On the Relationship between a Novel Prorocentrum sp. and Colonial Phaeocystis antarctica under Iron and Vitamin B12 Limitation: Ecological Implications for Antarctic Waters
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Phytoplankton Collection, Isolation, and Culture
2.2. Molecular Analysis and Identification of the Two Species
2.3. Experimental Setup
2.3.1. Experiment 1: Iron and B12 Limitation
- For the DFB treatment, we prepared L1 medium without iron (II). As natural oligotrophic seawater was used as a base for the medium, we added DFB as a ligand to chelate all dissolved iron, to achieve a final DFB/iron (II) ratio of 100:1, assuming an iron concentration of 5 nM.
- For the -B12 treatment, modified 100% L1 medium was prepared by excluding vitamin B12 from the vitamin stock solution. The amount of vitamin B12 present in the prefiltered (0.2 μm mesh size) seawater used as the culture medium before the addition of L1 medium would be negligible as seawater samples were sterilized by autoclaving at 1.06 kg cm−2 for 20 min.
- For the CTRL treatment (i.e., with both iron and B12) 100% L1 medium was used.
2.3.2. Experiment 2: Exposure to Varying Light Intensity
2.4. Pigment Analysis
2.5. Photosynthetic Efficiency
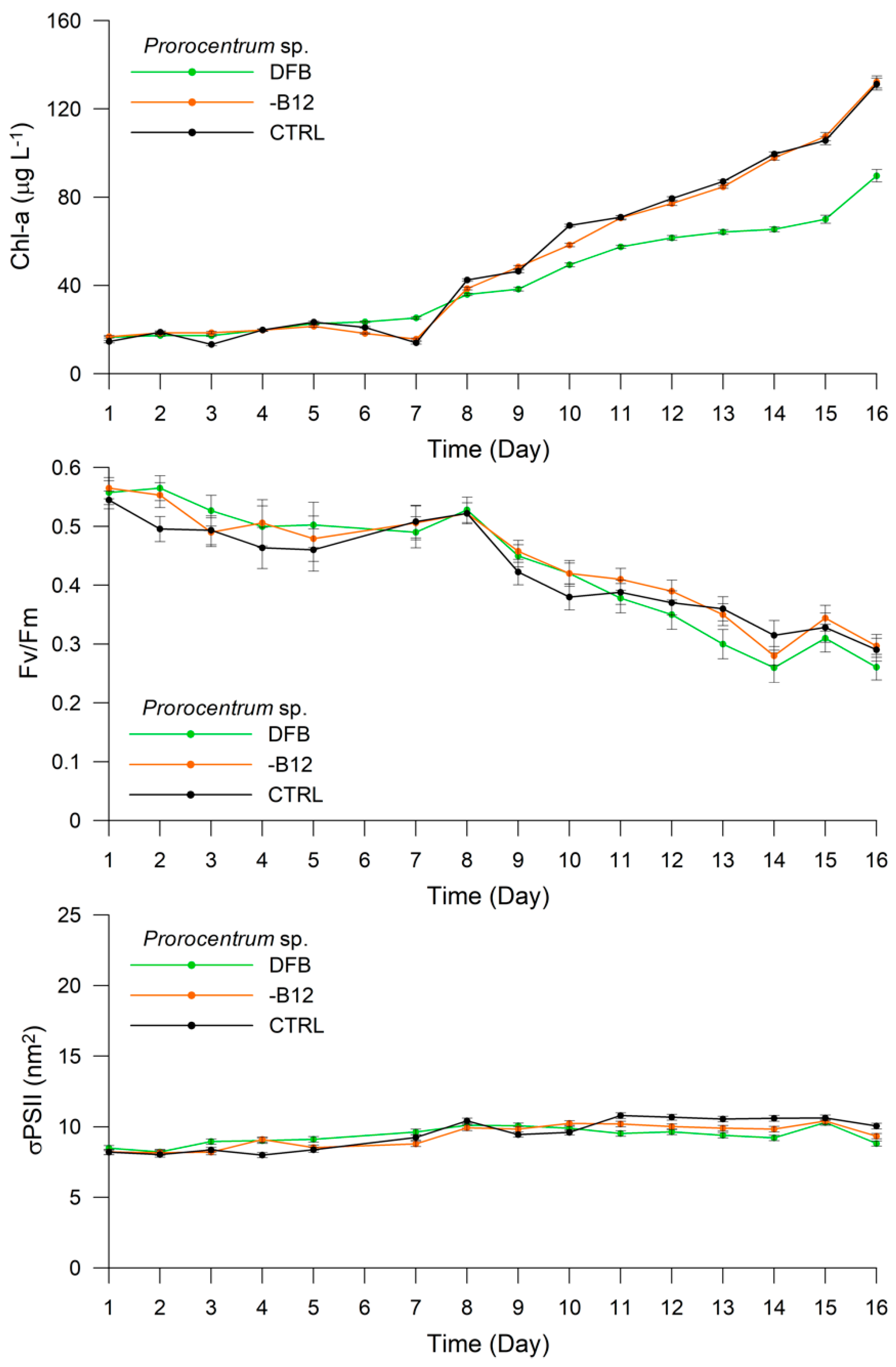
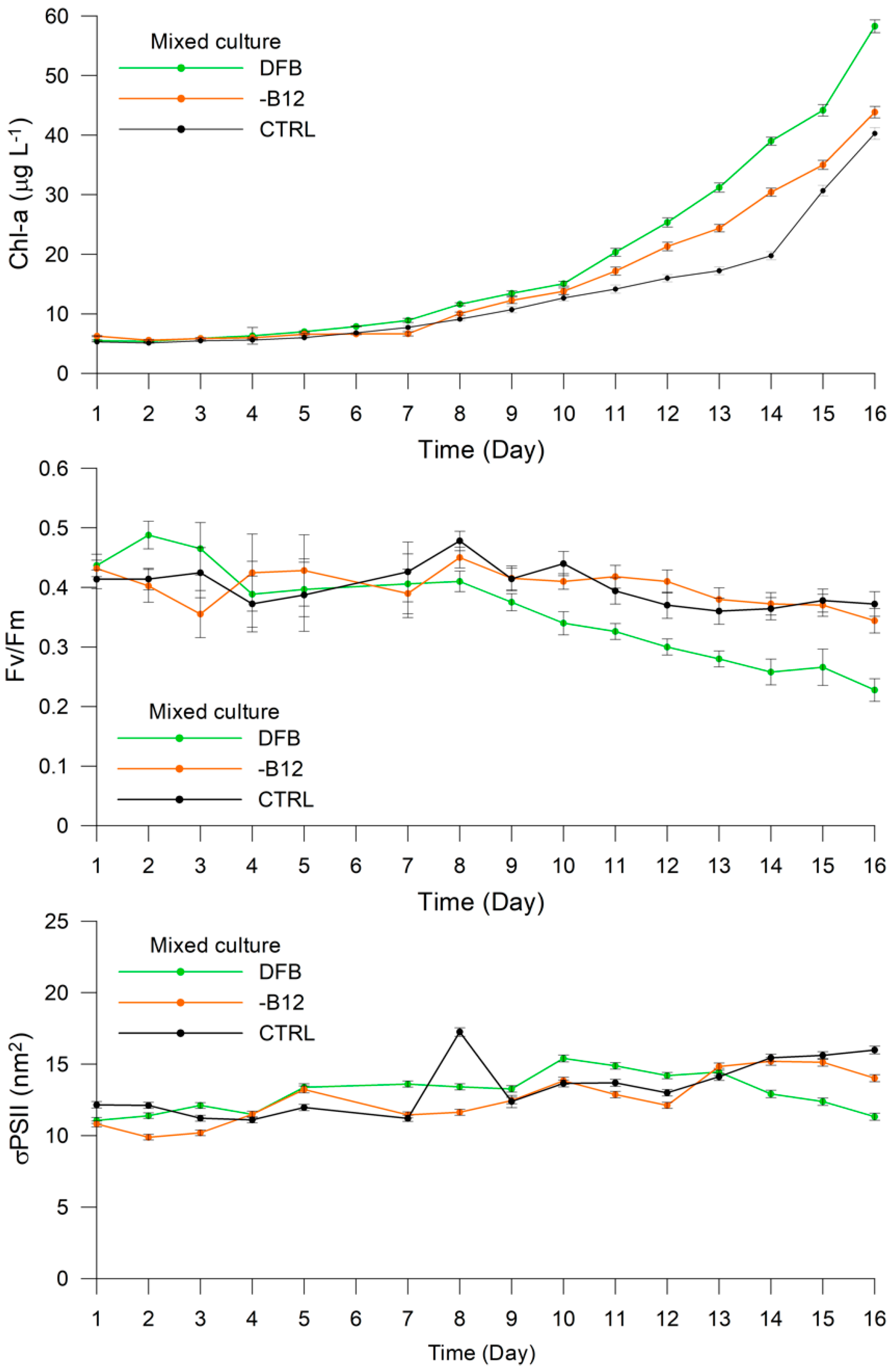
3. Results
3.1. Identification of a Novel Prorocentrum sp. from Phaeocystis Antarctica Colonies
3.2. Experiment 1
3.3. Experiment 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, W.O.; Nelson, D.M. Phytoplankton Bloom Produced by a Receding Ice Edge in the Ross Sea: Spatial Coherence with the Density Field. Science 1985, 227, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, K.R. Phytoplankton Community Structure and the Drawdown of Nutrients and CO2 in the Southern Ocean. Science 1999, 283, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Peloquin, J.A.; Smith, W.O. Phytoplankton blooms in the Ross Sea, Antarctica: Interannual variability in magnitude, temporal patterns, and composition. J. Geophys. Res. 2007, 112, C08013. [Google Scholar] [CrossRef]
- Tremblay, J.-E.; Smith, W.O. Chapter 8 Primary Production and Nutrient Dynamics in Polynyas. In Polynyas: Windows to the World; Smith, W.O., Barber, D.G., Eds.; Elsevier Oceanography Series; Elsevier: Amsterdam, The Netherlands, 2007; Volume 74, pp. 239–269. [Google Scholar] [CrossRef]
- Smith, W.O.; Ainley, D.G.; Arrigo, K.R.; Dinniman, M.S. The Oceanography and Ecology of the Ross Sea. Annu. Rev. Mar. Sci. 2014, 6, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.O.; Shields, A.R.; Peloquin, J.A.; Catalano, G.; Tozzi, S.; Dinniman, M.S.; Asper, V.A. Interannual variations in nutrients, net community production, and biogeochemical cycles in the Ross Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 815–833. [Google Scholar] [CrossRef]
- DiTullio, G.R.; Smith, W.O. Spatial patterns in phytoplankton biomass and pigment distributions in the Ross Sea. J. Geophys. Res. Oceans 1996, 101, 18467–18477. [Google Scholar] [CrossRef]
- Smith, W.O.; Marra, J.; Hiscock, M.R.; Barber, R.T. The seasonal cycle of phytoplankton biomass and primary productivity in the Ross Sea, Antarctica. Deep Sea Res. Part II Top. Stud. Oceanogr. 2000, 47, 3119–3140. [Google Scholar] [CrossRef]
- Bertrand, E.M.; Saito, M.A.; Rose, J.M.; Riesselman, C.R.; Lohan, M.C.; Noble, A.E.; Lee, P.A.; DiTullio, G.R. Vitamin B12 and iron colimitation of phytoplankton growth in the Ross Sea. Limnol. Oceanogr. 2007, 52, 1079–1093. [Google Scholar] [CrossRef]
- Bertrand, E.M.; McCrow, J.P.; Moustafa, A.; Zheng, H.; McQuaid, J.B.; Delmont, T.O.; Post, A.F.; Sipler, R.E.; Spackeen, J.L.; Xu, K.; et al. Phytoplankton–bacterial interactions mediate micronutrient colimitation at the coastal Antarctic sea ice edge. Proc. Natl. Acad. Sci. USA 2015, 112, 9938–9943. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Z.; Koch, F.; Gobler, C.J. Most harmful algal bloom species are vitamin B1 and B12 auxotrophs. Proc. Natl. Acad. Sci. USA 2010, 107, 20756–20761. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.; Marcoval, M.A.; Panzeca, C.; Bruland, K.W.; Sañudo-Wilhelmy, S.A.; Gobler, C.J. The effect of vitamin B12 on phytoplankton growth and community structure in the Gulf of Alaska. Limnol. Oceanogr. 2011, 56, 1023–1034. [Google Scholar] [CrossRef]
- Delmont, T.O.; Hammar, K.M.; Ducklow, H.W.; Yager, P.L.; Post, A.F. Phaeocystis antarctica blooms strongly influence bacterial community structures in the Amundsen Sea polynya. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Schoemann, V.; Becquevort, S.; Stefels, J.; Rousseau, V.; Lancelot, C. Phaeocystis blooms in the global ocean and their controlling mechanisms: A review. J. Sea Res. 2005, 53, 43–66. [Google Scholar] [CrossRef]
- Sedwick, P.N.; Garcia, N.S.; Riseman, S.F.; Marsay, C.M.; DiTullio, G.R. Evidence for high iron requirements of colonial Phaeocystis antarctica at low irradiance. Biogeochemistry 2007, 83, 83–97. [Google Scholar] [CrossRef]
- Garcia, N.; Sedwick, P.; DiTullio, G. Influence of irradiance and iron on the growth of colonial Phaeocystis antarctica: Implications for seasonal bloom dynamics in the Ross Sea, Antarctica. Aquat. Microb. Ecol. 2009, 57, 203–220. [Google Scholar] [CrossRef]
- Alderkamp, A.-C.; Kulk, G.; Buma, A.G.J.; Visser, R.J.W.; Van Dijken, G.L.; Mills, M.M.; Arrigo, K.R. The effect of iron limitation on the photophysiology of Phaeocystis antarctica (Prymnesiophyceae) and Fragilariopsis cylindrus (Bacillariophyceae) under dynamic irradiance. J. Phycol. 2012, 48, 45–59. [Google Scholar] [CrossRef]
- Bender, S.J.; Moran, D.M.; McIlvin, M.R.; Zheng, H.; McCrow, J.P.; Badger, J.; DiTullio, G.R.; Allen, A.E.; Saito, M.A. Colony formation in Phaeocystis antarctica: Connecting molecular mechanisms with iron biogeochemistry. Biogeosciences 2018, 15, 4923–4942. [Google Scholar] [CrossRef]
- Mathiot, P.; Jourdain, N.C.; Barnier, B.; Gallée, H.; Molines, J.M.; Le Sommer, J.; Penduff, T. Sensitivity of coastal polynyas and high-salinity shelf water production in the Ross Sea, Antarctica, to the atmospheric forcing. Ocean Dyn. 2012, 62, 701–723. [Google Scholar] [CrossRef]
- Dennett, M.R.; Mathot, S.; Caron, D.A.; Smith, W.O.; Lonsdale, D.J. Abundance and distribution of phototrophic and heterotrophic nano- and microplankton in the southern Ross Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 4019–4037. [Google Scholar] [CrossRef]
- Rousseau, V.; Chrétiennot-Dinet, M.-J.; Jacobsen, A.; Verity, P.; Whipple, S. The life cycle of Phaeocystis: State of knowledge and presumptive role in ecology. Biogeochemistry 2007, 83, 29–47. [Google Scholar] [CrossRef]
- Hamm, C.; Simson, D.; Merkel, R.; Smetacek, V. Colonies of Phaeocystis globosa are protected by a thin but tough skin. Mar. Ecol. Prog. Ser. 1999, 187, 101–111. [Google Scholar] [CrossRef]
- Andreoli, C.; Tolomio, C.; Moro, I.; Radice, M.; Moschin, E.; Bellato, S. Diatoms and dinoflagellates in Terra Nova Bay (Ross Sea-Antarctica) during austral summer 1990. Polar Biol. 1995, 15. [Google Scholar] [CrossRef]
- Waters, R.L.; van den Enden, R.; Marchant, H.J. Summer microbial ecology off East Antarctica (80–150° E): Protistan community structure and bacterial abundance. Deep Sea Res. Part II Top. Stud. Oceanogr. 2000, 47, 2401–2435. [Google Scholar] [CrossRef]
- Davidson, A.T.; Scott, F.J.; Nash, G.V.; Wright, S.W.; Raymond, B. Physical and biological control of protistan community composition, distribution and abundance in the seasonal ice zone of the Southern Ocean between 30 and 80° E. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 828–848. [Google Scholar] [CrossRef]
- Escalera, L.; Mangoni, O.; Bolinesi, F.; Saggiomo, M. Austral Summer Bloom of Loricate Choanoflagellates in the Central Ross Sea Polynya. J. Eukaryot. Microbiol. 2019, 66, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Balech, E. Contribución al conocimiento del plancton antártico. Plancton del Mar de Bellingshausen. Physis 1947, 75–91. [Google Scholar]
- Balech, E. Clave ilustrada de dinoflagelados antárticos. Publ. Inst. Antárt. Argent. 1976, 11, 1–99. [Google Scholar]
- Gast, R.J.; Moran, D.M.; Dennett, M.R.; Caron, D.A. Kleptoplasty in an Antarctic dinoflagellate: Caught in evolutionary transition? Environ. Microbiol. 2007, 9, 39–45. [Google Scholar] [CrossRef]
- Stamatakis, K.; Broussos, P.-I.; Panagiotopoulou, A.; Gast, R.J.; Pelecanou, M.; Papageorgiou, G.C. Light-adaptive state transitions in the Ross Sea haptophyte Phaeocystis antarctica and in dinoflagellate cells hosting kleptoplasts derived from it. Biochim. Biophys. Acta BBA—Bioenerg. 2019, 1860, 102–110. [Google Scholar] [CrossRef]
- Phan-Tan, L.; Nguyen-Ngoc, L.; Smith, W.O.; Doan-Nhu, H. A new dinoflagellate species, Protoperidinium smithii H. Doan-Nhu, L. Phan-Tan et L. Nguyen-Ngoc sp. nov., and an emended description of Protoperidinium defectum (Balech 1965) Balech 1974 from the Ross Sea, Antarctica. Polar Biol. 2018, 41, 983–992. [Google Scholar] [CrossRef]
- DiTullio, G.R.; Smith, W.O. Relationship between dimethylsulfide and phytoplankton pigment concentrations in the Ross Sea, Antarctica. Deep Sea Res. Part Oceanogr. Res. Pap. 1995, 42, 873–892. [Google Scholar] [CrossRef]
- DiTullio, G.R.; Grebmeier, J.M.; Arrigo, K.R.; Lizotte, M.P.; Robinson, D.H.; Leventer, A.; Barry, J.P.; VanWoert, M.L.; Dunbar, R.B. Rapid and early export of Phaeocystis antarctica blooms in the Ross Sea, Antarctica. Nature 2000, 404, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.; Thomas, D.; Marchant, H.; Higgins, H.; Mackey, M.; Mackey, D. Analysis of phytoplankton of the Australian sector of the Southern Ocean: Comparisons of microscopy and size frequency data with interpretations of pigment HPLC data using the “CHEMTAX” matrix factorisation program. Mar. Ecol. Prog. Ser. 1996, 144, 285–298. [Google Scholar] [CrossRef]
- Roy, S.; Llewellyn, C.; Egeland, E.S.; Johnsen, G. (Eds.) Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge University Press: Cambridge, UK, 2011; p. 845. ISBN 978-0-511-73226-3. [Google Scholar]
- Mangoni, O.; Saggiomo, V.; Bolinesi, F.; Margiotta, F.; Budillon, G.; Cotroneo, Y.; Misic, C.; Rivaro, P.; Saggiomo, M. Phytoplankton blooms during austral summer in the Ross Sea, Antarctica: Driving factors and trophic implications. PLoS ONE 2017, 12, e0176033. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J. Isolation of plant DNA from fresh tissue. Focus 1999, 13–15. [Google Scholar]
- Wakeman, K.C.; Yamaguchi, A.; Horiguchi, T. Molecular Phylogeny and Morphology of Haplozoon ezoense n. sp. (Dinophyceae): A Parasitic Dinoflagellate with Ultrastructural Evidence of Remnant Non-photosynthetic Plastids. Protist 2018, 169, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Scholin, C.A.; Herzog, M.; Sogin, M.; Anderson, D.M. Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU Rrna Gene1. J. Phycol. 1994, 30, 999–1011. [Google Scholar] [CrossRef]
- Vandersea, M.W.; Kibler, S.R.; Holland, W.C.; Tester, P.A.; Schultz, T.F.; Faust, M.A.; Holmes, M.J.; Chinain, M.; Wayne Litaker, R. Development of semi-quantitative pcr assays for the detection and enumeration of Gambierdiscus species (Gonyaulacales, dinophyceae)1: Gambierdiscus qPCR. J. Phycol. 2012, 48, 902–915. [Google Scholar] [CrossRef]
- Nakayama, T.; Watanabe, S.; Mitsui, K.; Uchida, H.; Inouye, I. The phylogenetic relationship between the Chlamydomonadales and Chlorococcales inferred from 18SrDNA sequence data. Phycol. Res. 1996, 44, 47–55. [Google Scholar] [CrossRef]
- Aceto, S.; Caputo, P.; Cozzolino, S.; Gaudio, L.; Moretti, A. Phylogeny and Evolution of Orchis and Allied Genera Based on ITS DNA Variation: Morphological Gaps and Molecular Continuity. Mol. Phylogenet. Evol. 1999, 13, 67–76. [Google Scholar] [CrossRef]
- Kogame, K.; Horiguchi, T.; Masuda, M. Phylogeny of the order Scytosiphonales (Phaeophyceae) based on DNA sequences of rbcL, partial rbcS, and partial LSU nrDNA. Phycologia 1999, 38, 496–502. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Anderson, D.M.; Cembella, A.D. Manual on Harmful Marine Microalgae; UNESCO Publishing: Paris, France, 2004; ISBN 978-92-3-103948-5. [Google Scholar]
- Holm-Hansen, O.; Lorenzen, C.J.; Holmes, R.W.; Strickland, J.D.H. Fluorometric Determination of Chlorophyll. ICES J. Mar. Sci. 1965, 30, 3–15. [Google Scholar] [CrossRef]
- Vidussi, F.; Claustre, H.; Bustillos-Guzmàn, J.; Cailliau, C.; Marty, J.-C. Determination of chlorophylls and carotenoids of marine phytoplankton: Separation of chlorophyll a from divinylchlorophyll a and zeaxanthin from lutein. J. Plankton Res. 1996, 18, 2377–2382. [Google Scholar] [CrossRef]
- Brunet, C.; Mangoni, O. Determinazione quali-quantitativa dei pigmenti fitoplanctonici mediante HPLC. In Metodologie di Studio del Plancton Marino; Manuali e Linee Guida 56/2010; Socal, G., Buttino, I., Cabrini, M., Mangoni, O., Penna, A., Totti, C., Eds.; ISPRA: Roma, Italy, 2010; pp. 379–385. ISBN 978-88-448-0427-5. [Google Scholar]
- DiTullio, G.R.; Geesey, M.E.; Leventer, A.; Lizotte, M.P. Algal pigment ratios in the Ross Sea: Implications for Chemtax analysis of Southern Ocean data. In Antarctic Research Series; DiTullio, G.R., Dunbar, R.B., Eds.; American Geophysical Union: Washington, DC, USA, 2003; Volume 78, pp. 35–51. ISBN 978-0-87590-972-1. [Google Scholar]
- DiTullio, G.R.; Garcia, N.; Riseman, S.F.; Sedwick, P.N. Effects of iron concentration on pigment composition in Phaeocystis antarctica grown at low irradiance. Biogeochemistry 2007, 83, 71–81. [Google Scholar] [CrossRef]
- van Leeuwe, M.A.; Visser, R.J.W.; Stefels, J. The pigment composition of Phaeocystis antarctica (Haptophyceae) under various conditions of light, temperature, salinity, and iron. J. Phycol. 2014, 50, 1070–1080. [Google Scholar] [CrossRef]
- Mangoni, O.; Saggiomo, M.; Bolinesi, F.; Castellano, M.; Povero, P.; Saggiomo, V.; DiTullio, G.R. Phaeocystis antarctica unusual summer bloom in stratified Antarctic coastal waters (Terra Nova Bay, Ross Sea). Mar. Environ. Res. 2019, 151, 104733. [Google Scholar] [CrossRef]
- Demers, S.; Roy, S.; Gagnon, R.; Vignault, C. Rapid light-induced changes in cell fluorescence and in xanthophyll-cycle pigments of Alexandrium excavatum (Dinophyceae) and Thalassiosira pseudonana (Bacillariophyceae): A photo-protection mechanism. Mar. Ecol. Prog. Ser. 1991, 76, 185–193. [Google Scholar] [CrossRef]
- Meyer, A.A.; Tackx, M.; Daro, N. Xanthophyll cycling in Phaeocystis globosa and Thalassiosira sp.: A possible mechanism for species succession. J. Sea Res. 2000, 43, 373–384. [Google Scholar] [CrossRef]
- Mangoni, O.; Carrada, G.C.; Modigh, M.; Catalano, G.; Saggiomo, V. Photoacclimation in Antarctic bottom ice algae: An experimental approach. Polar Biol. 2009, 32, 325–335. [Google Scholar] [CrossRef]
- Mauzerall, D.; Greenbaum, N.L. The absolute size of a photosynthetic unit. Biochim. Biophys. Acta BBA—Bioenerg. 1989, 974, 119–140. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis; Blackwell Science: Malden, MA, USA, 1997; ISBN 978-0-86542-387-9. [Google Scholar]
- Cullen, J.J.; Davis, R.F. The blank can make a big difference in oceanographic measurements. Limnol. Oceanogr. Bull. 2003, 12, 29–35. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U.; Klughammer, C.; Kolbowski, J. Assessment of wavelength-dependent parameters of photosynthetic electron transport with a new type of multi-color PAM chlorophyll fluorometer. Photosynth. Res. 2012, 113, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, K.R.; DiTullio, G.R.; Dunbar, R.B.; Robinson, D.H.; VanWoert, M.; Worthen, D.L.; Lizotte, M.P. Phytoplankton taxonomic variability in nutrient utilization and primary production in the Ross Sea. J. Geophys. Res. Oceans 2000, 105, 8827–8846. [Google Scholar] [CrossRef]
- Bertrand, E.M.; Saito, M.A.; Lee, P.A.; Dunbar, R.B.; Sedwick, P.N.; DiTullio, G.R. Iron Limitation of a Springtime Bacterial and Phytoplankton Community in the Ross Sea: Implications for Vitamin B12 Nutrition. Front. Microbiol. 2011, 2. [Google Scholar] [CrossRef]
- Ryan-Keogh, T.J.; DeLizo, L.M.; Smith, W.O.; Sedwick, P.N.; McGillicuddy, D.J.; Moore, C.M.; Bibby, T.S. Temporal progression of photosynthetic-strategy in phytoplankton in the Ross Sea, Antarctica. J. Mar. Syst. 2017, 166, 87–96. [Google Scholar] [CrossRef]
- van Boekel, W.H.M. Phaeocystis colony mucus components and the importance of calcium ions for colony stability. Mar. Ecol.-Prog. Ser. 1992, 87, 301–305. [Google Scholar] [CrossRef]
- Smith, W.O.; Dinniman, M.S.; Klinck, J.M.; Hofmann, E. Biogeochemical climatologies in the Ross Sea, Antarctica: Seasonal patterns of nutrients and biomass. Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 3083–3101. [Google Scholar] [CrossRef]
- Tang, K.W.; Smith, W.O., Jr.; Elliott, D.T.; Shields, A.R. Colony size of Phaeocystis antarctica (Prymnesiophyceae) as influenced by zooplankton grazers. J. Phycol. 2008, 44, 1372–1378. [Google Scholar] [CrossRef]
- Lohr, M.; Wilhelm, C. Xanthophyll synthesis in diatoms: Quantification of putative intermediates and comparison of pigment conversion kinetics with rate constants derived from a model. Planta 2001, 212, 382–391. [Google Scholar] [CrossRef]
- Thingstad, F.; Billen, G. Microbial degradation of Phaeocystis material in the water column. J. Mar. Syst. 1994, 5, 55–65. [Google Scholar] [CrossRef]
- Solomon, C.M.; Lessard, E.J.; Keil, R.G.; Foy, M.S. Characterization of extracellular polymers of Phaeocystis globosa and P. antarctica. Mar. Ecol. Prog. Ser. 2003, 250, 81–89. [Google Scholar]
- Lubbers, G.; Gieskes, W.; Castilho, P.; del Salomons, W.; Bril, J. Manganese accumulation in the high pH microenvironment of Phaeocystis sp. (Haptophyceae) colonies from the North Sea. Mar. Ecol. Prog. Ser. 1990, 59, 285–293. [Google Scholar] [CrossRef]
- Smayda, T.J.; Reynolds, C.S. Strategies of marine dinoflagellate survival and some rules of assembly. J. Sea Res. 2003, 49, 95–106. [Google Scholar] [CrossRef]
- Ross, O.; Sharples, J. Phytoplankton motility and the competition for nutrients in the thermocline. Mar. Ecol. Prog. Ser. 2007, 347, 21–38. [Google Scholar] [CrossRef]
- Stoecker, D.K.; Hansen, P.J.; Caron, D.A.; Mitra, A. Mixotrophy in the Marine Plankton. Annu. Rev. Mar. Sci. 2017, 9, 311–335. [Google Scholar] [CrossRef]
- Stamatakis, K.; Vayenos, D.; Kotakis, C.; Gast, R.J.; Papageorgiou, G.C. The extraordinary longevity of kleptoplasts derived from the Ross Sea haptophyte Phaeocystis antarctica within dinoflagellate host cells relates to the diminished role of the oxygen-evolving Photosystem II and to supplementary light harvesting by mycosporine-like amino acid/s. Biochim. Biophys. Acta BBA—Bioenerg. 2017, 1858, 189–195. [Google Scholar] [CrossRef]
- Gast, R.J.; Fay, S.A.; Sanders, R.W. Mixotrophic Activity and Diversity of Antarctic Marine Protists in Austral Summer. Front. Mar. Sci. 2018, 5, 13. [Google Scholar] [CrossRef]
- Bird, D.F.; Kalff, J. Bacterial Grazing by Planktonic Lake Algae. Science 1986, 231, 493–495. [Google Scholar] [CrossRef]
- Nygaard, K.; Tobiesen, A. Bacterivory in algae: A survival strategy during nutrient limitation. Limnol. Oceanogr. 1993, 38, 273–279. [Google Scholar] [CrossRef]
- Dolan, J.R.; PÉrez, M.T. Costs, benefits and characteristics of mixotrophy in marine oligotrichs: Mixotrophy in marine oligotrichs. Freshw. Biol. 2000, 45, 227–238. [Google Scholar] [CrossRef]
- Gast, R.J.; Moran, D.M.; Beaudoin, D.J.; Blythe, J.N.; Dennett, M.R.; Caron, D.A. Abundance of a novel Dinoflagellate phylotype in the Ross Sea, Antarctica. J. Phycol. 2006, 42, 233–242. [Google Scholar] [CrossRef]
- Bolinesi, F.; Saggiomo, M.; Ardini, F.; Castagno, P.; Cordone, A.; Fusco, G.; Rivaro, P.; Saggiomo, V.; Mangoni, O. Spatial-Related Community Structure and Dynamics in Phytoplankton of The Ross Sea, Antarctica. Front. Mar. Sci. submitted.
- Deppeler, S.L.; Davidson, A.T. Southern Ocean Phytoplankton in a Changing Climate. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]







| (A) | ||||||||||||||
| Lines Growth | Chl-c3/ Chl-a | Chl-c2/ Chl-a | Perid/ Chl-a | But/ Chl-a | Fuco/ Chl-a | Hex/ Chl-a | Viola-like/ Chl-a | Lut/ Chl-a | ß-car/ Chl-a | Hex: Chl-c2 | Hex: Chl-c3 | Perid: Hex | Dt/(Dd + Dt) × 100 | |
| Prorocentrum sp. | DFB | 0.00 | 0.36 | 1.40 | - | - | - | 0.02 | - | 0.01 | - | - | - | 5.55 |
| -B12 | 0.00 | 0.34 | 1.30 | - | - | - | 0.03 | - | 0.01 | - | - | - | 4.75 | |
| CTRL | 0.03 | 0.01 | 1.27 | - | - | - | 0.03 | - | 0.01 | - | - | - | 4.62 | |
| P. antarctica | DFB | 0.28 | 0.44 | - | 0.01 | 0.02 | 0.53 | - | 0.01 | 0.01 | 1.20 | 1.89 | - | 0 |
| -B12 | 0.24 | 0.35 | - | 0.01 | 0.02 | 0.47 | - | 0.01 | 0.01 | 1.33 | 1.94 | - | 0 | |
| CTRL | 0.26 | 0.38 | - | 0.01 | 0.02 | 0.42 | - | 0.01 | 0.01 | 1.11 | 1.63 | - | 0 | |
| Mixed culture | DFB | 0.21 | 0.48 | 0.62 | 0.02 | 0.01 | 0.73 | - | 0.01 | 0.01 | 1.52 | 3.51 | 0.85 | 0.00 |
| -B12 | 0.20 | 0.43 | 0.93 | 0.00 | 0.00 | 0.21 | - | 0.00 | 0.01 | 0.49 | 1.04 | 4.43 | 2.78 | |
| CTRL | 0.08 | 0.37 | 0.88 | 0.00 | 0.00 | 0.21 | - | 0.00 | 0.01 | 0.56 | 2.45 | 4.19 | 0.00 | |
| (B) | ||||||||||||||
| Lines Growth | Chl-c3/ Chl-a | Chl-c2/ Chl-a | Perid/ Chl-a | But/ Chl-a | Fuco/ Chl-a | Hex/ Chl-a | Viola-like/ Chl-a | Lut/ Chl-a | ß-car/ Chl-a | Hex/ Chl-c2 | Hex/ Chl-c3 | Hex/ Perid | Dt/(Dd + Dt) × 100 | |
| Prorocentrum sp. | HL | - | 0.26 | 1.29 | - | - | - | 0.02 | 0.00 | 0.02 | - | - | - | 8.78 |
| L1 | - | 0.25 | 1.10 | - | - | - | 0.02 | 0.00 | 0.02 | - | - | - | 8.22 | |
| L2 | - | 0.34 | 1.33 | - | - | - | 0.03 | 0.00 | 0.01 | - | - | - | 4.85 | |
| L3 | - | 0.22 | 1.26 | - | - | - | 0.03 | 0.00 | 0.01 | - | - | - | 3.46 | |
| P. antarctica | HL | 0.33 | 0.38 | - | 0.01 | 0.01 | 0.36 | - | 0.00 | 0.01 | 0.95 | 1.09 | - | 53.07 |
| L1 | 0.21 | 0.36 | - | 0.01 | 0.01 | 0.41 | - | 0.01 | 0.01 | 1.14 | 1.96 | - | 33.89 | |
| L2 | 0.29 | 0.44 | - | 0.01 | 0.02 | 0.55 | - | 0.01 | 0.01 | 1.23 | 1.91 | - | 11.70 | |
| L3 | 0.27 | 0.42 | - | 0.01 | 0.02 | 0.49 | - | 0.01 | 0.01 | 1.15 | 1.79 | - | 5.59 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolinesi, F.; Saggiomo, M.; Aceto, S.; Cordone, A.; Serino, E.; Valoroso, M.C.; Mangoni, O. On the Relationship between a Novel Prorocentrum sp. and Colonial Phaeocystis antarctica under Iron and Vitamin B12 Limitation: Ecological Implications for Antarctic Waters. Appl. Sci. 2020, 10, 6965. https://doi.org/10.3390/app10196965
Bolinesi F, Saggiomo M, Aceto S, Cordone A, Serino E, Valoroso MC, Mangoni O. On the Relationship between a Novel Prorocentrum sp. and Colonial Phaeocystis antarctica under Iron and Vitamin B12 Limitation: Ecological Implications for Antarctic Waters. Applied Sciences. 2020; 10(19):6965. https://doi.org/10.3390/app10196965
Chicago/Turabian StyleBolinesi, Francesco, Maria Saggiomo, Serena Aceto, Angelina Cordone, Emanuela Serino, Maria Carmen Valoroso, and Olga Mangoni. 2020. "On the Relationship between a Novel Prorocentrum sp. and Colonial Phaeocystis antarctica under Iron and Vitamin B12 Limitation: Ecological Implications for Antarctic Waters" Applied Sciences 10, no. 19: 6965. https://doi.org/10.3390/app10196965
APA StyleBolinesi, F., Saggiomo, M., Aceto, S., Cordone, A., Serino, E., Valoroso, M. C., & Mangoni, O. (2020). On the Relationship between a Novel Prorocentrum sp. and Colonial Phaeocystis antarctica under Iron and Vitamin B12 Limitation: Ecological Implications for Antarctic Waters. Applied Sciences, 10(19), 6965. https://doi.org/10.3390/app10196965






