Crosslinking Collagen Constructs: Achieving Cellular Selectivity Through Modifications of Physical and Chemical Properties
Abstract
:1. Introduction
2. Considerations in Selecting a Chemical Crosslinker
3. An Overview of Selected Chemical Crosslinkers
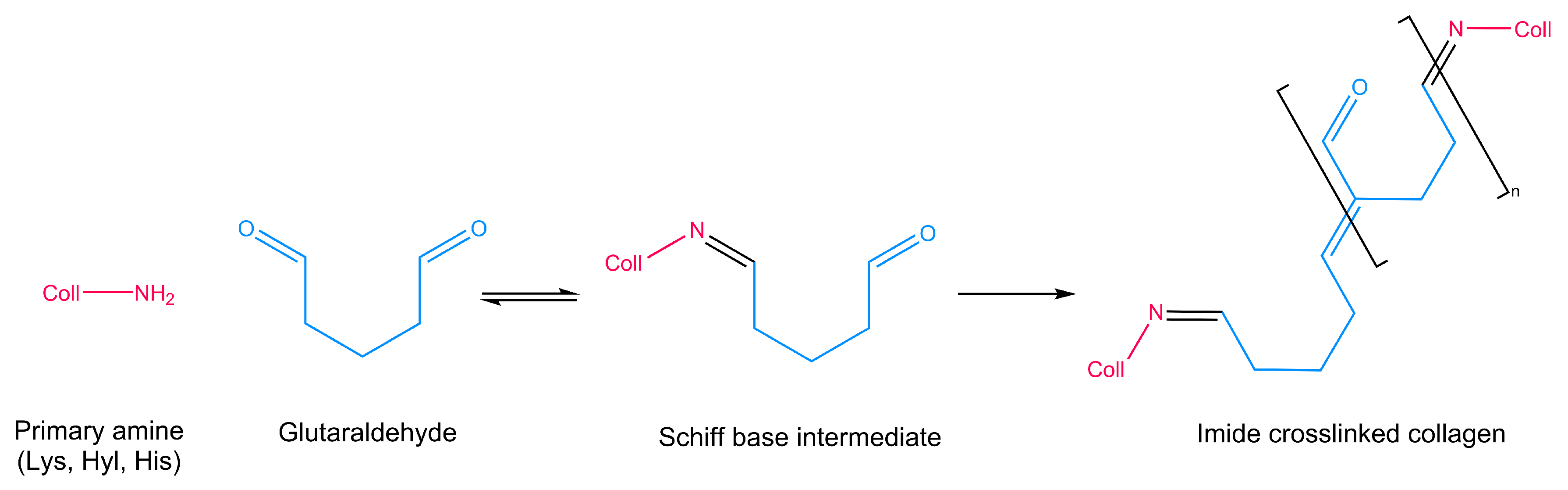
3.1. Glutaraldehyde
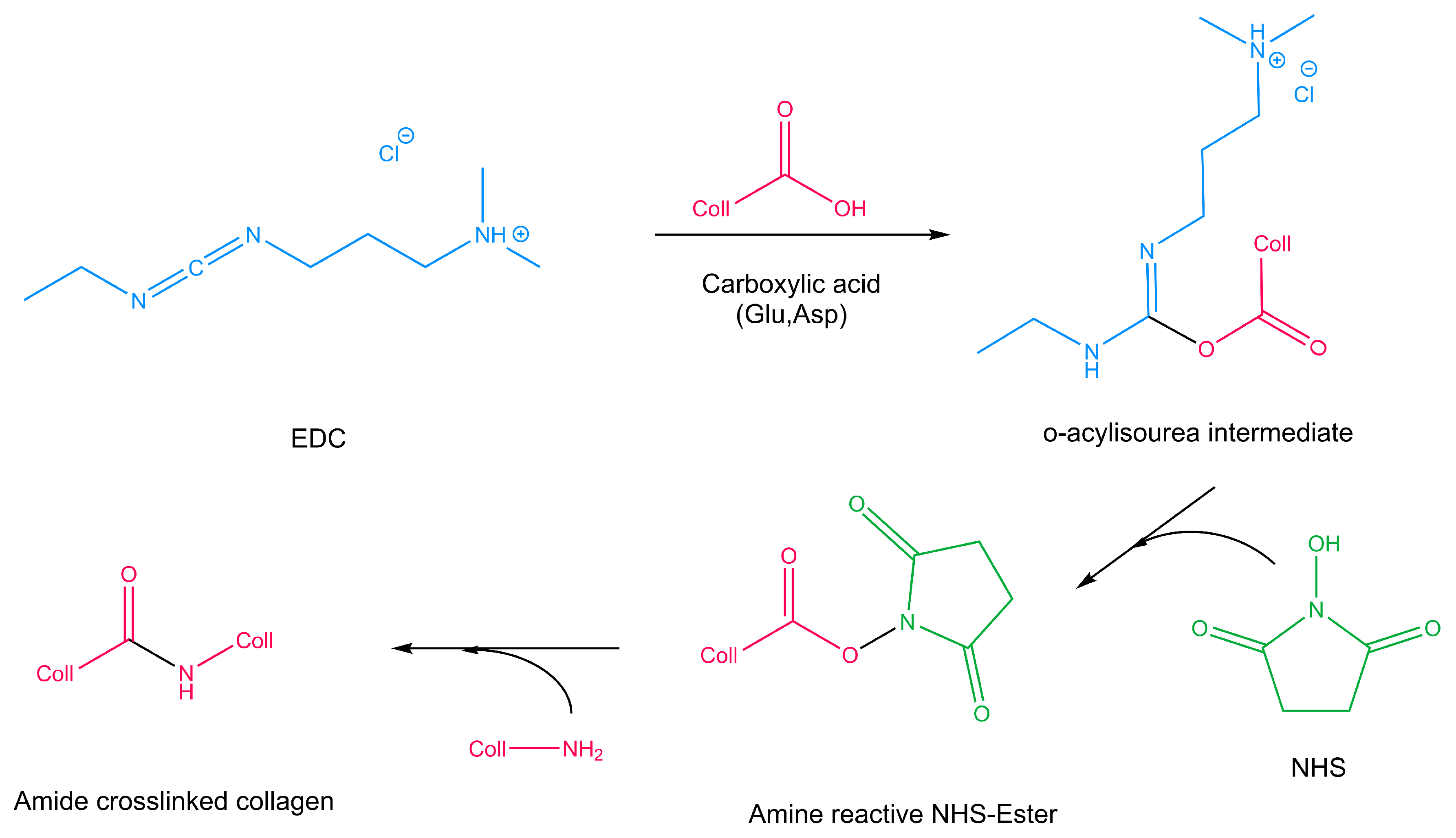
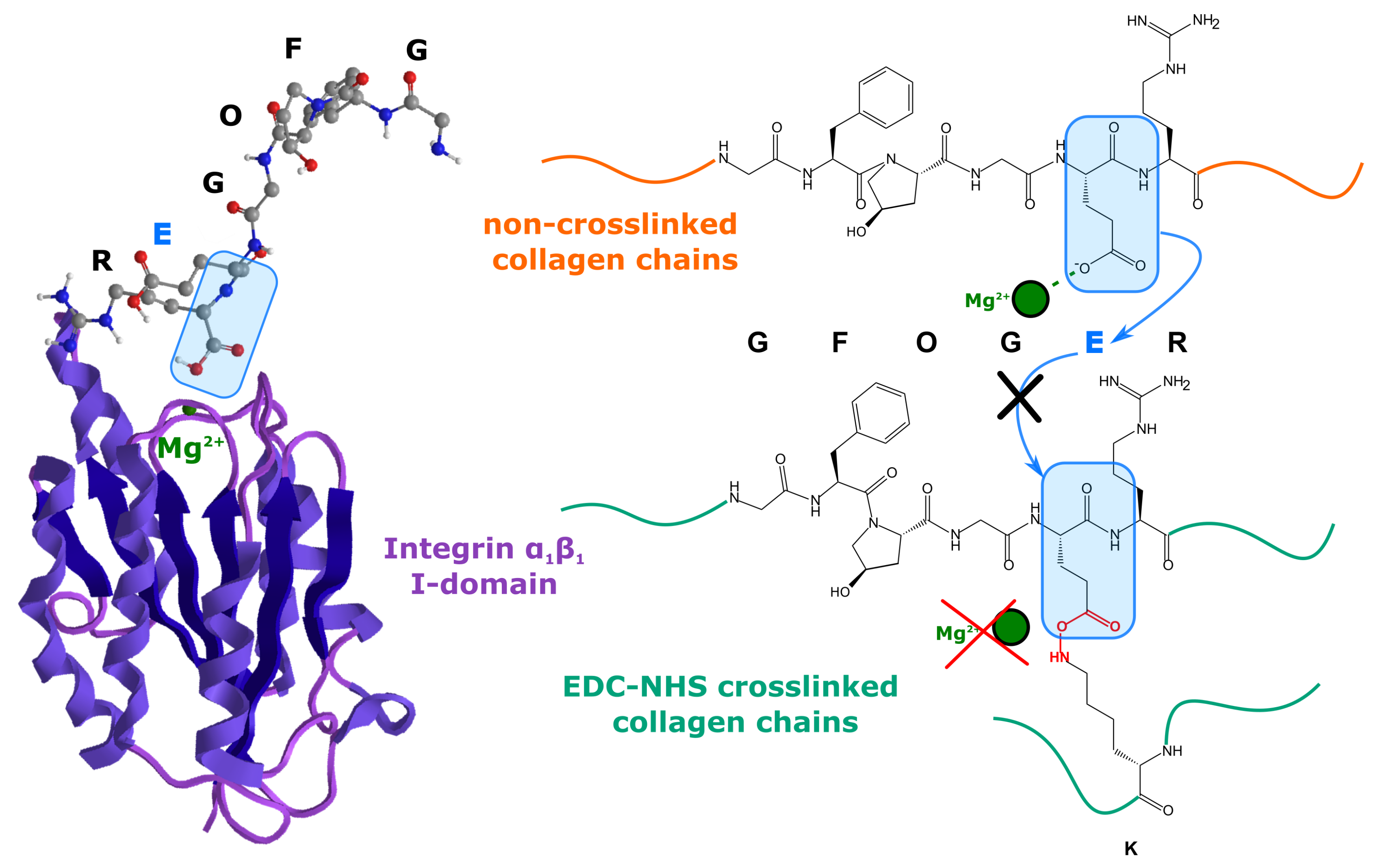
3.2. EDC-NHS
3.3. Dehydrothermal Treatment
3.4. Ultraviolet Radiation
3.5. Genipin
3.6. Riboflavin
Transglutaminase
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Olde Damink, L.; Dijkstra, P.; Van Luyn, M.; Van Wachem, P.; Nieuwenhuis, P.; Feijen, J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci. Mater. Med. 1995, 6, 460–472. [Google Scholar] [CrossRef] [Green Version]
- Olde Damink, L.; Dijkstra, P.; Van Luyn, M.; Van Wachem, P.; Nieuwenhuis, P.; Feijen, J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials 1996, 17, 765–773. [Google Scholar] [CrossRef] [Green Version]
- Mekhail, M.; Wong, K.K.H.; Padavan, D.T.; Wu, Y.; O’Gorman, D.B.; Wan, W. Genipin-cross-linked electrospun collagen fibers. J. Biomater. Sci. Polym. Ed. 2011, 22, 2241–2259. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, D.; San Chau, D.Y.; Wang, Z.; Collighan, R.J.; Griffin, M. Cross-linking of collagen I by tissue transglutaminase provides a promising biomaterial for promoting bone healing. Amino Acids 2014, 46, 1751–1761. [Google Scholar] [CrossRef]
- Smith-Mungo, L.I.; Kagan, H.M. Lysyl oxidase: Properties, regulation and multiple functions in biology. Matrix Biol. 1998, 16, 387–398. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771. [Google Scholar] [CrossRef]
- Bax, D.V.; Davidenko, N.; Gullberg, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental insight into the effect of carbodiimide crosslinking on cellular recognition of collagen-based scaffolds. Acta Biomater. 2017, 49, 218–234. [Google Scholar] [CrossRef]
- Takigawa, T.; Endo, Y. Effects of glutaraldehyde exposure on human health. J. Occup. Health 2006, 48, 75–87. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Baier, J.M. Acellular vascular tissues: Natural biomaterials for tissue repair and tissue engineering. Biomaterials 2000, 21, 2215–2231. [Google Scholar] [CrossRef]
- Sheu, M.T.; Huang, J.C.; Yeh, G.C.; Ho, H.O. Characterization of collagen gel solutions and collagen matrices for cell culture. Biomaterials 2001, 22, 1713–1719. [Google Scholar] [CrossRef]
- Chen, D.C.; Lai, Y.L.; Lee, S.Y.; Hung, S.L.; Chen, H.L. Osteoblastic response to collagen scaffolds varied in freezing temperature and glutaraldehyde crosslinking. J. Biomed. Mater. Res. Part A 2007, 80, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.; Ghose, S.; Kew, S.; Moavenian, A.; Best, S.M.; Cameron, R.E. Effect of fiber crosslinking on collagen-fiber reinforced collagen–chondroitin-6-sulfate materials for regenerating load-bearing soft tissues. J. Biomed. Mater. Res. Part A 2013, 101, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Johal, R.K.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Tunable bioactivity and mechanics of collagen-based tissue engineering constructs: A comparison of EDC-NHS, genipin and TG2 crosslinkers. Biomaterials 2020, 254, 120109. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.; Bax, D.; Raynal, N.; Farndale, R.; Best, S.; Cameron, R. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Nair, M.; Calahorra, Y.; Kar-Narayan, S.; Best, S.M.; Cameron, R.E. Self-assembly of collagen bundles and enhanced piezoelectricity induced by chemical crosslinking. Nanoscale 2019, 11, 15120–15130. [Google Scholar] [CrossRef] [Green Version]
- Grover, C.N.; Gwynne, J.H.; Pugh, N.; Hamaia, S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films. Acta Biomater. 2012, 8, 3080–3090. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, Z.; Shepherd, J.H.; Shepherd, D.V.; Ghose, S.; Kew, S.J.; Cameron, R.E.; Best, S.M.; Brooks, R.A.; Wardale, J.; Rushton, N. Effect of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and N-hydroxysuccinimide concentrations on the mechanical and biological characteristics of cross-linked collagen fibres for tendon repair. Regen. Biomater. 2015, 2, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, D.; Shepherd, J.; Ghose, S.; Kew, S.; Cameron, R.; Best, S. The process of EDC-NHS cross-linking of reconstituted collagen fibres increases collagen fibrillar order and alignment. APL Mater. 2015, 3, 014902. [Google Scholar] [CrossRef] [Green Version]
- Imhof, C.; Schloesser, L.; Stiefel, N.; Wüest, M. Collagen Sponge. EP Patent 3,055,000, 21 December 2016. [Google Scholar]
- Madaghiele, M.; Calò, E.; Salvatore, L.; Bonfrate, V.; Pedone, D.; Frigione, M.; Sannino, A. Assessment of collagen crosslinking and denaturation for the design of regenerative scaffolds. J. Biomed. Mater. Res. Part A 2016, 104, 186–194. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, L.; Xu, H.; Yamamoto, M.; Shinoda, M.; Kishimoto, M.; Tanaka, T.; Yamane, H. Effect of the Application of a Dehydrothermal Treatment on the Structure and the Mechanical Properties of Collagen Film. Materials 2020, 13, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soller, E.C.; Tzeranis, D.S.; Miu, K.; So, P.T.; Yannas, I.V. Common features of optimal collagen scaffolds that disrupt wound contraction and enhance regeneration both in peripheral nerves and in skin. Biomaterials 2012, 33, 4783–4791. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Bax, D.V.; Schuster, C.F.; Farndale, R.W.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds. J. Mater. Sci. Mater. Med. 2016, 27, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, Y.; Nishikawa, T.; Kawakishi, S. Formation of protein-bound 3,4-dihydroxyphenylalanine in collagen types i and iv exposed to ultraviolet light. Photochem. Photobiol. 1995, 61, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A. Modification of collagen films by ultraviolet irradiation. Polym. Degrad. Stab. 2000, 68, 147–151. [Google Scholar] [CrossRef]
- Ohan, M.P.; Weadock, K.S.; Dunn, M.G. Synergistic effects of glucose and ultraviolet irradiation on the physical properties of collagen. J. Biomed. Mater. Res. 2002, 60, 384–391. [Google Scholar] [CrossRef]
- Bax, D.V.; Davidenko, N.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Impact of UV-and carbodiimide-based crosslinking on the integrin-binding properties of collagen-based materials. Acta Biomater. 2019, 100, 280–291. [Google Scholar] [CrossRef]
- Manickam, B.; Sreedharan, R.; Elumalai, M. ‘Genipin’–the natural water soluble cross-linking agent and its importance in the modified drug delivery systems: An overview. Curr. Drug Deliv. 2014, 11, 139–145. [Google Scholar] [CrossRef]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Tambe, N.; Di, J.; Zhang, Z.; Bernacki, S.; El-Shafei, A.; King, M.W. Novel genipin-collagen immobilization of polylactic acid (PLA) fibers for use as tissue engineering scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1188–1197. [Google Scholar] [CrossRef]
- Sundararaghavan, H.G.; Monteiro, G.A.; Lapin, N.A.; Chabal, Y.J.; Miksan, J.R.; Shreiber, D.I. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res. Part A 2008, 87, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Yang, T.; Zhang, N.; Dong, L.; Ma, S.; Liu, X.; Zhou, M.; Li, B. The effects of different crossing-linking conditions of genipin on type I collagen scaffolds: An in vitro evaluation. Cell Tissue Bank. 2014, 15, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Racadio, J.; Brown, M.; Hedman, T. Six-Month Safety and Efficacy of Genipin Crosslinking Treatment for Chronic Low Back Pain. Spine J. 2017, 17, S206. [Google Scholar] [CrossRef]
- Rich, H.; Odlyha, M.; Cheema, U.; Mudera, V.; Bozec, L. Effects of photochemical riboflavin-mediated crosslinks on the physical properties of collagen constructs and fibrils. J. Mater. Sci. Mater. Med. 2014, 25, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.J.; Bax, D.V.; Best, S.M.; Cameron, R.E. Blue light activated crosslinking of collagen. 2020; pending publishing. [Google Scholar]
- Cheema, U.; Rong, Z.; Kirresh, O.; MacRobert, A.J.; Vadgama, P.; Brown, R.A. Oxygen diffusion through collagen scaffolds at defined densities: Implications for cell survival in tissue models. J. Tissue Eng. Regen. Med. 2012, 6, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Chau, D.Y.; Collighan, R.J.; Verderio, E.A.; Addy, V.L.; Griffin, M. The cellular response to transglutaminase-cross-linked collagen. Biomaterials 2005, 26, 6518–6529. [Google Scholar] [CrossRef]
- Keillor, J.W.; Clouthier, C.M.; Apperley, K.Y.; Akbar, A.; Mulani, A. Acyl transfer mechanisms of tissue transglutaminase. Bioorg. Chem. 2014, 57, 186–197. [Google Scholar] [CrossRef]




| Crosslinker | Advantages | Disadvantages |
|---|---|---|
| GTA | • Increased elastic modulus [1,13] | • Non-zero length crosslinker [1] • Cytotoxic [8] |
| EDC-NHS | • Increased elastic modulus [13,17] • Zero length crosslinker [2,12] | • Loss of some integrin-specific binding [7] |
| UV | • Integrin binding unaffected [24] • Zero length crosslinking [24,25] | • Non-specific reaction sites [24,25] • Low crosslink densities [24] • Collagen fragmentation [26] • Some denaturing [26] without use of glucose [24,27] |
| DHT | • Integrin binding unaffected [13] • Zero length crosslinking | • Denaturation of collagen [21] |
| Genipin | • Non-cytotoxic [34] • Increases in elastic modulus [33] • Mechanism should not affect GXOGER motifs in collagen [31] | • Not a zero-length crosslinker [31] • Imparts a blue colour [33] |
| Riboflavin | • Mechanism should not affect GXOGER motifs in collagen [36] • Comparable ultimate tensile strength to EDC-NHS [36] | • Some, albeit limited, loss of mediated binding |
| TG2 | • Mechanism do not affect GXOGER motifs in collagen [39] | • Can cause morphological changes to collagen ultrastructure [38] • Can result in a reduction of Young’s modulus |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, M.; Best, S.M.; Cameron, R.E. Crosslinking Collagen Constructs: Achieving Cellular Selectivity Through Modifications of Physical and Chemical Properties. Appl. Sci. 2020, 10, 6911. https://doi.org/10.3390/app10196911
Nair M, Best SM, Cameron RE. Crosslinking Collagen Constructs: Achieving Cellular Selectivity Through Modifications of Physical and Chemical Properties. Applied Sciences. 2020; 10(19):6911. https://doi.org/10.3390/app10196911
Chicago/Turabian StyleNair, Malavika, Serena M. Best, and Ruth E. Cameron. 2020. "Crosslinking Collagen Constructs: Achieving Cellular Selectivity Through Modifications of Physical and Chemical Properties" Applied Sciences 10, no. 19: 6911. https://doi.org/10.3390/app10196911





