.9) | 36.7 (0.9) | 36.6 (0.9) | 36.6 (0.9) |
| Active | 36.5 (1.0) | 37.2 (1.0) | 37.0 (1.0) | 36.8 (1.0) | 36.7 (1.0) | 36.7 (1.0) | |
| Passive | 36.6 (0.9) | 37.2 (1.0) | 37.1 (1.0) | 36.9 (1.0) | 36.9 (1.0) | 36.8 (1.0) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.S.; Park, J. Immediate Effects of an Inverted Body Position on Energy Expenditure and Blood Lactate Removal after Intense Running. Appl. Sci. 2020, 10, 6645. https://doi.org/10.3390/app10196645
Kim MS, Park J. Immediate Effects of an Inverted Body Position on Energy Expenditure and Blood Lactate Removal after Intense Running. Applied Sciences. 2020; 10(19):6645. https://doi.org/10.3390/app10196645
Chicago/Turabian StyleKim, Moo Sung, and Jihong Park. 2020. "Immediate Effects of an Inverted Body Position on Energy Expenditure and Blood Lactate Removal after Intense Running" Applied Sciences 10, no. 19: 6645. https://doi.org/10.3390/app10196645





