Comparison of the Effects Caused by Three Different Mandibular Advancement Devices on the Periodontal Ligaments and Teeth for the Treatment of Osa: A Finite Element Model Study
Abstract
1. Introduction
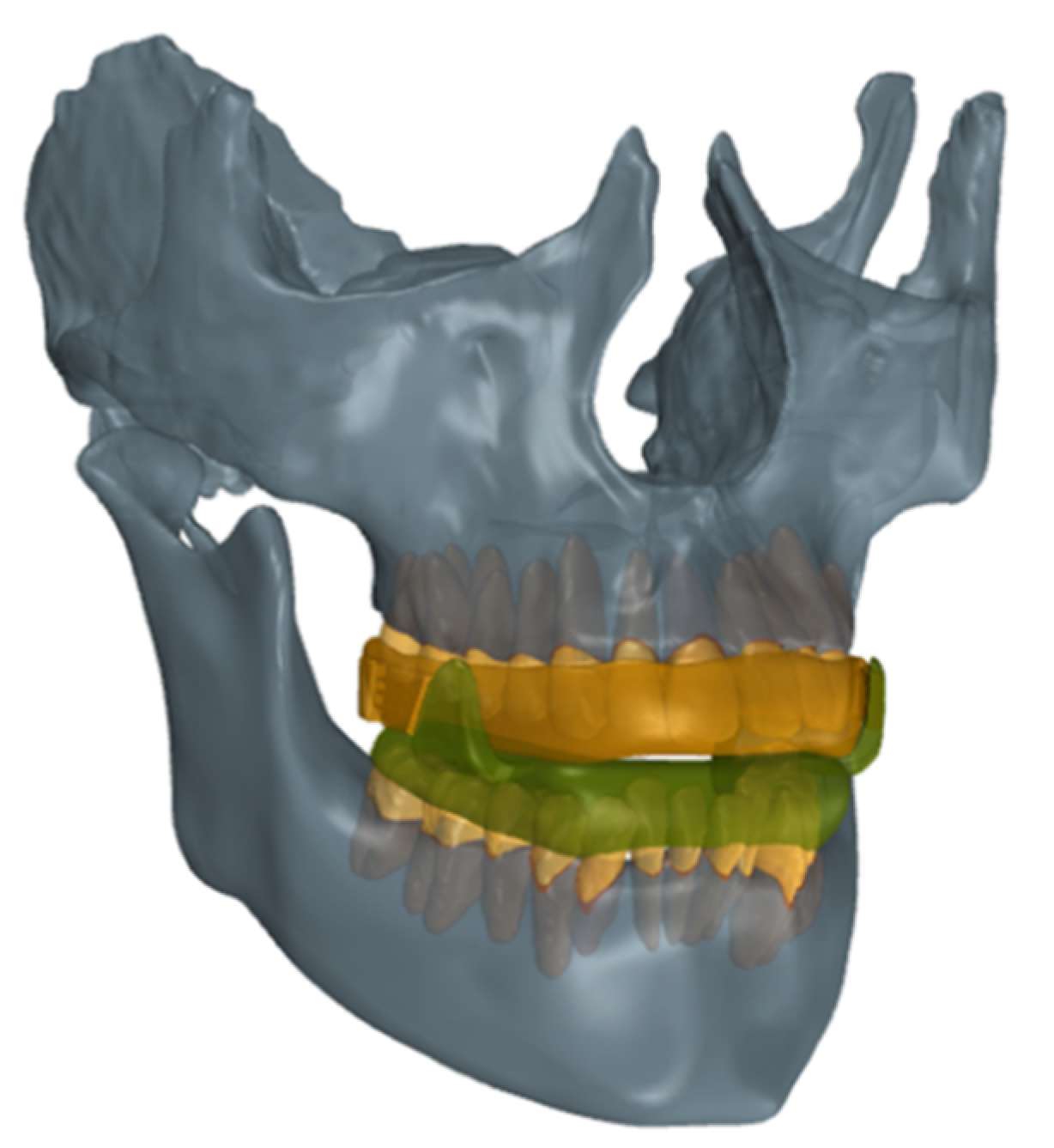
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Laratta, C.R.; Ayas, N.T.; Povitz, M.; Pendharkar, S.R. Diagnosis and treatment of obstructive sleep apnea in adults. CMAJ 2017, 189, 1481–1488. [Google Scholar] [CrossRef]
- Mayer, P.; Pépin, J.L.; Bettega, G.; Veale, D.; Ferretti, G.; Deschaux, C.; Lévy, P. Relationship between body mass index, age and upper airway measurements in snorers and sleep apnoea patients. Eur. Respir. J. 1996, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- White, D.P. Pathogenesis of obstructive and central sleep apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Horner, R.L. Contributions of passive mechanical loads and active neuromuscular compensation to upper airway collapsibility during sleep. J. Appl. Physiol. 2007, 102, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Destors, M.; Tamisier, R.; Galerneau, L.M.; Lévy, P.; Pepin, J.L. Pathophysiology of obstructive sleep apnea syndrome and its cardiometabolic consequences. Presse Med. 2017, 46, 395–403. [Google Scholar] [CrossRef]
- Schwartz, R.N.; Payne, R.J.; Forest, V.I.; Hier, M.P.; Fanous, A.; Vallée-Gravel, C. The relationship between upper airway collapse and the severity of obstructive sleep apnea syndrome: A chart review. J. Otolaryngol. Head Neck Surg. 2015, 44, 32. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 6–14. [Google Scholar] [CrossRef]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Lurie, A. Obstructive Sleep Apnea in Adults; Karger: Basel, Switzerland, 2011. [Google Scholar]
- Chowdhuri, S.; Quan, S.F.; Almeida, F.; Ayappa, I.; Batool-Anwar, S.; Budhiraja, R.; Cruse, P.E.; Drager, L.F.; Griss, B.; Marshall, N.; et al. An official american thoracic society research statement: Impact of mild obstructive sleep apnea in adults. Am. J. Respir. Crit. Care Med. 2016, 193, 37–54. [Google Scholar] [CrossRef]
- Fleetham, J.; Ayas, N.; Bradley, D.; Fitzpatrick, M.; Oliver, T.K.; Morrison, D.; Ryan, F.; Series, F.; Skomro, R.; Tsai, W.; et al. Canadian thoracic Society 2011 guideline update: Diagnosis and treatment of sleep disordered breathing. Can. Respir. J. 2011, 18, 25–47. [Google Scholar] [CrossRef]
- Salmina, D.; Ogna, A.; Wuerzner, G.; Heinzer, R.; Ogna, V.F. Arterial hypertension and obstructive sleep apnea syndrome: State of knowledge. Rev. Med. Suisse 2019, 11, 1620–1624. [Google Scholar]
- Gaines, J.; Vgontzas, A.N.; Fernandez-Mendoza, J.; Bixler, E.O. Obstructive sleep apnea and the metabolic syndrome: The road to clinically-meaningful phenotyping, improved prognosis, and personalized treatment. Sleep Med. Rev. 2018, 42, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Amra, B.; Rahmati, B.; Soltaninejad, F.; Feizi, A. Screening questionnaires for obstructive sleep apnea: An updated systematic review. Oman Med. J. 2018, 33, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Nagappa, M.; Liao, P.; Wong, J.; Auckley, D.; Ramachandran, S.K.; Memtsoudis, S.; Mokhlesi, B.; Chung, F. Validation of the stop-bang questionnaire as a screening tool for obstructive sleep apnea among different populations: A systematic review and meta-Analysis. PLoS ONE 2015, 10, 14–36. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.Y.; Chen, P.Y.; Chuang, L.P.; Chen, N.H.; Tu, Y.K.; Hsieh, Y.J.; Wang, Y.C.; Guilleminault, C. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med. Rev. 2017, 36, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Lonia, L.; Scalese, M.; Rossato, G.; Bruno, G.; Zalunardo, F.; De Stefani, A.; Gracco, A. Validity of the stop-bang questionnaire in identifying osa in a dental patient cohort. Medicina 2020, 56, 324. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 472–477. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.C.T.; Martinez, D.; Vasconcelos, L.F.T.; Gonçalves, S.C.; do Carmo Lenz, M.; Fuchs, S.C.; Gus, M.; de Abreu-Silva, E.O.; Moreira, L.B.; Fuchs, F.D. Diagnosis of obstructive sleep apnea syndrome and its outcomes with home portable monitoring. Chest 2009, 135, 330–336. [Google Scholar] [CrossRef]
- Su, S.; Baroody, F.M.; Kohrman, M.; Suskind, D. A comparison of polysomnography and a portable home sleep study in the diagnosis of obstructive sleep apnea syndrome. Otolaryngol. Head Neck Surg. 2004, 131, 44–50. [Google Scholar] [CrossRef]
- De Stefani, A.; Bruno, G.; Agostini, L.; Mezzofranco, L.; Gracco, A. Resolution of a Severe Grade of Obstructive Sleep Apnea Syndrome with Mandibular Advancement Device: A Case Report. Sleep Med. Res. 2020, 11, 44–48. [Google Scholar] [CrossRef]
- Luzzi, V.; Brunori, M.; Terranova, S.; Di Paolo, C.; Ierardo, G.; Vozza, I.; Polimeni, A. Difficult-to-treat OSAS: Combined continuous positive airway pressure (CPAP) and mandibular advancement devices (MADs) therapy. A case report. Cranio J. Craniomandib. Pract. 2020, 38, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.L.; Grunstein, R.R.; Darendeliler, M.A.; Mihailidou, A.S.; Srinivasan, V.K.; Yee, B.J.; Marks, G.B.; Cistulli, P.A. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2013, 187, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.A.; Cartwright, R.; Rogers, R.; Schmidt-Nowara, W. Oral appliances for snoring and obstructive sleep apnea: A review. Sleep 2006, 29, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K.; Vanderveken, O.M.; Tsuda, H.; Marklund, M.; Gagnadoux, F.; Kushida, C.A.; Cistulli, P.A. Oral appliance treatment for obstructive sleep apnea: An update. J. Clin. Sleep Med. 2014, 10, 15–27. [Google Scholar] [CrossRef]
- De Stefani, A.; Bruno, G.; Mezzofranco, L.; Perri, A.; Marchese Ragona, R.; Gracco, A. Multidisciplinary ent-orthodontic treatment in a hypertensive patient affected by severe OSAS. ORAL Implantol. 2018, 11, 59–63. [Google Scholar]
- Mickelson, S.A. Oral Appliances for Snoring and Obstructive Sleep Apnea. Otolaryngol. Clin. N. Am. 2020, 53, 397–407. [Google Scholar] [CrossRef]
- Ramar, K.; Dort, L.C.; Katz, S.G.; Lettieri, C.J.; Harrod, C.G.; Thomas, S.M.; Chervin, R.D. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015: An American Academy of Sleep Medicine and American Academy of Dental Sleep Medicine clinical practice guideline. J. Dent. Sleep Med. 2015, 11, 773–827. [Google Scholar] [CrossRef]
- De Martins, O.F.M.; Chaves Junior, C.M.; Rossi, R.R.P.; Cunali, P.A.; Dal-Fabbro, C.; Bittencourt, L. Side effects of mandibular advancement splints for the treatment of snoring and obstructive sleep apnea: A systematic review. Dent. Press J. Orthod. 2018, 23, 45–54. [Google Scholar] [CrossRef]
- Lang, N.P.; Bartold, P.M. Periodontal health. J. Clin. Periodontol. 2018, 89, 9–16. [Google Scholar] [CrossRef]
- Savage, A.; Eaton, K.A.; Moles, D.R.; Needleman, I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J. Clin. Periodontol. 2009, 36, 458–467. [Google Scholar] [CrossRef]
- Eke, P.I.; Page, R.C.; Wei, L.; Thornton-Evans, G.; Genco, R.J. Update of the Case Definitions for Population-Based Surveillance of Periodontitis. J. Periodontol. 2012, 78, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Holtfreter, B.; Schwahn, C.; Biffar, R.; Kocher, T. Epidemiology of periodontal diseases in the study of health in Pomerania. J. Clin. Periodontol. 2009, 36, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Holtfreter, B.; Kocher, T.; Hoffmann, T.; Desvarieux, M.; Micheelis, W. Prevalence of periodontal disease and treatment demands based on a German dental survey (DMS IV). J. Clin. Periodontol. 2010, 37, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Perotto, S.; Castiglione, A.; Mariani, G.M.; Ferrarotti, F.; Romano, F. Prevalence of periodontitis in an adult population from an urban area in North Italy: Findings from a cross-sectional population-based epidemiological survey. J. Clin. Periodontol. 2015, 42, 622–631. [Google Scholar] [CrossRef]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 11–22. [Google Scholar] [CrossRef]
- Kushida, C.A.; Morgenthaler, T.I.; Littner, M.R.; Alessi, C.A.; Bailey, D.; Coleman, J., Jr.; Friedman, L.; Hirshkowitz, M.; Kapen, S.; Kramer, M.; et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: An update for 2005. Sleep 2006, 29, 240–243. [Google Scholar] [CrossRef]
- Lee, J.S.; Choi, H.I.; Lee, H.; Ahn, S.J.; Noh, G. Biomechanical effect of mandibular advancement device with different protrusion positions for treatment of obstructive sleep apnoea on tooth and facial bone: A finite element study. J. Oral Rehabil. 2018, 45, 948–958. [Google Scholar] [CrossRef]
- Chandra, S.; Chandra, S.; Chandra, M.; Chandra, N. Textbook of Dental and Oral Histology with Embryology; Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi, India, 2007. [Google Scholar]
- Dorow, C.; Schneider, J.; Sander, F.G. Finite element simulation of in vivo tooth mobility in comparison with experimental results. J. Mech. Med. Biol. 2003, 3, 79–94. [Google Scholar] [CrossRef]
- Ammar, H.H.; Ngan, P.; Crout, R.J.; Mucino, V.H.; Mukdadi, O.M. Three-dimensional modeling and finite element analysis in treatment planning for orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2011, 139, e59–e71. [Google Scholar] [CrossRef]
- Liu, Z.; Qian, Y.; Zhang, Y.; Fan, Y. Effects of several temporomandibular disorders on the stress distributions of temporomandibular joint: A finite element analysis. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 137–143. [Google Scholar] [CrossRef]
- Wu, B.; Tang, W.; Yan, B. Study on stress distribution in periodontal ligament of impacted tooth based on hyperelastic model. In Proceedings of the 2009 International Conference on Information Engineering and Computer Science, ICIECS, Wuhan, China, 19–20 December 2009; pp. 1–4. [Google Scholar]
- Huang, H.; Tang, W.; Yan, B.; Wu, B. Mechanical responses of periodontal ligament under a realistic orthodontic loading. Procedia Eng. 2012, 31, 828–833. [Google Scholar]
- Roostaie, M.; Soltani, M. Mechanical responses of maxillary canine and surrounding tissues under orthodontic loading: A non-linear three-dimensional finite element analysis. J. Braz. Soc. Mech. Sci. Eng. 2017, 39, 2353–2369. [Google Scholar] [CrossRef]
- Natali, A.N.; Carniel, E.L.; Pavan, P.G.; Bourauel, C.; Ziegler, A.; Keilig, L. Experimental-numerical analysis of minipig’s multi-rooted teeth. J. Biomech. 2007, 40, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; De Stefani, A.; Conte, E.; Caragiuli, M.; Mandolini, M.; Landi, D.; Gracco, A. A procedure for analyzing mandible roto-translation induced by mandibular advancement devices. Materials (Basel) 2020, 13, 1826. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Levy, J.; Pételle, B.; Pinguet, J.; Limerat, E.; Fleury, B. Forces created by mandibular advancement devices in OSAS patients: A pilot study during sleep. Sleep Breath. 2013, 17, 781–789. [Google Scholar] [CrossRef]
- Azagra-Calero, E.; Espinar-Escalona, E.; Barrera-Mora, J.M.; Llamas-Carreras, J.M.; Solano-Reina, E. Obstructive sleep apnea syndrome (OSAS). Review of the literature. Med. Oral Patol. Oral Cir. Bucal 2012, 17, 925–929. [Google Scholar] [CrossRef]
- Serra-Torres, S.; Bellot-Arcís, C.; Montiel-Company, J.M.; Marco-Algarra, J.; Almerich-Silla, J.M. Effectiveness of mandibular advancement appliances in treating obstructive sleep apnea syndrome: A systematic review. Laryngoscope 2016, 126, 507–514. [Google Scholar] [CrossRef]
- Crivellin, G.; Bruno, G.; De Stefani, A.; Mazzoli, A.; Mandolini, M.; Brunzini, A.; Gracco, A. Strength distribution on TMJ using mandibular advancement device for OSAS treatment: A finite element study. Dent. Cadmos 2018, 86, 757–764. [Google Scholar] [CrossRef]
- Lu, R.J.; Tian, N.; Wang, J.Z.; Zou, X.; Wang, J.J.; Zhang, M.; Bai, C.Q.; Yu, K.T. The effectiveness of adjustable oral appliance for older adult patients with obstructive sleep apnea syndrome. Ann. Cardiothorac. Surg. 2020, 9, 2178–2186. [Google Scholar]
- Pliska, B.T.; Nam, H.; Chen, H.; Lowe, A.A.; Almeida, F.R. Obstructive sleep apnea and mandibular advancement splints: Occlusal effects and progression of changes associated with a decade of treatment. J. Clin. Sleep Med. 2014, 10, 1285–1291. [Google Scholar] [CrossRef]
- Liao, Z.; Chen, J.; Li, W.; Darendeliler, M.A.; Swain, M.; Li, Q. Biomechanical investigation into the role of the periodontal ligament in optimising orthodontic force: A finite element case study. Arch. Oral Biol. 2016, 66, 98–107. [Google Scholar] [CrossRef]
- Ueda, H.; Almeida, F.R.; Lowe, A.A.; Ruse, N.D. Changes in occlusal contact area during oral appliance therapy assessed on study models. Angle Orthod. 2008, 78, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Uniken Venema, J.A.M.; Doff, M.H.J.; Joffe-Sokolova, D.S.; Wijkstra, P.J.; van der Hoeven, J.H.; Stegenga, B.; Hoekema, A. Dental side effects of long-term obstructive sleep apnea therapy: A 10-year follow-up study. Clin. Oral Investig. 2019, 24, 3069–3076. [Google Scholar] [CrossRef] [PubMed]
- Brunzini, A.; Gracco, A.; Mazzoli, A.; Mandolini, M.; Manieri, S.; Germani, M. Preliminary simulation model toward the study of the effects caused by different mandibular advancement devices in OSAS treatment. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Toms, S.R.; Eberhardt, A.W. A nonlinear finite element analysis of the periodontal ligament under orthodontic tooth loading. Am. J. Orthod. Dentofac. Orthop. 2003, 123, 657–665. [Google Scholar]
- Jeon, P.D.; Turley, P.K.; Ting, K. Three-dimensional finite element analysis of stress in the periodontal ligament of the maxillary first molar with simulated bone loss. Am. J. Orthod. Dentofac. Orthop. 2001, 119, 498–504. [Google Scholar]
- Venema, J.; Stellingsma, C.; Doff, M.; Hoekema, A. Dental Side Effects of Long-Term Obstructive Sleep Apnea Therapy: A Comparison of Three Therapeutic Modalities. J. Dent. Sleep Med. 2018, 5, 39–46. [Google Scholar] [CrossRef]
- Tsuda, H.; Lowe, A.A.; Chen, H.; Fleetham, J.A.; Ayas, N.T.; Almeida, F.R. The relationship between mouth opening and sleep stage-related sleep disordered breathing. J. Clin. Sleep Med. 2011, 7, 181–186. [Google Scholar] [CrossRef]
- Kim, E.J.; Choi, J.H.; Kim, K.W.; Kim, T.H.; Lee, S.H.; Lee, H.M.; Shin, C.; Lee, K.Y.; Lee, S.H. The impacts of open-mouth breathing on upper airway space in obstructive sleep apnea: 3-D MDCT analysis. Eur. Arch. Oto-Rhino-Laryngol. 2011, 268, 533–539. [Google Scholar] [CrossRef]
- Toniolo, I.; Salmaso, C.; Bruno, G.; De Stefani, A.; Stefanini, C.; Gracco, A.L.T.; Carniel, E.L. Anisotropic computational modelling of bony structures from CT data: An almost automatic procedure. Comput. Methods Programs Biomed. 2020, 189, 105319. [Google Scholar]




| Material | Young’s Modulus [MPa] | Poisson’s Ratio |
|---|---|---|
| Acrylic MAD | 8300 | 0.28 |
| Tooth | 18,300 | 0.31 |
| Model | Parameter | Value |
|---|---|---|
| Hyperelastic-Ogden | α1 | −3.4761 |
| α2 | 18.679 | |
| µ1 | −0.034004 | |
| µ2 | 0.00088691 | |
| d1 | 0 | |
| d1 | 0 |
| Mesh Size (mm) | Nodes/Elements | Von Mises Stress (MPa) | Elapsed Time (hour) | Memory Used (GB) |
|---|---|---|---|---|
| 0.26 | 631,362/3,317,272 | 8.07 × 10−3 | 3 | 18.972 |
| 0.20 | 1,073,911/5,706,491 | 4.26 × 10−3 | 22 | 33.212 |
| 0.15 | 1,928,454/10,404,873 | 4.48 × 10−3 | 36 | 70.366 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, G.; de Stefani, A.; Caragiuli, M.; Zalunardo, F.; Mazzoli, A.; Landi, D.; Mandolini, M.; Gracco, A. Comparison of the Effects Caused by Three Different Mandibular Advancement Devices on the Periodontal Ligaments and Teeth for the Treatment of Osa: A Finite Element Model Study. Appl. Sci. 2020, 10, 6932. https://doi.org/10.3390/app10196932
Bruno G, de Stefani A, Caragiuli M, Zalunardo F, Mazzoli A, Landi D, Mandolini M, Gracco A. Comparison of the Effects Caused by Three Different Mandibular Advancement Devices on the Periodontal Ligaments and Teeth for the Treatment of Osa: A Finite Element Model Study. Applied Sciences. 2020; 10(19):6932. https://doi.org/10.3390/app10196932
Chicago/Turabian StyleBruno, Giovanni, Alberto de Stefani, Manila Caragiuli, Francesca Zalunardo, Alida Mazzoli, Daniele Landi, Marco Mandolini, and Antonio Gracco. 2020. "Comparison of the Effects Caused by Three Different Mandibular Advancement Devices on the Periodontal Ligaments and Teeth for the Treatment of Osa: A Finite Element Model Study" Applied Sciences 10, no. 19: 6932. https://doi.org/10.3390/app10196932
APA StyleBruno, G., de Stefani, A., Caragiuli, M., Zalunardo, F., Mazzoli, A., Landi, D., Mandolini, M., & Gracco, A. (2020). Comparison of the Effects Caused by Three Different Mandibular Advancement Devices on the Periodontal Ligaments and Teeth for the Treatment of Osa: A Finite Element Model Study. Applied Sciences, 10(19), 6932. https://doi.org/10.3390/app10196932









