Abstract
In this work, the production of xylitol from a hemicellulosic hydrolysate of exhausted olive pomace (EOP), a residue originated in the olive oil production process by Candida boidinii, was assessed. The hydrolysate was obtained by dilute acid pretreatment of EOP at 170 °C and 2% H2SO4 (w/v). A previous detoxification step of the hydrolysate was necessary, and its treatment with activated charcoal and ion-exchange resin was evaluated. Prior to fermentation of the hydrolysate, fermentation tests in synthetic media were performed to determine the maximum xylitol yield and productivity that could be obtained if inhibitory compounds were not present in the medium. In addition, the glucose existing in the media exerted a negative influence on xylitol production. A maximum xylitol yield of 0.52 g/g could be achieved in absence of inhibitor compounds. Fermentation of the hemicellulosic hydrolysate from EOP after detoxification with ion-exchange resin resulted in a xylitol yield of 0.43 g/g.
1. Introduction
Xylitol is a sugar alcohol with five carbon atoms and similar sweetening properties to sucrose. Xylitol is widely known for its various health benefits, including a great capacity to decrease the risk of dental caries. In addition, xylitol stands out for its high efficiency in the treatment of illnesses, such as diabetes, osteoporosis, iron deficiency, anaemia, muscle inflammation, or problems in respiratory system and colon [1]. The use of xylitol has increasingly gained interest for food and medicine manufacturing in the past several years [2]. Traditionally, it has been produced using a chemical synthesis process, reducing pure xylose at very high temperatures and pressures. However, due to the complexity of this chemical process, the need for pure xylose as feedstock and the associated high-energy requirements [3], novel biological processes, where xylitol is produced by xylose fermentation with microorganisms, have emerged. These alternative processes can be more environmentally friendly and lower energy consumption, making biological production of xylitol economically viable [4,5]. To reach these objectives, the use of xylose sources coming from lignocellulosic biomass residues, originating in different industrial sectors (in many cases abundant and of low economic value), can be an important contribution.
In this context, an interesting lignocellulosic residue for biological production of xylitol could be exhausted olive pomace (EOP) because it contains a significant amount of carbon sources, cellulose, and hemicellulose [6]. EOP is generated in the the olive oil sector together with other residues, such as olive stones, olive leaves, and olive pomace. EOP is generated in specific extraction industries, as a result of extraction with hexane of the residual oil contained in olive pomace, which in turn, is produced in olive mills [7,8]. Olive pomace extraction industries in Spain are mainly located in the south of the country and can generate more than 1,182,000 tons per annum [8]. However, even though EOP can be employed in home heaters or small industrial boilers, due to strict environmental standards for emissions, it is now used less, and there is a certain surplus of this residue [9].
EOP contains cellulose, hemicellulose, lignin, and extractives (rich in value-added compounds, such as phenolic and antioxidants compounds) as its main components. Due to its high content in extractives (about 42%), an initial water extraction step is desirable in its conversion process, aimed at recovering these valuable compounds. Moreover, a pre-treatment stage is necessary, in order to disturb its structure and fractionate its main components [6].
In the pre-treatment stage, different types of technologies, such as autohydrolysis, dilute acid, alkalis, microwave, extrusion, organosolv, ionic liquid, deep eutectic solvents, steam explosion, or biologic treatment, have been tested with a vast variety lignocellulosic material [10,11,12]. Between them, dilute acid pretreatment is highlighted, as it is able to achieve high solubilization of hemicellulosic fractions (>85%) and generate a pretreated solid composed mainly of cellulose, which is more easily accessible for enzymes in the subsequent enzymatic hydrolysis step [11,13]. Nevertheless, fermentation inhibitory compounds, such as acetic and formic acids, furfural, 5-hydroxymethylfurfural (HMF), and phenolic compounds, might be generated during acid pre-treatment [11,14]. Therefore, in order to minimize the generation of these undesirable compounds, the choice of adequate acid pretreatment conditions, such as temperature, time, and acid concentration, is essential [11]. Even so, in many cases the production of such compounds is unavoidable if good fractionation results are desired. Therefore, the treatment of acid hydrolysates with detoxification methods may be a suitable way of reducing the presence of inhibitor compounds and, thus, favouring its utilization as a sugar source. From these detoxification methods, over-liming, liquid-liquid extraction, and treatments with ion-exchange resins, activated charcoal, and organic solvents stand out [15,16].
The purpose of this work was to study xylitol production from the hemicellulosic hydrolysate generated in the dilute sulfuric acid pretreatment of EOP. In order to decrease the inhibitor compound concentrations in the acid hydrolysate, different detoxification methods were tested prior to fermentation assays. C. boidinii was the yeast used in the fermentation, which is considered one of the best microorganism producers of xylitol [17]. Fermentation tests, using synthetic media instead of hydrolysate, were conducted to determine the maximum xylitol yield and effect of glucose in the fermentation performance. To the best of our knowledge, this is the first study on xylitol generation from EOP using C. boidinii yeast.
2. Materials and Methods
2.1. Raw Material
EOP residue was generated in a pomace oil extracting industry (“Spuny SA,” Jaén, Spain). The EOP composition was (% w/w) 10.4 ± 0.34 cellulose, 11.5 ± 0.28 hemicellulose (9.5 ± 0.28 xylan, 1.0 ± 0.02 galactan, and 1.0 ± 0.01 arabinan), 22.1 ± 1.41 acid insoluble lignin, 1.6 ± 0.03 acid soluble lignin, 2.1 ± 0.03 acetyl groups, 9.4 ± 0.05 ashes, and 42.0 ± 1.18 extractives [6].
2.2. Production and Conditioning of EOP Hemicellulosic Hydrolysate
EOP was first extracted with water (at 100 °C, 30 min and 15% (w/v) solid-liquid ratio) and, then, pretreated with 2% (w/v) sulfuric acid (H2SO4) (Sigma-Aldrich, Madrid, Spain) at 170 °C and 20% biomass loading in a 1 L Parr reactor (Parr Instr. Co., Illinois, USA), according to the process scheme proposed by Manzanares et al. [6]. The sulfuric acid pretreatment was started once the temperature was set, with a heating rate of 5 °C/min. The resulting mixture was vacuum filtrated. The hydrolysate was separated and employed in the fermentation assay for the production of xylitol. In order to reduce the presence of inhibitor compounds contained in the EOP hydrolysate, two different detoxification methods were applied: activated charcoal and ion-exchange resin treatment.
Detoxification by activated charcoal was carried out in an orbital shaker at 45 °C, with a 3.5% (w/v) loading of activated charcoal (100-mesh particle size, Sigma-Aldrich, Madrid, Spain), and 200 rpm for 1 h. The residual activated charcoal was removed by vacuum filtration.
The treatment with ion-exchange resin was performed at room temperature by adding hydrolysate to a plastic column (7 cm wide × 13 cm length) with 20% (w/v) ion-exchange resin (Microionex MB200, Rohm and Haas, Madrid, Spain). The resin was previously subjected to a washing process with distilled water and dried. Prior to treatment with resins, solid KOH was added to the hydrolysate to a pH of 6.
Sugar and inhibitor (such as formic acid, acetic acid, furfural, HMF, and total phenolic compounds) concentrations in the liquors after detoxification were measured to evaluate the effectiveness of each detoxification method. Next, the detoxified liquors were submitted to xylitol fermentation.
2.3. Microorganism
Candida boidinii (NCAIM Y.01308) yeast was maintained at 4 °C in agar culture medium composed of (g/L): yeast extract, 3; peptone, 10; glucose, 10; and agar, 20. The microorganism was grown in an orbital shaker (Certomat-R, B-Braun, Melsungen, Germany) at 30 °C and 220 rpm for 24 h. In fermentation tests, 250 mL Erlenmeyer flasks were used, containing 50 mL of medium with the following composition (g/L): xylose, 30; yeast extract, 10; KH2PO4, 5; MgSO4·7H2O, 1; and (NH4)2HPO4, 3. Previously, the medium was sterilized at 121 °C for 20 min. Once the inoculum had grown, it was recovered by centrifugation (3000× g, 10 min) and re-suspended in the fermentation medium.
2.4. Xylitol Fermentation
Fermentation tests were carried out in 100 mL Erlenmeyer flasks in an orbital shaker (Certomat-R, B-Braun, Melsungen, Germany) at 30 °C and 150 rpm, employing 50 mL of fermentation medium. The initial cell concentration used was 5 g/L. Samples were withdrawn each 24 h during the fermentation, centrifuged (550× g, 10 min), and analysed for sugar concentration, cell growth, and ethanol and xylitol production. Tests were performed in triplicate, and average values and standard deviations are shown.
On one hand, two synthetic media were used as fermentation media: synthetic medium with the sugar concentration contained in acid hydrolysate before detoxification, called SM1 (g/L: glucose, 4.9; xylose, 23.7; galactose, 4.4; arabinose, 3.4; and mannose, 0.7), and synthetic media with the same composition as SM1 but without glucose (SM2). These synthetic media were previously adjusted to pH 6 with solid KOH, sterilized by filtration with 0.22 μm membranes, and supplemented with the same nutrients used for microorganism growth. On the other hand, fermentation assays were carried out as described previously, using the acid hydrolysates (before and after each detoxification) as fermentation media.
2.5. Analytical Methods
The composition in sugars (glucose, xylose, galactose, arabinose, and mannose) and inhibitory compounds (acetic acid, formic acid, furfural, and HMF) in the hydrolysates before and after detoxification were measured by high performance liquid chromatography (HPLC) using a Waters 2695 liquid chromatograph (Mildford, MA, USA) equipped with a refractive index detector (Waters 2414). Sugar quantification was performed with a Transgenomic CHO-782 carbohydrate analysis column (70 °C and 0.6 mL/min ultrapure water as the mobile phase), while the inhibitors were analysed with a Bio-Rad HPX-87H column (65 °C and 0.6 mL/min, 5 mM H2SO4). The Folin-Ciocalteu method was employed to quantify the total phenolic compound concentration in the hydrolysates [18].
Sugars, ethanol, and xylitol concentrations contained in fermentation samples were also measured by HPLC using the Bio-Rad HPX-87H column mentioned previously. In order to analyse the cell content in the fermentation samples, they were filtered using 0.2 μm cellulose nitrate filters (Sartorius stedim Biotech, Göttingen, Germany). The biomass concentration was determined as the ratio between the mass of dried biomass and the volume of filtered inoculum [19]. All analytical determinations were performed in triplicate, and the average results and relative standard deviations were below 1%.
3. Results and Discussion
3.1. EOP Hydrolysate Composition and Detoxification
Table 1 displays the composition in monomeric sugars of the hydrolysate obtained from sulfuric acid pretreatment of EOP. As seen, a hydrolysate rich in hemicellulosic sugars was generated, with a total sugar concentration of 37.1 g/L. Among the sugars detected, the most abundant one was xylose, accounting for 64% of the total sugars contained in the acid hydrolysate. Therefore, this hydrolysate shows potential to be used as media for xylitol production, as xylitol can be biologically generated from xylose through fermentation with different microorganisms [4,5].

Table 1.
Sugar and inhibitor compound concentrations in acid hydrolysate before and after detoxification.
The inhibitor compounds composition of the EOP hydrolysate is also shown in Table 1. According to Kumar et al. [11], the specific type of lignocellulosic biomass, as well as the pretreatment conditions used, influence the type and concentrations of inhibitor compounds originated. In this study, under the work conditions employed, an insignificant presence of HMF (0.1 g/L) and low concentration of formic acid (0.8 g/L) were found, while a higher concentration of furfural (1.9 g/L) was detected. HMF and furfural are known to be generated as a result of the hexose and pentose degradation, respectively, coming from the formic acid from furfural [15]. Our results indicate a significant effect of acid pretreatment conditions in xylose decay, though hexoses were affected to a minor extent. On the other hand, acetic acid and total phenols were detected in much higher levels (5.6 and 4.1 g/L, respectively). Acetic acid comes from acetyl groups contained in the hemicellulose fraction, whereas the lignin breakdown could lead to the generation of phenolic compounds [15]. According to Manzanares et al. [6], raw EOP contains 2.1% acetyl groups in its composition that explain the presence of acetic acid in the hydrolysate.
The inhibitory effect of compounds, such as furans, acetic acid, and phenolic compounds, on the metabolism of xylitol-producing microorganisms has been previously reported, and several detoxification methods have been tested [20,21]. In this work, treatment with activated charcoal and ion-exchange resin were assayed to reduce the presence of these type of compounds in EOP hydrolysate.
The efficiency of each detoxification method was evaluated considering its effect on the reduction of toxic compounds and resulting loss of sugars (Figure 1) and, specially, on its fermentability for xylitol production. The sugar and toxic compound concentrations obtained as a result of each detoxification method are showed in Table 1.
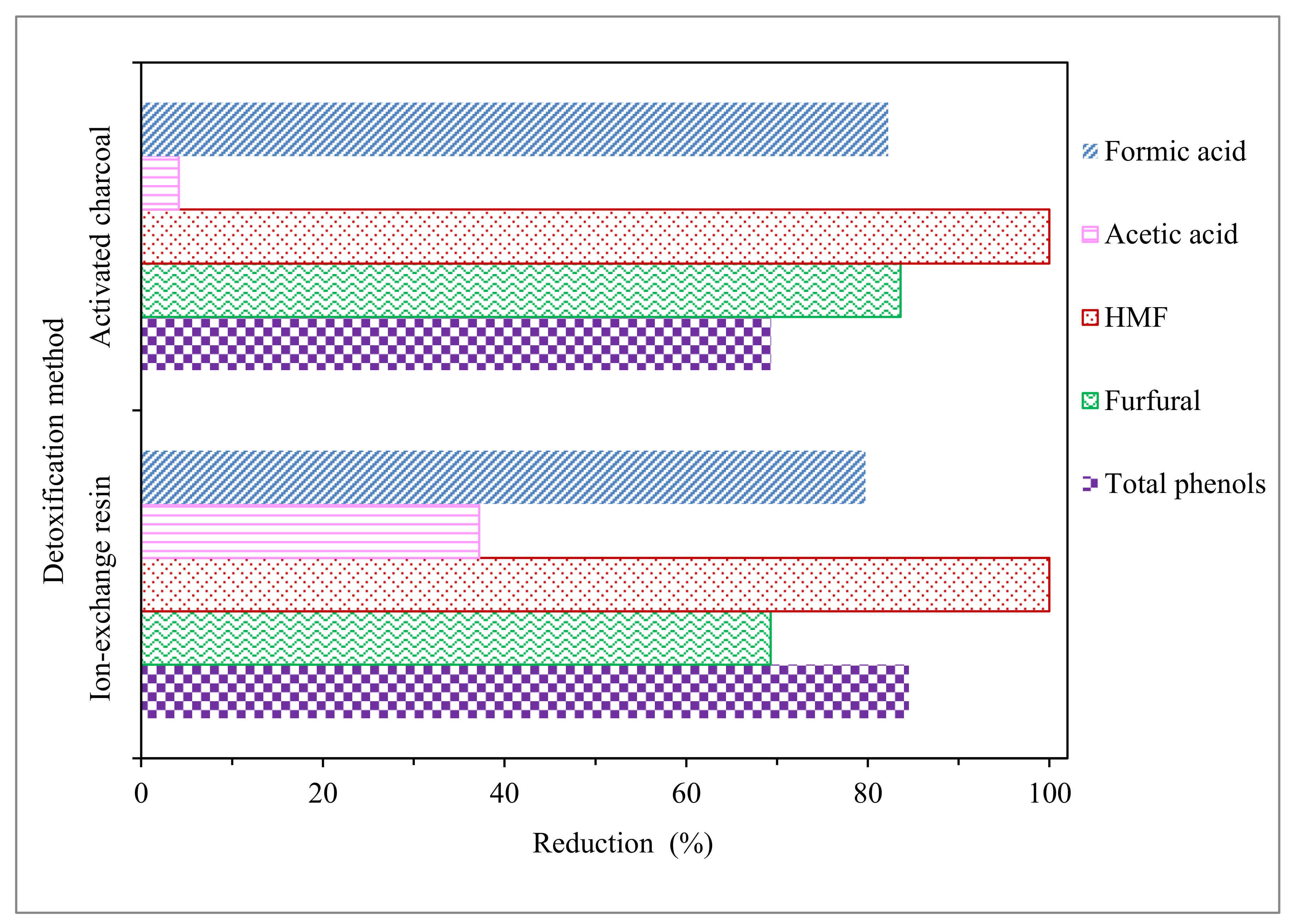
Figure 1.
Removal percentages of total sugars and inhibitor compounds in the acid hydrolysate after diverse detoxification methods. HMF: 5-hydroximethylfurfural.
According to Jönsson et al. [22], treatment by activated charcoal is considered one of the more economical and effective detoxification methods. Our results support this statement since, as seen in Table 1 and Figure 1, the treatment allows for very high reductions of HMF, furfural, and formic acid (100, 84, and 82%, respectively) and is able to significantly reduce the total phenol concentration (69%). On the contrary, acetic acid is only reduced on a very low level (only 4%), as activated charcoal detoxification is able to effectively reduce inhibitors, such as HMF, furfural, formic acid, and phenols, but there is not much evidence that this method is effective for acetic acid reduction [22]. Substantial furan and phenolic compound reductions by activated charcoal detoxification were also achieved from hydrolysates of the brewery’s spent grain [23], sweet sorghum bagasse [24], olive tree pruning biomass [25], and corn pericarp [26].
Regarding the treatment with ion-exchange resin, as seen in Table 1 and Figure 1, a noticeable reduction of acetic acid content (37 versus 4%) and improvement in the results in phenolic compounds detoxification, compared with activated charcoal (85 versus 69%), was observed. Moreover, high reductions were also achieved in furans and formic acid (100% HMF, 69% furfural, and 80% formic acid). A high efficiency of ion-exchange resin detoxification to remove acetic acid and phenolic compounds was also observed in the detoxification of hydrolysates from rapeseed straw [19], cocoa pod husk [17], and corn cob [27].
Furthermore, considering sugar concentrations in the EOP hydrolysate before, and after, detoxification (Table 1), the two methods were effective for avoiding loss of sugars, with both cases lower than 8%. However, ion-exchange resin seemed to provide slightly better protection against sugar loss than activated charcoal. Taking into account the previous comments, the best method used in this work to reduce the presence of inhibitor compounds in EOP acid hydrolysate was the ion-exchange resin.
3.2. Xylitol Fermentation from Synthetic Media
The aim of the fermentation tests carried out in synthetic media (SM1 and SM2, see Section 2.4) was to evaluate how the presence of glucose affects xylitol production by C. boidinii. Moreover, these experiments were useful in estimating the highest xylitol yield and productivity that could be obtained in the fermentation of EOP hydrolysate in the absence of inhibitor compounds.
The influence that sugars other than xylose (especially glucose) exert in xylose-to-xylitol bioconversion by yeast has been previously studied. For instance, Mussatto et al. [28], evaluated the influence of glucose and arabinose on the biological production of xylitol by Candida guilliermondii. The authors found that glucose decreased the xylose uptake rate by 30%, while arabinose did not affect xylose consumption. The negative effect of glucose has been suggested to originate from a partial inhibition of the xylose reductase of the yeast [29,30,31].
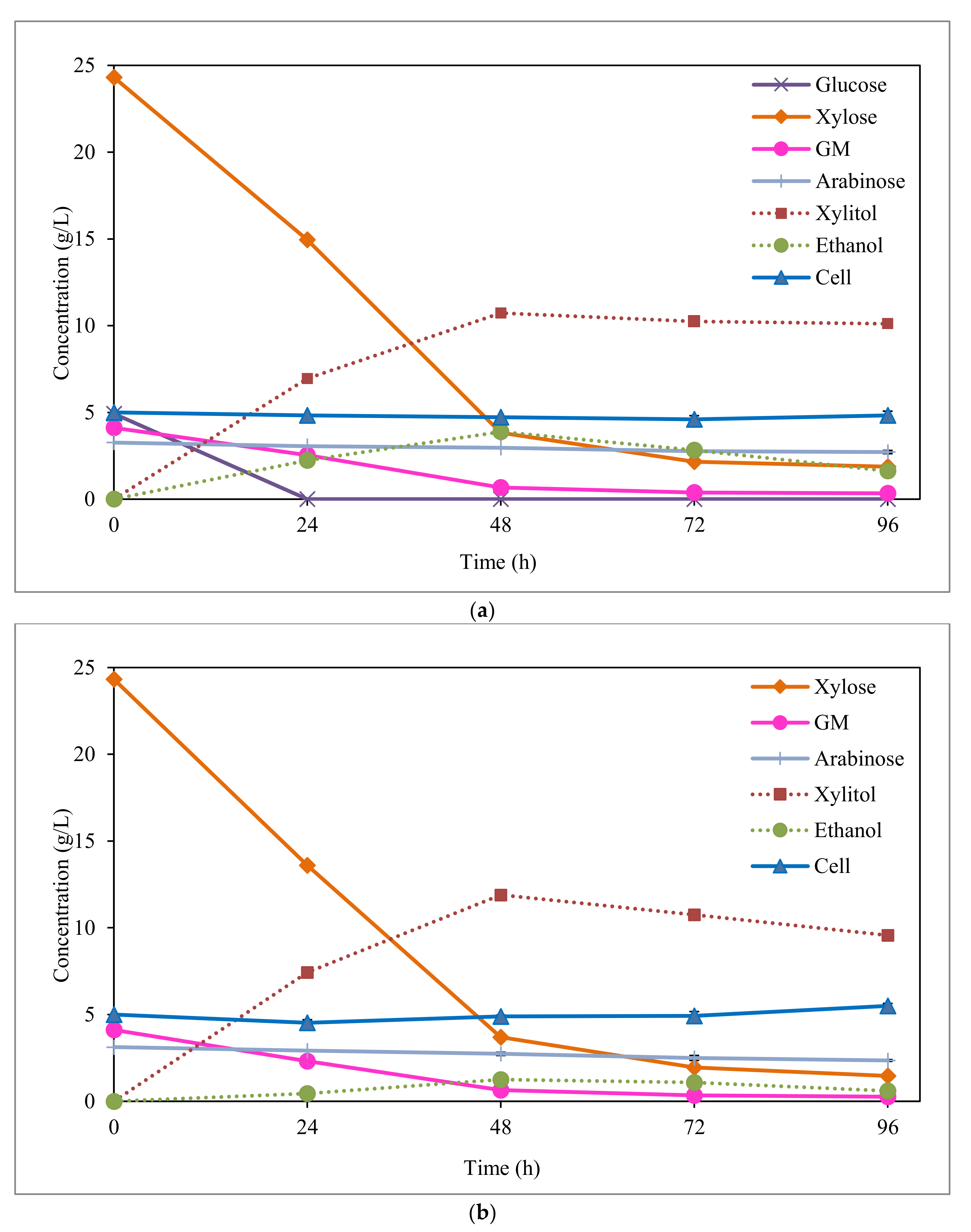
Figure 2 shows sugar uptake and xylitol, ethanol, and cell production during the fermentation of synthetic media (SM1 and SM2). As seen, in both media, the highest xylitol concentrations were achieved for fermentation times as short as 48 h. At this fermentation time, most xylose had already been consumed by C. boidinii (xylose consumption > 84%), and without that, the subsequent uptake of remaining xylose resulted in higher xylitol production. Moreover, in absence of glucose (SM2 medium), significant xylitol consumption after 48 h fermentation was observed, which could be used for the growth of the microorganism. In this context, a slightly higher cell yield was obtained in SM2 medium compared with SM1 medium (0.08 vs. 0.05 g/g).
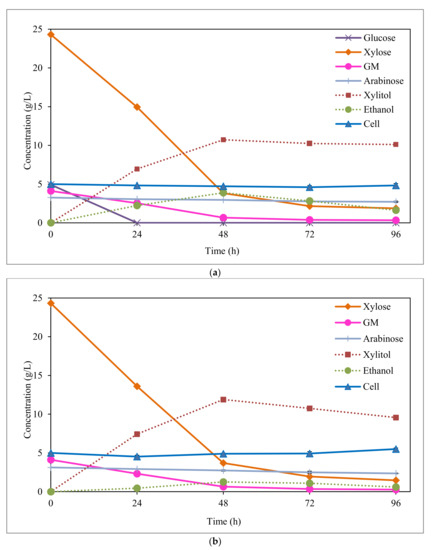
Figure 2.
Fermentation of SM1; and (a) SM2; (b) synthetic media by C. boidinii. GM: sum of galactose and mannose. SM1: synthetic medium with the same sugars concentration contained in acid hydrolysate before detoxification. SM2: same composition of SM1 but without glucose.
By comparing the two synthetic media (Figure 2 and Table 2), it can be appreciated that the highest xylitol concentration and yield values (11.9 g/L, and 0.58 g/g, respectively) were obtained when glucose was not present in the synthetic medium (SM2), supporting the observation of glucose’s negative effect on xylose metabolism by C. boidinii. Vandeska et al. [31] also attained a lower xylitol concentration in the fermentation by C. boidinii with a synthetic media containing glucose and xylose than when only xylose was present (39.4 vs. 59.3 g xylitol/L). These results were obtained in fermentation runs under fed-batch conditions, which the authors claim is a suitable technique to improve productivities and yields in microbial fermentation. This behaviour was also reported by López-Linares et al. [32] in xylitol fermentation of synthetic media by Debaryomyces hansenii and Candida guilliermondii, well-known microorganisms used for xylitol production.

Table 2.
Fermentation parameters attained by the xylitol fermentation by C. boidinii of synthetic media and EOP acid hydrolysate after detoxification.
As seen in Table 1, raw EOP acid hydrolysate and SM1 synthetic medium contain a glucose/xylose ratio above 21%. Glucose/xylose ratios > 10% have been reported to negatively affect xylose transportation into the strain, inhibiting the action of the most important enzymes, such as the xylose reductase, of the yeast [33]. On the contrary, glucose/xylose ratios < 10% could enhance the enzyme action and, thus, the xylitol production.
On the other hand, xylitol fermentation by C. boidinii of SM1 synthetic medium also afforded knowledge of the maximum xylitol yield and productivity values that could be obtained in the fermentation of the EOP acid hydrolysate if any inhibitor compound was present. In this way, as seen in Table 2, maxima values for xylitol yield and productivity of 0.52 g/g and 0.22 g/L/h were achieved, respectively.
3.3. Xylitol Fermentation from EOP Acid Hydrolysate
C. boidinii was not able to produce xylitol from the EOP acid hydrolysate without a previous detoxification step (data not shown). The inhibitor compounds contained in this hydrolysate (as described in Section 3.2), mainly furans, acetic acid, and phenolic compounds, are seemingly the underlying reason for this finding. Moreover, a possible synergistic effect between them could also take place, significantly increasing the inhibition of C. boidinii [34]. In this work, two different detoxification methods were applied: Activated charcoal and ion-exchange resin. The resulting detoxified hydrolysates were fermented by C. boidinii in order to evaluate its fermentability.
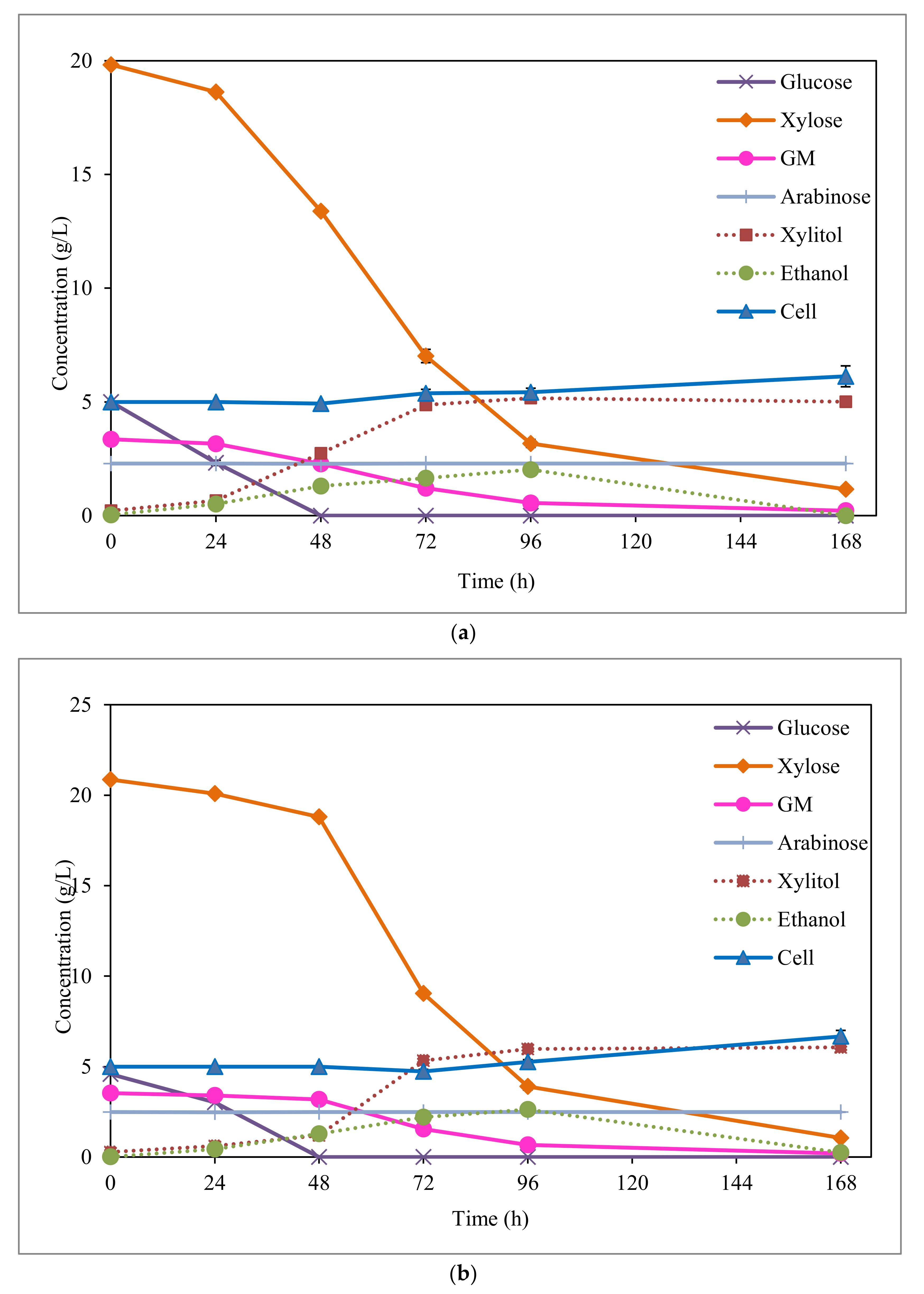
Sugar consumption, as well as xylitol, ethanol, and cell production values attained during the fermentation process of EOP hydrolysates, are displayed in Figure 3 for each detoxification method. As observed, the maximum xylitol concentration was achieved at 96 h fermentation for the two detoxification methods applied. Although, at this fermentation time, a small xylose content remained without being consumed (between 16–19%). In all cases, C. boidinii was able to consume almost the totality of this sugar at the end of the fermentation (Figure 3). However, after 96 h fermentation, no significant increase in xylitol production (<1.5%) was achieved in any case. Fehér et al. [35] also reported good results in the fermentation of hemicellulosic hydrolysates of corn fibre by C. boidinii, after treatment with activated charcoal. The authors focused on the removal of phenolic compounds by the treatment applied, which reduced almost 96% of the total initial content. In the same way, López-Linares et al. [32] proved the success of using a detoxification treatment with organic solvent (ethyl acetate) in the fermentation, by D. hansenii and C. guilliermondii, of rapeseed straw hydrolysates containing toxic compounds (mainly acetic acid and phenolic compounds), although in a higher concentration than in EOP hydrolysate. This detoxification method reached a 62% reduction of the acetic acid level.
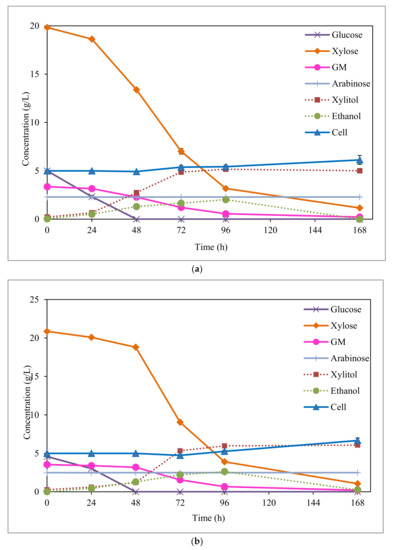
Figure 3.
Fermentation of EOP acid hydrolysate by C. boidinii after detoxification by activated charcoal; and (a) ion-exchange resin (b).
By comparing fermentations with the different detoxified hydrolysates, the highest xylitol concentration (Figure 3) and yield (Table 2) were achieved with ion-exchange resin (6.0 vs. 5.2 g/L and 0.43 vs. 0.36 g/g, respectively). However, as seen in Table 2, similar xylitol productivities and cell growths were obtained in both cases (0.07 g/L/h and 0.04 g/g, respectively). The best fermentation results are most likely attained for ion-exchange resin detoxified hydrolysate were due to the lowest levels of acetic acid (3.5 vs. 5.4 g/L) and phenolic compounds (0.6 vs. 1.3 g/L) found in this case compared to activated charcoal detoxified hydrolysate (Table 1). Therefore, the ion-exchange resin provides advantages over activated charcoal treatment by achieving a more effective removal of inhibitory compounds, particularly in the case of acetic acid. In relation to the inhibition by acetic acid, the literature reported that the presence of acetic acid concentrations higher than 3–3.5 g/L in liquors can negatively affect the xylitol fermentation process by typical microorganisms, such as C. guilliermondii [36], C. magnoliae TISTR 5663 [37], or D. hansenii [32]. Considering the presence of phenolic compounds, according to Zhang et al. [38], C. athensensis growth is only possible when the levels of these types of compounds are reduced below 1 g/L, resulting also in a more prolonged lag phase. Moreover, a possible synergistic effect between acetic acid and phenolic compounds can also take place in the fermentation of activated charcoal detoxified hydrolysate, which has been previously reported in these types of xylitol producing microorganisms [34].
Even though acetic acid was generated at the onset of the fermentation with ion-exchange resin detoxified hydrolysate, C. boidinii was able to consume this compound once the xylose concentrations were low (data not shown). According to Camargo et al. [39], when acetic acid concentrations are not high (<3–3.5 g/L), microorganisms are able to consume this compound as a nutrient. C. boidinii was also able to consume acetic acid contained in the hemicellulosic hydrolysate of corn fibre during the fermentation of this hydrolysate to xylitol [35].
On the other hand, the xylitol yield and productivities obtained from ion-exchange resin detoxified hydrolysate (considered as the best attained in this work for EOP hemicellulosic hydrolysate) can be compared with those achieved in the SM1 synthetic medium (Table 2), which were regarded as the maximum values that could be obtained if toxic compounds were not present in the hydrolysate. As described in Section 3.3, the xylitol production with C. boidinii is not only inhibited by toxic compounds, but it also is negatively affected by a considerable presence of glucose (glucose/xylose ratios > 10%). Then, as seen in Table 2, a decrease in the xylitol yield occurred because of the presence of residual toxic compounds in the EOP ion-exchange resin detoxified hydrolysate (0.43 vs. 0.52 g/g for EOP ion-exchange resin detoxified hydrolysate and SM1, respectively), reaching 83% of the maximum possible xylitol yield in SM1. In addition, fermentation of EOP ion-exchange resin detoxified hydrolysate was slower than for SM1 due to the presence of inhibitors, with its productivity threefold lower compared to SM1 (0.07 versus 0.22 g/L/h, respectively). However, similar cell yields were obtained in both cases (0.04–0.05 g/g).
In summary, taking into account that a previous detoxification method (for instance, ion-exchange resin) is essential to reduce the toxic compounds, EOP hemicellulosic hydrolysate can be successfully employed as fermentation medium to generate xylitol. Xylitol yields reported in the literature for the fermentation of diverse hemicellulosic hydrolysates from different lignocellulosic biomass and by different xylitol producing microorganisms are compared in Table 3. As seen, in all examples the different raw materials were pretreated using H2SO4, mainly in low concentrations. In most studies, a previous detoxification step, prior to fermentation, was necessary, with the most employed methods being chemical methods, like activated charcoal, ion-exchange resin, or ethyl acetate extraction. Regarding xylitol production, yields ranging between 0.11 and 0.55 g/g were reported, which were comparable to the results achieved in this work for EOP hydrolysates detoxified with both activated charcoal and ion-exchange resin. Moreover, the results achieved in this work were favourable compared to those attained by Brás et al. [40] with the same raw material. These authors reached a yield of 0.26 g/g in the xylitol fermentation with Debaryomyces hansenii of hemicellulosic hydrolysate from EOP obtained by pretreating with sulfuric acid (130 °C, 130 min, 3.5% H2SO4, 33.3% solids), but a previous detoxification step by diananofiltration was also necessary.

Table 3.
Xylitol yields reported in the fermentation of hemicellulosic hydrolysates from diverse lignocellulosic. residues, using different types of microorganisms.
3.4. Ethanol Sub-Production
In both fermentations of EOP hemicellulosic hydrolysates and synthetic media by C. boidinii, ethanol was produced as a secondary product, due to the consumption of hexoses (like glucose, galactose, and mannose). In relation to the synthetic media fermentations (Figure 2 and Table 2), as expected, the lowest ethanol production was obtained in absence of glucose (SM2 synthetic media). C. boidinii was able to produce a small amount of ethanol (1.3 g/L) by consumption of galactose and mannose with a yield of 0.05 g/g. In the two synthetic media, the highest ethanol production was achieved at 48 h of fermentation (Figure 2), having consumed the totality of glucose (in SM1) and most of the galactose and mannose (84%, in both SM1 and SM2) by this time. As seen in Figure 2a, glucose was the first hexose completely consumed by the yeast, being exhausted in only 24 h of fermentation.
This same behaviour was also appreciated in the fermentation of EOP hemicellulosic hydrolysates, but in this case, glucose was not totally consumed until 48 h of fermentation, probably due to the presence of toxic compounds (Figure 3). Moreover, based on the same reason, the maximum ethanol concentrations were attained at fermentation times as long as 96 h. By comparing the ethanol results achieved with EOP hemicellulosic hydrolysates detoxified by activated charcoal and ion-exchange resin, slightly higher ethanol concentrations (2.6 versus 2.0 g/L, Figure 3) and yields (0.12 versus 0.08 g/g, Table 2) were obtained for ion-exchange resin detoxification. This is most likely due to the lowest toxic compound concentrations (mainly acetic acid and phenolic compounds) contained in the ion-exchange resin detoxified hydrolysate (Table 1).
On the other hand, as seen in Figure 2 and Figure 3, glucose, xylose, galactose, and mannose were consumed simultaneously by C. boidinii, being able to produce both xylitol and ethanol at the same time. However, as expected, the main product generated was xylitol, being produced in much higher levels than ethanol. Regarding arabinose, C. boidinii was not able to consume this sugar, neither in synthetic media nor in EOP hemicellulosic hydrolysates fermentations.
This behaviour of C. boidinii was also reported by Féher et al. [35] in the xylitol fermentation of corn fibre hydrolysates. Arabinose was not consumed in the fermentation of rice straw hydrolysates by a recombinant Saccharomyces cerevisiae YPH499 strain [45].
Moreover, as can be appreciated in Figure 2 and Figure 3, once most of the sugars were used up by C. boidinii, the yeast started to consume xylitol and, above all, ethanol in both synthetic media and EOP hemicellulosic hydrolysate fermentations, being employed probably to cell growth. The use of ethanol as a second carbon source has also been reported by Gírio et al. [46] in single and mixed substrate fermentations by D. hansenii. In this case, fermentation tests were carried out in a glucose-containing media, and ethanol was used when the glucose concentration in the media was close to 3 g/L. In this work, C. boidinii first consumed galactose and mannose, and once all hexose sugars were depleted, the ethanol concentration started to decrease. Partial consumption of ethanol and xylitol after depletion of the main carbon sources (xylose and glucose) was found in the fermentation of rapeseed straw hemicellulosic hydrolysates by C. guilliermondii [32].
To sum up, the results of the present work prove the suitability of the yeast C. boidinii for the production of xylitol from EOP hemicellulosic hydrolysate, previous a detoxification step by activated charcoal or ion-exchange resin treatment. This is a novel and valuable finding for increasing the value of all sugars contained in EOP biomass that are separated into the different streams generated after pretreatment, in this case, an acid pre-treatment. In this regard, apart from the EOP hydrolysate rich in hemicellulosic sugars, a cellulose and lignin-enriched solid was obtained as a result of the dilute acid pretreatment. This pre-treated solid could be used as substrate for ethanol production after enzymatic hydrolysis. Previous work carried out by the authors have shown the good perspectives of this approach [6]. Furthermore, the residual solid, rich in lignin, after the biological conversion of pretreated EOP to ethanol might also be employed in the processing facility to generate energy. In this way, EOP could be successfully used as a feedstock in a potential biorefinery for the production of xylitol, ethanol, and energy.
4. Conclusions
Hemicellulosic hydrolysate from EOP was a suitable fermentation medium for xylitol production by C. boidinii. However, a previous detoxification step was necessary, with ion-exchange resin being the most effective method. C. boidinii was able to reach a maximum xylitol yield of 0.43 g/g. Fermentation of synthetic media showed that toxic compounds negatively affected xylitol production, but the presence of glucose also caused a negative impact on the fermentation. Future investigation could focus on the adaptation of yeast to the presence of glucose in the medium to increase the xylitol production.
Author Contributions
Conceptualization, E.R.; investigation, J.C.L.-L.; resources, E.R.; writing—original draft preparation, J.C.L.-L.; writing—review and editing, P.M. and I.R.; supervision, E.C.; project administration, I.R., P.M. and E.R.; funding acquisition, P.M., I.R. and E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agencia Estatal de Investigación (MICINN, Spain) and Fondo Europeo de Desarrollo Regional, reference projects ENE2017-85819-C2-1-R and ENE2017-85819-C2-2-R.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Albuquerque, T.L.; Da Silva, I.J.; De MacEdo, G.R.; Rocha, M.V.P. Biotechnological production of xylitol from lignocellulosic wastes: A review. Process Biochem. 2014, 49, 1779–1789. [Google Scholar] [CrossRef]
- Salli, K.; Lehtinen, M.J.; Tiihonen, K.; Ouwehand, A.C. Xylitol’s Health Benefits beyond Dental Health: A Comprehensive Review. Nutrients 2019, 11, 1813. [Google Scholar] [CrossRef] [PubMed]
- Barathikannan, K.; Agastian, P. Xylitol: Production, Optimization and Industrial Application. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 324–339. [Google Scholar] [CrossRef][Green Version]
- Antunes, F.A.F.; dos Santos, J.C.; da Cunha, M.A.A.; Brumano, L.P.; dos Santos Milessi, T.S.; Terán-Hilares, R.; Peres, G.F.D.; Medina, K.J.D.; da Silva, D.D.V.; Dalli, S.S.; et al. Biotechnological Production of Xylitol from Biomass; Springer: Singapore, 2017; pp. 311–342. [Google Scholar]
- Torres-Mayanga, P.C.; Lachos-Perez, D.; Mudhoo, A.; Kumar, S.; Brown, A.B.; Tyufekchiev, M.; Dragone, G.; Mussatto, S.I.; Rostagno, M.A.; Timko, M.; et al. Production of biofuel precursors and value-added chemicals from hydrolysates resulting from hydrothermal processing of biomass: A review. Biomass Bioenergy 2019, 130, 105397. [Google Scholar] [CrossRef]
- Manzanares, P.; Ballesteros, I.; Negro, M.J.; González, A.; Oliva, J.M.; Ballesteros, M. Processing of extracted olive oil pomace residue by hydrothermal or dilute acid pretreatment and enzymatic hydrolysis in a biorefinery context. Renew. Energy 2020, 145, 1235–1245. [Google Scholar] [CrossRef]
- Ruiz, E.; Romero-García, J.M.; Romero, I.; Manzanares, P.; Negro, M.J.; Castro, E. Olive-derived biomass as a source of energy and chemicals. Biofuels Bioprod. Biorefining 2017, 11, 1077–1094. [Google Scholar] [CrossRef]
- Manzanares, P.; Ruiz, E.; Ballesteros, M.; Negro, M.J.; Gallego, F.J.; López-Linares, J.C.; Castro, E. Residual biomass potential in olive tree cultivation and olive oil industry in Spain: Valorization proposal in a biorefinery context. Spanish J. Agric. Res. 2017, 15, e0206. [Google Scholar] [CrossRef]
- Cruz-Peragón, F.; Palomar, J.; Ortega, A. Ciclo energético integral del sector oleícola de Jaén (España). Grasas y Aceites 2006, 57, 219–228. [Google Scholar]
- Alvarez-Chavez, B.J.; Godbout, S.; Palacios-Rios, J.H.; Le Roux, É.; Raghavan, V. Physical, chemical, thermal and biological pre-treatment technologies in fast pyrolysis to maximize bio-oil quality: A critical review. Biomass Bioenergy 2019, 128, 105333. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Naresh Kumar, M.; Ravikumar, R.; Thenmozhi, S.; Ranjith Kumar, M.; Kirupa Shankar, M. Choice of Pretreatment Technology for Sustainable Production of Bioethanol from Lignocellulosic Biomass: Bottle Necks and Recommendations. Waste Biomass Valorization 2019, 10, 1693–1709. [Google Scholar] [CrossRef]
- Sen, B.; Chou, Y.-P.; Wu, S.-Y.; Liu, C.-M. Pretreatment conditions of rice straw for simultaneous hydrogen and ethanol fermentation by mixed culture. Int. J. Hydrogen Energy 2016, 41, 4421–4428. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. A review of biological delignification and detoxification methods for lignocellulosic bioethanol production. Crit. Rev. Biotechnol. 2015, 35, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Santana, N.B.; Dias, J.C.T.; Rezende, R.P.; Franco, M.; Oliveira, L.K.S.; Souza, L.O. Production of xylitol and bio-detoxification of cocoa pod husk hemicellulose hydrolysate by Candida boidinii XM02G. PLoS ONE 2018, 13, e0195206. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, S.A. Colorimetric of total phenolics with phosphomolibicphosphotungstic acid reagents. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- López-Linares, J.C.; Romero, I.; Cara, C.; Castro, E. Bioconversion of rapeseed straw: Enzymatic hydrolysis of whole slurry and cofermentation by an ethanologenic Escherichia coli. Energy Fuels 2016, 30, 9532–9539. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Lopes, S.; Parajó, J.C.; Pereira, H.; Gírio, F.M. Evaluation of the detoxification of brewery’s spent grain hydrolysate for xylitol production by Debaryomyces hansenii CCMI 941. Process Biochem. 2005, 40, 1215–1223. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Optimal Experimental Condition for Hemicellulosic Hydrolyzate Treatment with Activated Charcoal for Xylitol Production. Biotechnol. Prog. 2004, 20, 134–139. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- López-Linares, J.C.; García-Cubero, M.; Lucas, S.; González-Benito, G.; Coca, M. Microwave assisted hydrothermal as greener pretreatment of brewer’s spent grains for biobutanol production. Chem. Eng. J. 2019, 368, 1045–1055. [Google Scholar] [CrossRef]
- Camargo, D.; Sydney, E.B.; Leonel, L.V.; Pintro, T.C.; Sene, L. Dilute acid hydrolysis of sweet sorghum bagasse and fermentability of the hemicellulosic hydrolysate. Braz. J. Chem. Eng. 2019, 36, 143–156. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Ruiz, E.; Cara, C.; Romero, I.; Castro, E. Advanced bioethanol production from olive tree biomass using different bioconversion schemes. Biochem. Eng. J. 2018, 137, 172–181. [Google Scholar] [CrossRef]
- Granados-Arvizu, J.A.; Melo-Sabogal, D.V.; Amaro-Reyes, A.; Gracida-Rodríguez, J.N.; García-Almendárez, B.E.; Castaño-Tostado, E.; Regalado-González, C. Corn pericarp pretreated with dilute acid: Bioconversion of sugars in the liquid fraction to ethanol and studies on enzymatic hydrolysis of the solid fraction. Biomass Convers. Biorefinery 2019, 1–9. [Google Scholar] [CrossRef]
- Kumar, V.; Krishania, M.; Preet Sandhu, P.; Ahluwalia, V.; Gnansounou, E.; Sangwan, R.S. Efficient detoxification of corn cob hydrolysate with ion-exchange resins for enhanced xylitol production by Candida tropicalis MTCC 6192. Bioresour. Technol. 2018, 251, 416–419. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Silva, C.J.S.M.; Roberto, I.C. Fermentation performance of Candida guilliermondii for xylitol production on single and mixed substrate media. Appl. Microbiol. Biotechnol. 2006, 72, 681–686. [Google Scholar] [CrossRef]
- Parajó, J.C.; Domínguez, H.; Domínguez, J. Biotechnological production of xylitol. Part 2: Operation in culture media made with commercial sugars. Bioresour. Technol. 1998, 65, 203–212. [Google Scholar] [CrossRef]
- Felipe, M.G.A.; Mancilha, I.M.; Vitolo, M.; Roberto, I.C.; Silva, S.S.; Rosa, S.A. Preparação de xilitol por fermentação de hidrolizado hemicelulosico de bagaço de cana-de-açúcar. Arq. Biotecnol. 1993, 36, 103–114. [Google Scholar]
- Vandeska, E.; Amartey, S.; Kuzmanova, S.; Jeffries, T.W. Fed-batch culture for xylitol production by Candida boidinii. Process Biochem. 1996, 31, 265–270. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Romero, I.; Cara, C.; Castro, E.; Mussatto, S.I. Xylitol production by Debaryomyces hansenii and Candida guilliermondii from rapeseed straw hemicellulosic hydrolysate. Bioresour. Technol. 2018, 247, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Tochampa, W.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Srinophakun, P.; Bakker, H.H.C.; Chisti, Y. A model of xylitol production by the yeast Candida mogii. Bioprocess Biosyst. Eng. 2005, 28, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, J.M.; Cruz, J.M.; Roca, E.; Domínguez, H.; Parajó, J.C. Xylitol Production from Wood Hydrolyzates by Entrapped Debaryomyces hansenii and Candida guilliermondii Cells. Appl. Biochem. Biotechnol. 1999, 81, 119–130. [Google Scholar] [CrossRef]
- Fehér, C. Integrated Process of Arabinose Biopurification and Xylitol Fermentation Based on the Diverse Action of Candida boidinii. Chem. Biochem. Eng. Q. 2016, 29, 587–597. [Google Scholar] [CrossRef]
- Felipe, M.G.A.; Vieira, D.C.; Vitolo, M.; Silva, S.S.; Roberto, I.C.; Manchilha, I.M. Effect of acetic acid on xylose fermentation to xylitol by Candida guilliermondii. J. Basic Microbiol. 1995, 35, 171–177. [Google Scholar] [CrossRef]
- Wannawilai, S.; Sirisansaneeyakul, S. Economical production of xylitol from Candida magnolia TISTR 5663 using sugarcane bagasse hydrolysate. Kasetsart J. Nat. Sci. 2015, 49, 583–596. [Google Scholar]
- Zhang, J.; Geng, A.; Yao, C.; Lu, Y.; Li, Q. Effects of lignin-derived phenolic compounds on xylitol production and key enzyme activities by a xylose utilizing yeast Candida athensensis SB18. Bioresour. Technol. 2012, 121, 369–378. [Google Scholar] [CrossRef]
- Camargo, D.; Sene, L.; Variz, D.I.L.S.; de Almeida Felipe, M.D.G. Xylitol Bioproduction in Hemicellulosic Hydrolysate Obtained from Sorghum Forage Biomass. Appl. Biochem. Biotechnol. 2015, 175, 3628–3642. [Google Scholar] [CrossRef]
- Brás, T.; Guerra, V.; Torrado, I.; Lourenço, P.; Carvalheiro, F.; Duarte, L.C.; Neves, L.A. Detoxification of hemicellulosic hydrolysates from extracted olive pomace by diananofiltration. Process Biochem. 2014, 49, 173–180. [Google Scholar] [CrossRef]
- Dalli, S.S.; da Silva, S.S.; Uprety, B.K.; Rakshit, S.K. Enhanced Production of Xylitol from Poplar Wood Hydrolysates Through a Sustainable Process Using Immobilized New Strain Candida tropicalis UFMG BX 12-a. Appl. Biochem. Biotechnol. 2017, 182, 1053–1064. [Google Scholar] [CrossRef]
- Ko, C.-H.; Chiang, P.-N.; Chiu, P.-C.; Liu, C.-C.; Yang, C.-L.; Shiau, I.-L. Integrated xylitol production by fermentation of hardwood wastes. J. Chem. Technol. Biotechnol. 2008, 83, 534–540. [Google Scholar] [CrossRef]
- Canilha, L.; Carvalho, W.; de Almeida Felipe, M.D.G.A.; de Almeida e Silva, J.B. Xylitol production from wheat straw hemicellulosic hydrolysate: Hydrolysate detoxification and carbon source used for inoculum preparation. Braz. J. Microbiol. 2008, 39, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Xylitol Production from Byproducts Generated During Sequential Acid-/Alkali-Pretreatment of Empty Palm Fruit Bunch Fiber by an Adapted Candida tropicalis. Front. Energy Res. 2019, 7, 72. [Google Scholar] [CrossRef]
- Guirimand, G.; Sasaki, K.; Inokuma, K.; Bamba, T.; Hasunuma, T.; Kondo, A. Cell surface engineering of Saccharomyces cerevisiae combined with membrane separation technology for xylitol production from rice straw hydrolysate. Appl. Microbiol. Biotechnol. 2016, 100, 3477–3487. [Google Scholar] [CrossRef] [PubMed]
- Gírio, F.; Amaro, C.; Azinheira, H.; Pelica, F.; Amaral-Collaço, M. Polyols production during single and mixed substrate fermentations in Debaryomyces hansenii. Bioresour. Technol. 2000, 71, 245–251. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).