The Microstructure Evolution and Mass Transfer in Mushy Zone during High-Pressure Solidifying Hypoeutectic Al-Ni Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
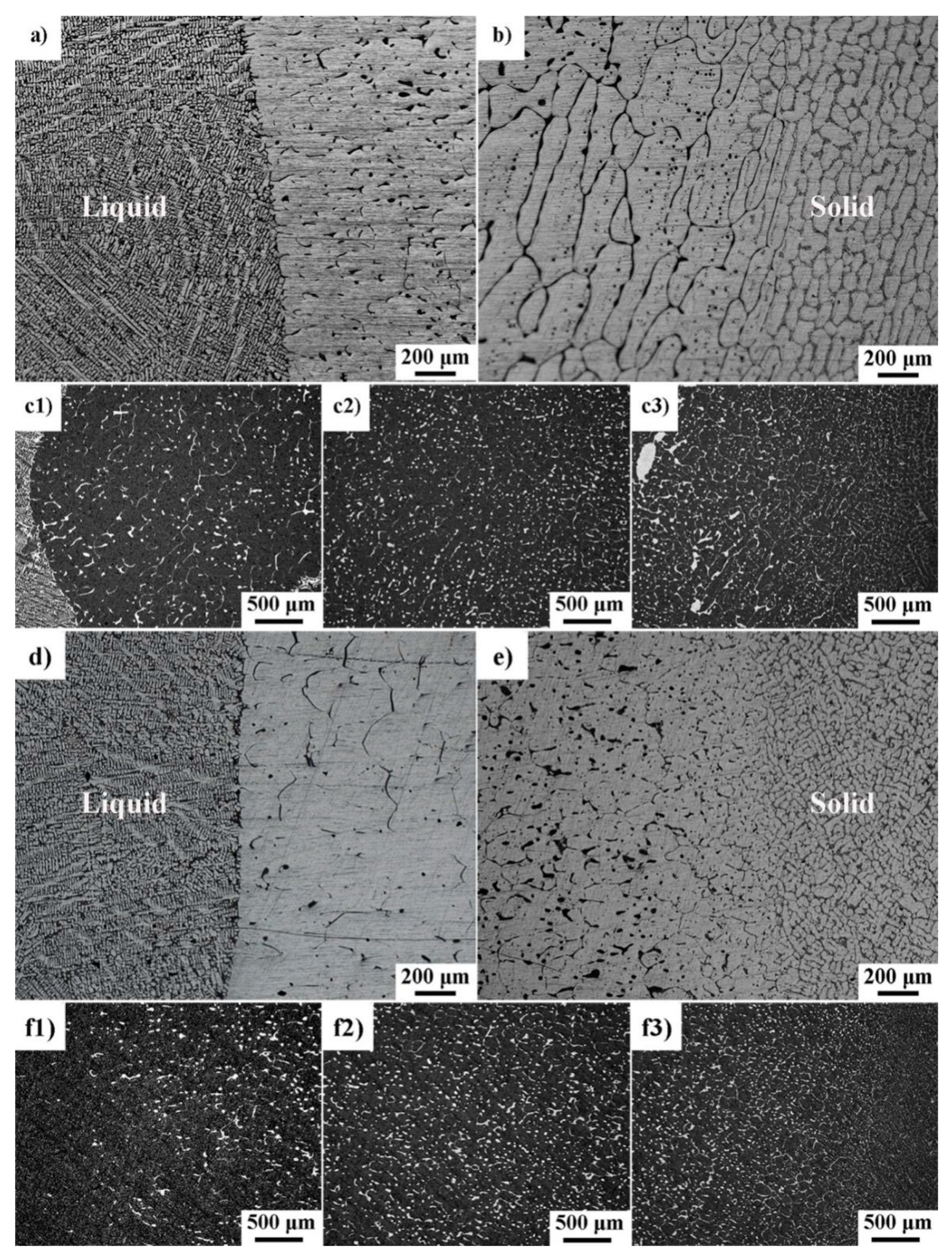
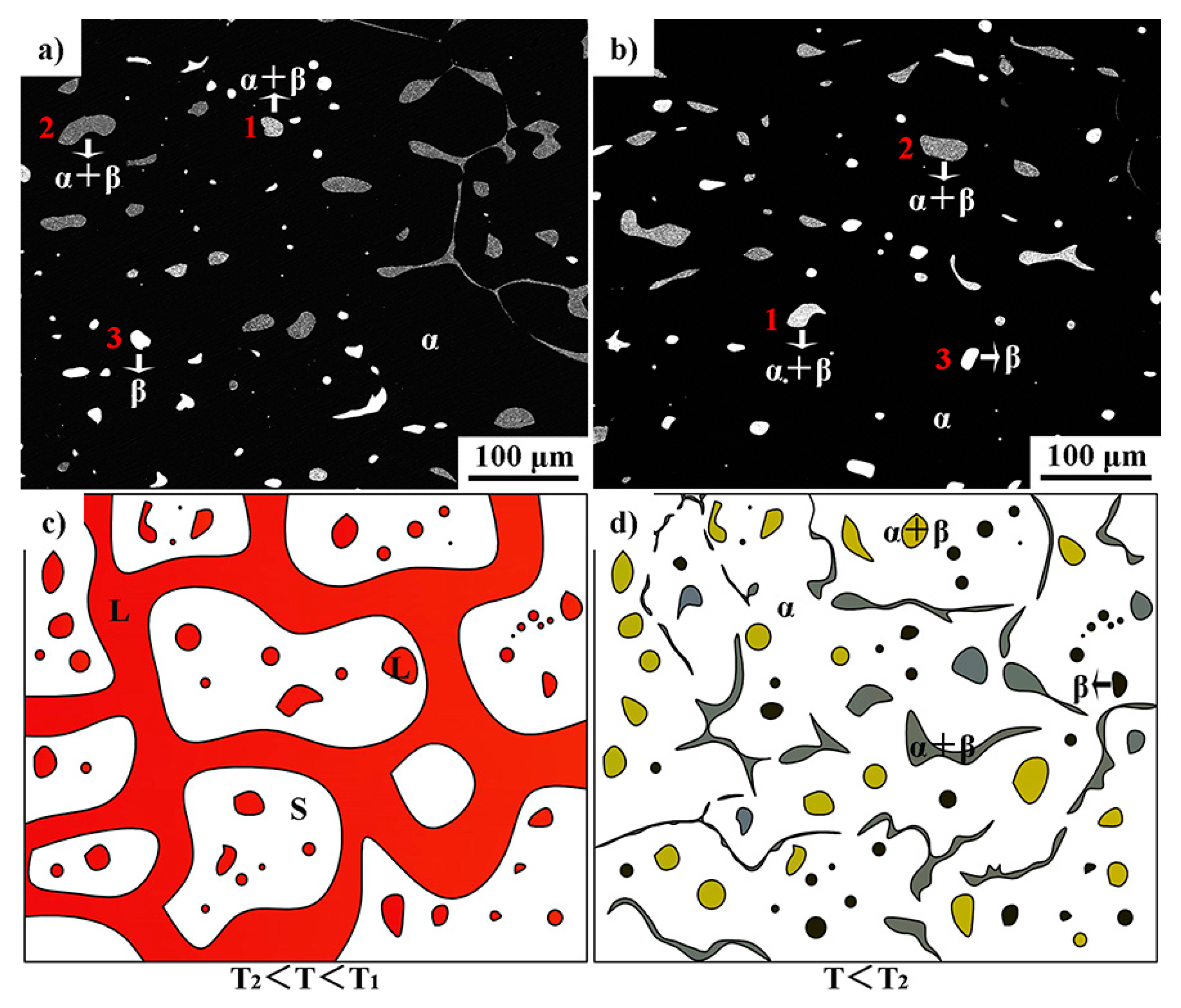
3.1. Microstructure and Phase Composition
3.2. The Microstructure Evolution of Mushy Zone during High-Pressure Solidification
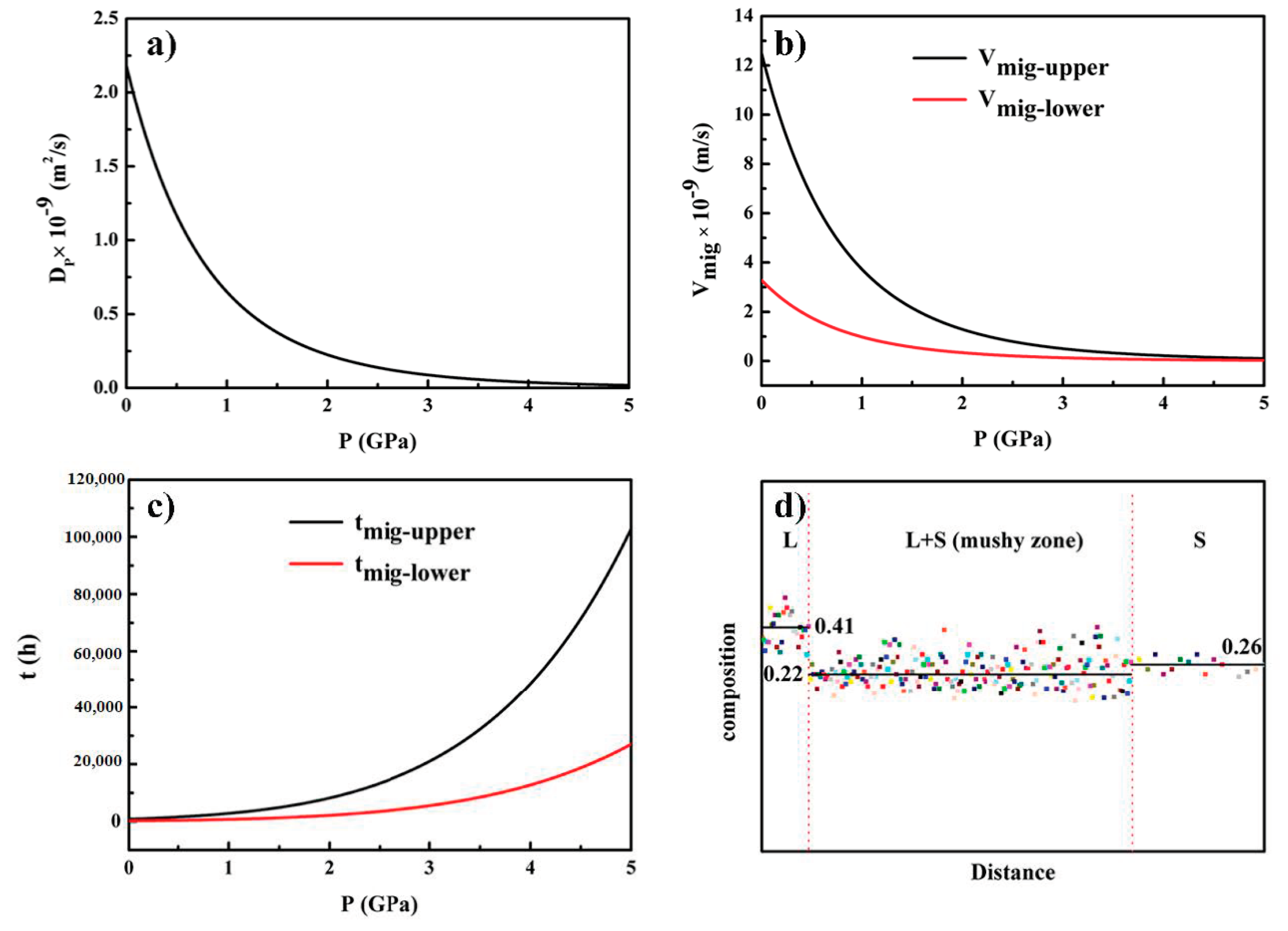
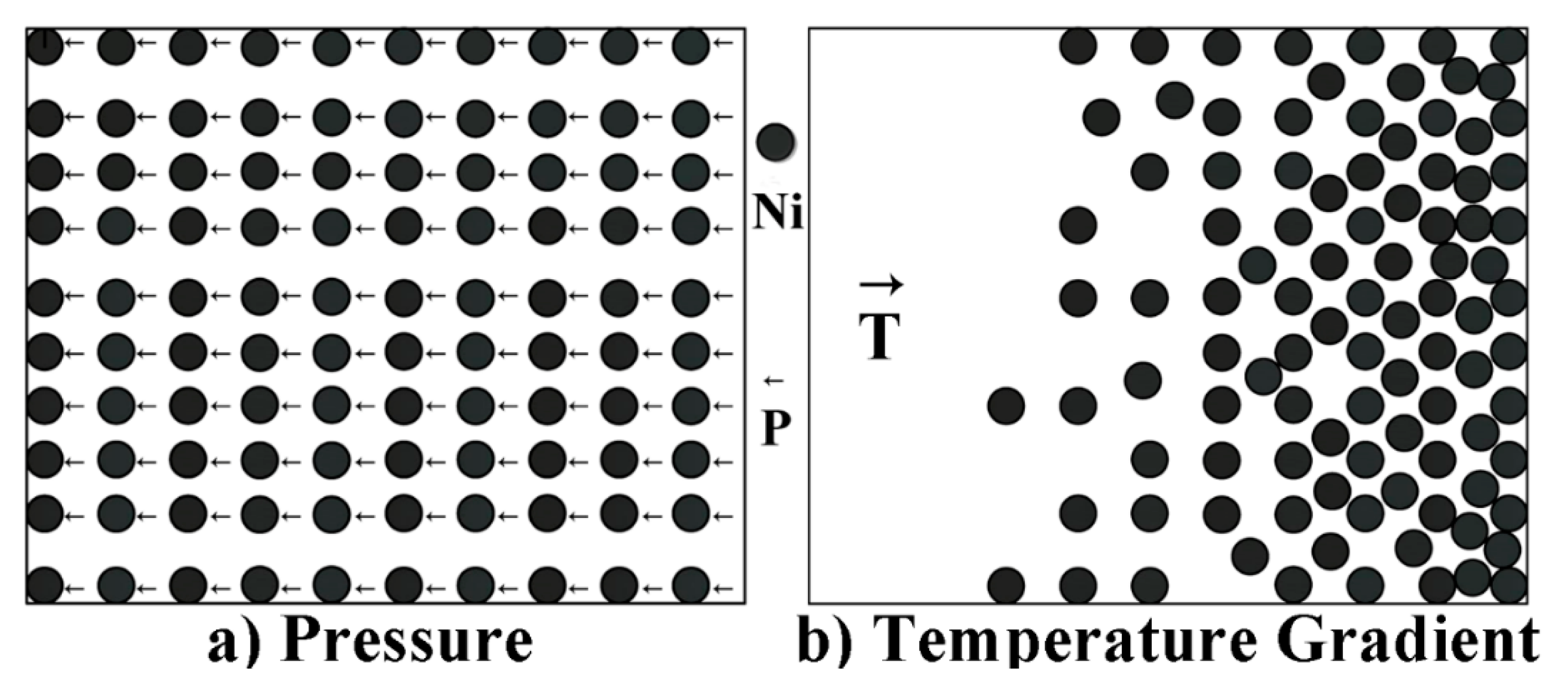
3.3. The Mass Transfer in the Mushy Zone during Thermal Stabilization Treatment under High Pressure
4. Conclusions
- (1)
- The change of the graphite heater structure is an effective way to fulfill high-pressure mushy-zone solidification.
- (2)
- Non-melted α-Al phase grains, intragranular Ni-rich liquid droplets, and intergranular liquid channels coexist in the mushy zone during thermal stabilization treatment. The black α-Al phase, bright bulk Al3Ni phase, and eutectic structures with different Ni content exist after solidification. Liquid channels disappeared gradually along with the temperature gradient.
- (3)
- Mass transfer was greatly inhibited under high pressure. After thermal stabilization treatment (within 30 min), the migration distance of the liquid pool in the mushy zone at 1 GPa was between 396 μm and 456 μm, while the value was between 9 μm and 216 μm when the pressure was 3 GPa. Furthermore, the minimum time required for a liquid pool to go through the whole mushy zone at 1 GPa and 3 GPa was about 746 h and 5523 h, respectively.
Author Contributions
Funding
Conflicts of Interest
References
- Chen, B.M.; Song, L.Q.; Gao, K.K.; Liu, F. Two zone model for mushy region of solid-liquid phase change based on Lattice Boltzmann method. Int. Commun. Heat Mass Transf. 2018, 98, 1–12. [Google Scholar] [CrossRef]
- Zhao, P.B.; Dai, R.K.; Li, W.; Wang, Q.W.; Zeng, M. Modeling the mushy zone during the melting process under Neumann boundary condition using the improved enthalpy-porosity method. Numer. Heat Transf. Part A 2020, 5, 23–29. [Google Scholar] [CrossRef]
- Yang, J.R.; Chen, R.R.; Ding, H.S.; Guo, J.J.; Han, J.C.; Fu, H.Z. Mechanism and evolution of heat transfer in mushy zone during cold crucible directionally solidifying TiAl alloys. Int. J. Heat Mass Transf. 2013, 63, 216–223. [Google Scholar] [CrossRef]
- Ding, B.; Zhu, X.; Wang, H.; He, X.Y.; Tan, Y. Experimental study on phase change heat transfer characteristics of alloys. Int. J. Heat Mass Transf. 2017, 105, 261–269. [Google Scholar] [CrossRef]
- Fadl, M.; Eames, P C. Numerical investigation of the influence of mushy zone parameter A(mush) on heat transfer characteristics in vertically and horizontally oriented thermal energy storage systems. Appl. Therm. Eng. 2019, 151, 90–99. [Google Scholar] [CrossRef]
- Yoshioka, H.; Tada, Y.; Hayashi, Y. Crystal growth and its morphology in the mushy zone. Acta Mater. 2004, 52, 1515–1523. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.L.; Liao, Z.R..; Li, P.D.; Yu, G.L.; Xu, C. The evolution of the mushy zone during the melting process of a binary nitrate salt. Int. J. Heat Mass Transf. 2019, 142. [Google Scholar] [CrossRef]
- Alexandrov, D.V.; Danilov, D.A.; Galenko, P.K. Selection criterion of a stable dendrite growth in rapid solidification. Int. J. Heat Mass Transf. 2016, 101, 789–799. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Drevet, B.; Debierre, J.M.; Camel, D.; Dabo, Y.; Billia, B. Preparation of The Initial Solid-Liquid Interface and Melt in Directional Solidification. J. Cryst. Growth 2003, 253, 539–548. [Google Scholar] [CrossRef]
- Rettenmayr, M. Melting and Remelting Phenomena. Int. Mater. Rev. 2009, 54, 1–17. [Google Scholar] [CrossRef]
- Yang, B.; Raza, A.; Bai, F.W.; Zhang, T.J.; Wang, Z.F. Microstructural evolution within mushy zone during paraffin’s melting and solidification. Int. J. Heat Mass Transf. 2019, 141, 769–778. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Reinhart, G.; Buffet, A.; Schenk, T.; Mangelinck-Noel, N.; Jung, N.; Bergeon, N.; Billia, B.; Hartwig, J.; Baruchel, J. In Situ and Real-Time Analysis of TGZM Phenomena by Synchrotron X-Ray Radiography. J. Cryst. Growth 2008, 310, 2906–2914. [Google Scholar] [CrossRef]
- Peng, P. Diffusion-induced liquid migration during thermal stabilization in presence of temperature gradients with different directions. Int. Commun. Heat Mass Transf. 2020, 113. [Google Scholar] [CrossRef]
- Peng, P. Determination of solid-liquid interfacial energy of Ni3Sn2 phase by grain boundary groove method in a temperature gradient. J. Alloy. Compd. 2019, 783, 156–163. [Google Scholar] [CrossRef]
- Batashef, A.E. Crystallization of Metals and Alloys at Pressure, 1st ed.; Moscow Metallurgy: Moscow, Russia, 1977; pp. 10–23. [Google Scholar]
- Huang, X.R.; Hab, Z.Q.; Liu, B.C. Study on the effect of pressure on the equilibrium and stability of the solid-liquid interface in solidification of binary alloys. Sci. China Tech. Sci. 2011, 54, 479–483. [Google Scholar] [CrossRef]
- Kawashima, Y.; Yagi, T. Temperature distribution in a cylindrical furnace for high-pressure use. Am. Inst. Phys. 1988, 59, 1186–1188. [Google Scholar] [CrossRef]
- Hernlund, J.; Leinenweber, K.; Locke, D.; Tyburczy, J.A. A numerical model for steady-state temperature distributions in solid-medium high-pressure cell assemblies. Am. Miner. 2006, 91, 295–305. [Google Scholar] [CrossRef]
- Okamoto, H. Supplemental Literature Review of Binary Phase Diagrams: Al-Ni, B-Hf, Ca-Sc, Cr-Sc, Fe-Rh, Hf-Mn, La-Sb, Ni-Re, Ni-Sm, Ni-Zr, Sb-Tb, and Ti-Zr. J. Phase Equilibria Diffus. 2019, 40, 830–841. [Google Scholar] [CrossRef]
- Li, C.; Yuan, Z.; Guo, R. Reaction diffusion in Ni–Al diffusion couples in steady magnetic fields. J. Alloys Compd. 2015, 641, 7–13. [Google Scholar] [CrossRef]
- Siquieri, R.; Emmerich, H. Morphology-dependent crossover effects in heterogeneous nucleation of peritectic materials studied via the phase-field method for Al-Ni. J. Phys. 2009, 21, 464105. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; White, J.D.E.; Kovalev, D. Dynamics of phase transformation during thermal explosion in the Al–Ni system: Influence of mechanical activation. Physica B 2010, 405, 778–784. [Google Scholar] [CrossRef]
- Yang, S.J.; Wang, W.L.; Wei, B.B. Growth mechanisms of dendrites and eutectics within undercooled liquid Al-Ni alloys. Acta Phys. Sin. 2015, 64, 056401. [Google Scholar] [CrossRef]
- Diao, Q.Z.; Tsai, H.L. Modeling of Solute Redistribution in the Mushy Zone during Solidification of Aluminum-Copper Alloys. Metall. Trans. A 1993, 24A, 963–973. [Google Scholar] [CrossRef]
- Zhong, H.; Li, S.M.; Lü, H.Y.; Liu, L.; Zou, G.R.; Fu, H.Z. Microstructure Evolution of Peritectic Nd14Fe79B7 Alloy During Directional Solidification. J. Cryst. Growth 2008, 310, 3366–3371. [Google Scholar] [CrossRef]
- Vernède, S.; Rappaz, M. Transition of the mushy zone from continuous liquid films to a coherent solid. Philos. Mag. 2006, 86, 3779–3794. [Google Scholar] [CrossRef] [Green Version]


| Pressure/GPa | Phase | Al/at% | Ni/at% |
|---|---|---|---|
| 1 | 1 | 85.75 | 14.25 |
| 2 | 93.54 | 6.46 | |
| 3 | 76.19 | 23.81 | |
| 3 | 1 | 83.96 | 16.04 |
| 2 | 91.90 | 8.10 | |
| 3 | 76.17 | 23.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Dong, D.; Zhu, D.; Wang, H.; Wei, Z. The Microstructure Evolution and Mass Transfer in Mushy Zone during High-Pressure Solidifying Hypoeutectic Al-Ni Alloy. Appl. Sci. 2020, 10, 7206. https://doi.org/10.3390/app10207206
Wang X, Dong D, Zhu D, Wang H, Wei Z. The Microstructure Evolution and Mass Transfer in Mushy Zone during High-Pressure Solidifying Hypoeutectic Al-Ni Alloy. Applied Sciences. 2020; 10(20):7206. https://doi.org/10.3390/app10207206
Chicago/Turabian StyleWang, Xiaohong, Duo Dong, Dongdong Zhu, Hongwei Wang, and Zunjie Wei. 2020. "The Microstructure Evolution and Mass Transfer in Mushy Zone during High-Pressure Solidifying Hypoeutectic Al-Ni Alloy" Applied Sciences 10, no. 20: 7206. https://doi.org/10.3390/app10207206






