How Do Plants and Climatic Conditions Control Soil Properties in Hypersaline Tidal Flats?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Soil Sampling and Field Measurements
2.3. Analytical Procedures
- (F1)
- Exchangeable and soluble Fe, extracted with MgCl2 1M (pH 7; 30 min shaking);
- (F2)
- Iron associated with carbonate extracted with NaOAc 1M (pH 5; 5 h shaking);
- (F3)
- Iron associated with poorly crystalline Fe (ferrihydrite) extracted with hydroxylamine 0.04 M + acetic acid 25% (6 h shaking at 30 °C);
- (F4)
- Iron associated with poorly crystalline Fe (lepidocrocite) extracted with hydroxylamine 0.04 M + acetic acid 25% (6 h shaking at 96 °C);
- (F5)
- Iron associated with crystalline oxyhydroxides extracted with sodium citrate 0.25 M + 0.25 M + sodium bicarbonate 0.11M + Sodium dithionite (30 min shaking at 75 °C);
- (F6)
- Iron associated with pyrite extracted with HNO3 (2 h shaking). This fraction was extracted following two pre-treatments (10 M HF and concentrated H2SO4), for the removal of Fe associated with silicate and organic matter.
2.4. Statistical Data Analysis
3. Results
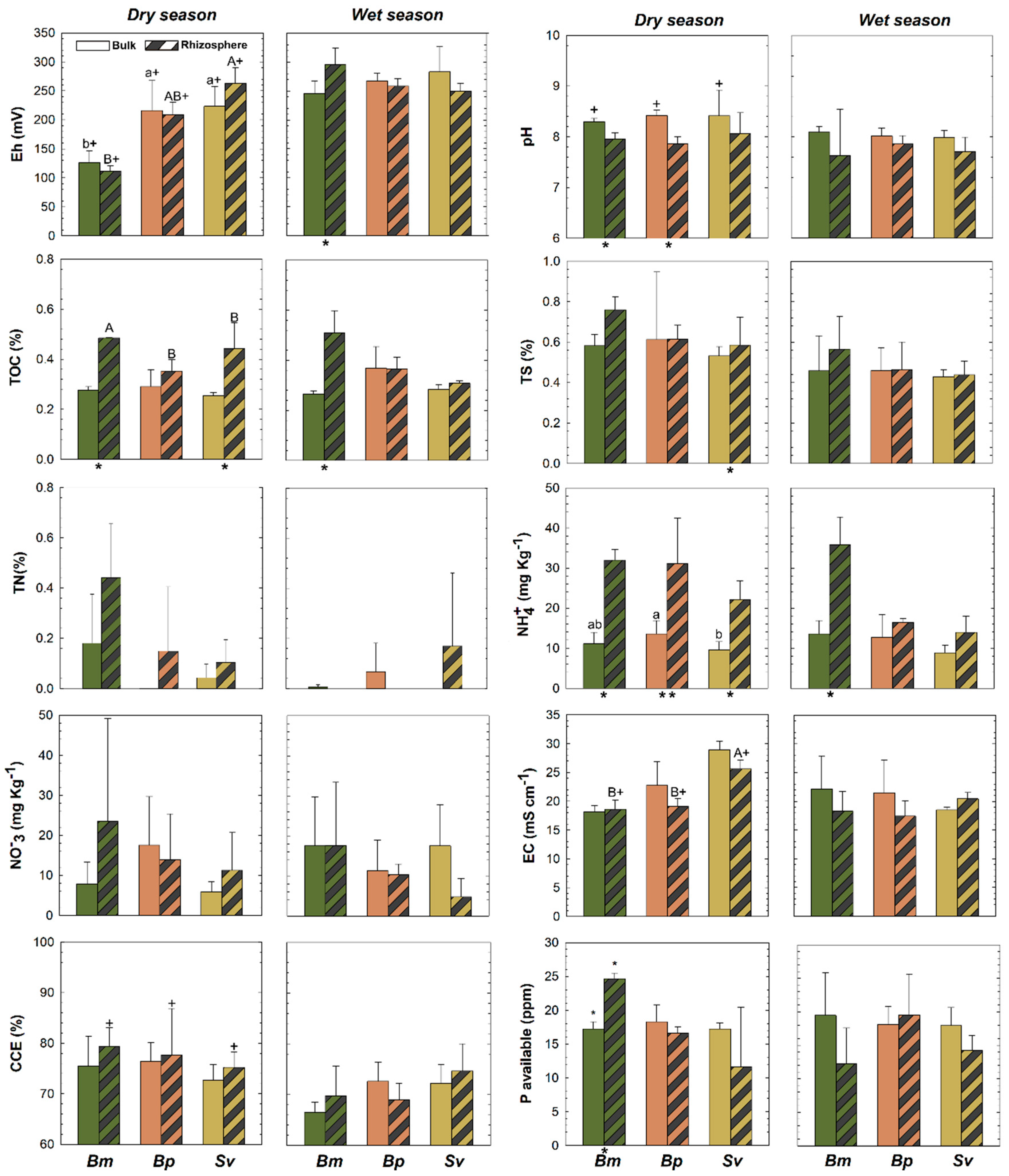
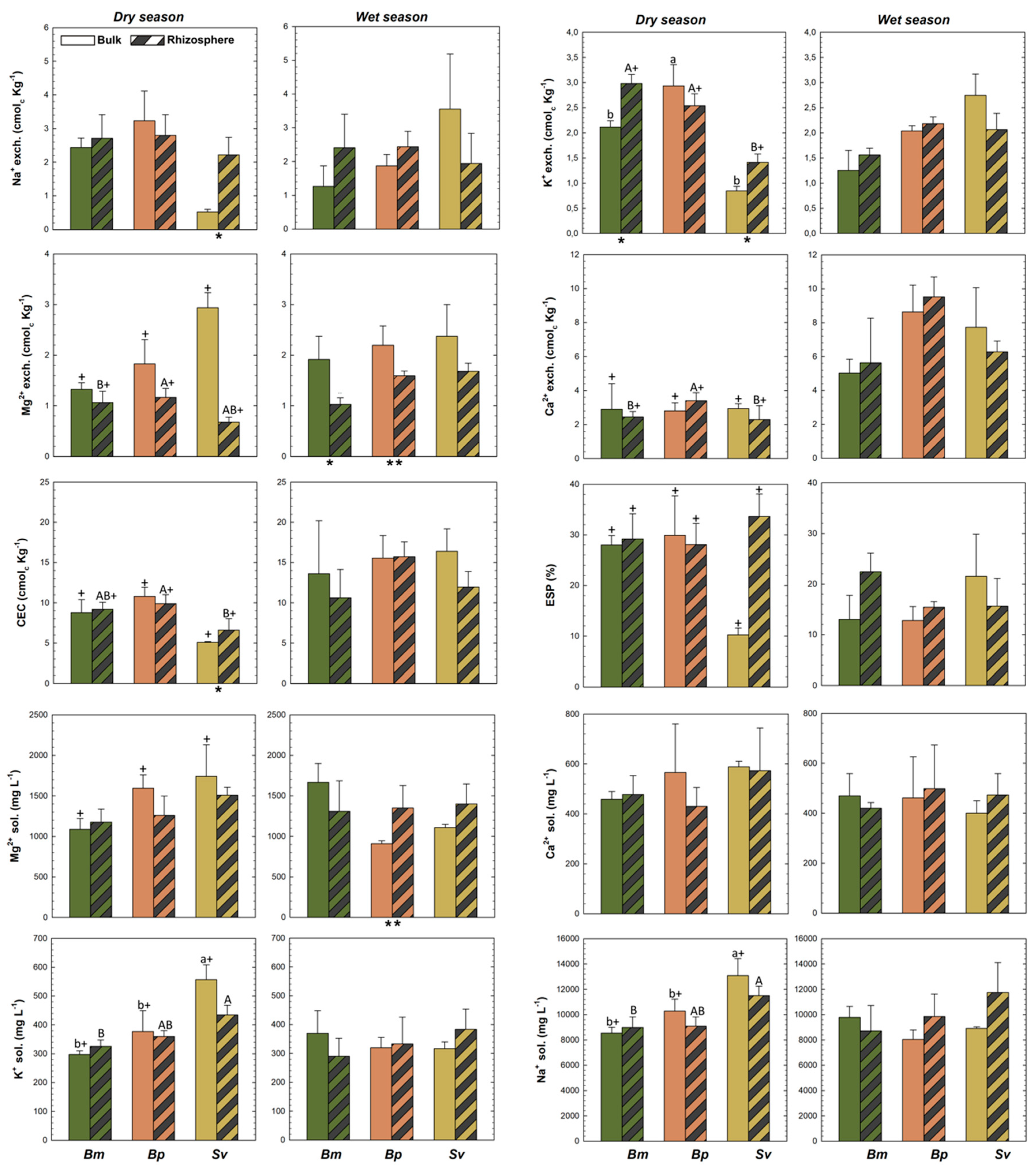
3.1. Differences between Rhizospheric and Bulk Soils
3.2. Soil Characteristics among the Three Plant Species in HTF
3.3. Seasonality and Soil Physicochemical Changes in HTF
4. Discussion
4.1. Rhizospheric and Bulk Soils in HTF
4.2. Plant Effects on Biogeochemical Soil Properties in HTF
4.3. Seasonal Effects on Soil Properties in HTF
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sartor, L.R.; Graham, R.C.; Ying, S.C.; Otero, X.L.; Montes, C.R.; Ferreira, T.O. Role of Redox Processes in the Pedogenesis of Hypersaline Tidal Flat Soils on the Brazilian Coast. Soil Sci. Soc. Am. J. 2018, 82, 1217–1230. [Google Scholar] [CrossRef] [Green Version]
- Schaeffer-Novelli, Y.; Soriano-Sierra, E.J.; Vale, C.C.D.; Bernini, E.; Rovai, A.S.; Pinheiro, M.A.A.; Schmidt, A.J.; De Almeida, R.; Júnior, C.C.; Menghini, R.P.; et al. Climate changes in mangrove forests and salt marshes. Braz. J. Oceanogr. 2016, 64, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Shao, T.; Zhu, T.; Long, X.-H.; Gao, X.; Liu, Z.; Shao, H.; Rengel, Z. Vegetation succession influences soil carbon sequestration in coastal alkali-saline soils in southeast China. Sci. Rep. 2018, 8, 9728. [Google Scholar] [CrossRef]
- Sartor, L.R.; Graham, R.C.; Ying, S.C.; Andrade, G.R.; Montes, C.R.; Ferreira, T.O. Are hypersaline tidal flat soils potential silicon sinks in coastal wetlands? Geoderma 2019, 337, 215–224. [Google Scholar] [CrossRef]
- Soares, R.H.R.D.M.; De Assunção, C.A.; Fernandes, F.D.O.; Marinho-Soriano, E. Identification and analysis of ecosystem services associated with biodiversity of saltworks. Ocean Coast. Manag. 2018, 163, 278–284. [Google Scholar] [CrossRef]
- Albuquerque, A.G.B.M.; Ferreira, T.O.; Cabral, R.L.; Nóbrega, G.N.; Romero, R.E.; Meireles, A.J.D.A.; Otero, X.L. Hypersaline tidal flats (apicum ecosystems): The weak link in the tropical wetlands chain. Environ. Rev. 2014, 22, 99–109. [Google Scholar] [CrossRef]
- Hadlich, G.; Ucha, J.; Celino, J. Apicuns na Baía de Todos os Santos: Distribuição espacial, descrição e caracterização física e química. In Avaliação de Ambientes na Baía de Todos os Santos: Aspectos Geoquímicos, Geofísicos e Biológicos; Queiroz, A., Celino, J., Eds.; UFBA: Salvador, Brazil, 2008; pp. 59–72. [Google Scholar]
- Augspurger, C.K. Light Requirements of Neotropical Tree Seedlings: A Comparative Study of Growth and Survival. J. Ecol. 1984, 72, 777. [Google Scholar] [CrossRef]
- Budowski, G. The distinction between old secondary and climax species in tropical Central American lowlands. Trop. Ecol. 1970, 11, 1–32. [Google Scholar]
- Rahnama, A.; James, R.A.; Poustini, K.; Munns, R. Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct. Plant Biol. 2010, 37, 255–263. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Haoliang, L.; Chongling, Y.; Liu, J. Low-molecular-weight organic acids exuded by Mangrove (Kandelia candel (L.) Druce) roots and their effect on cadmium species change in the rhizosphere. Environ. Exp. Bot. 2007, 61, 159–166. [Google Scholar] [CrossRef]
- Dayton, P.K. Experimental Evaluation of Ecological Dominance in a Rocky Intertidal Algal Community. Ecol. Monogr. 1975, 45, 137–159. [Google Scholar] [CrossRef]
- Bertness, M.D.; Hacker, S.D. Physical Stress and Positive Associations Among Marsh Plants. Am. Nat. 1994, 144, 363–372. [Google Scholar] [CrossRef]
- Caçador, M.I.; Madureira, M.J.; Vale, C. Effects of plant roots on salt-marsh sediment geochemistry. In Proceedings in Marine Science; Flemming, B.W., Delafontaine, M.T., Liebezeit, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 2, pp. 197–204. [Google Scholar]
- Araújo, E.D.S.; Da Silva, J.B.; Oliveira, T.D.S.; De Santana, N.M.G.; Freire, M.B.G.D.F. Apicum do estuário de Barra de Gramame-PB: Análises físicas e químicas. Rev. Bras. Geogr. Fís. 2019, 12, 112–123. [Google Scholar] [CrossRef]
- Lebigre, J. Les Marais à Mangrove et lês Tannes. Available online: http://www.futura-sciences.com/magazines/voyage/infos/dossiers/d/geographie-marais-mangrove-tannes-683/ (accessed on 20 August 2020).
- Barcellos, D.; Queiroz, H.M.; Nóbrega, G.N.; Filho, R.L.D.O.; Santaella, S.T.; Otero, X.L.; Ferreira, T.O. Phosphorus enriched effluents increase eutrophication risks for mangrove systems in northeastern Brazil. Mar. Pollut. Bull. 2019, 142, 58–63. [Google Scholar] [CrossRef]
- Nóbrega, G.N.; Otero, X.L.; Macías, F.; Ferreira, T.O. Phosphorus geochemistry in a Brazilian semiarid mangrove soil affected by shrimp farm effluents. Environ. Monit. Assess. 2014, 186, 5749–5762. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, H.M.; Artur, A.G.; Taniguchi, C.A.K.; Da Silveira, M.R.S.; Nascimento, J.C.D.; Nóbrega, G.N.; Otero, X.L.; Ferreira, T.O. Hidden contribution of shrimp farming effluents to greenhouse gas emissions from mangrove soils. Estuar. Coast. Shelf Sci. 2019, 221, 8–14. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Ferreira, T.O.; Taniguchi, C.A.K.; Barcellos, D.; Nascimento, J.C.D.; Nóbrega, G.N.; Otero, X.L.; Artur, A.G. Nitrogen mineralization and eutrophication risks in mangroves receiving shrimp farming effluents. Environ. Sci. Pollut. Res. 2020, 27, 34941–34950. [Google Scholar] [CrossRef]
- Cohen, M.C.; Rodrigues, E.; Rocha, D.O.; Freitas, J.; Fontes, N.A.; Pessenda, L.C.; De Souza, A.V.; Gomes, V.L.; França, M.C.; Bonotto, D.M.; et al. Southward migration of the austral limit of mangroves in South America. Catena 2020, 195, 104775. [Google Scholar] [CrossRef]
- Feher, L.C.; Osland, M.J.; Griffith, K.T.; Grace, J.B.; Howard, R.J.; Stagg, C.L.; Enwright, N.M.; Krauss, K.W.; Gabler, C.A.; Day, R.H.; et al. Linear and nonlinear effects of temperature and precipitation on ecosystem properties in tidal saline wetlands. Ecosphere 2017, 8, e01956. [Google Scholar] [CrossRef]
- Vidal-Torrado, P.; Ferreira, T.; Otero, X.; Souza, V., Jr.; Ferreira, F.; Andrade, G.; Macías, F. Pedogenetic processes in mangrove soils. In Biogeochemistry and Pedogenetic Process in Saltmarsh and Mangrove Systems; Otero, X., Macías, F., Eds.; MDPI: Basel, Switzerland, 2010; p. 27. [Google Scholar]
- Nóbrega, G.N.; Ferreira, T.O.; Neto, M.S.; Mendonça, E.D.S.; Romero, R.E.; Otero, X.L. The importance of blue carbon soil stocks in tropical semiarid mangroves: A case study in Northeastern Brazil. Environ. Earth Sci. 2019, 78, 369. [Google Scholar] [CrossRef]
- Otero, X.L.; Araújo, J.M.C.A.; Barcellos, D.; Queiroz, H.M.; Romero, D.J.; Nóbrega, G.N.; Siqueira-Neto, M.; Ferreira, T.O. Crab Bioturbation and Seasonality Control Nitrous Oxide Emissions in Semiarid Mangrove Forests (Ceará, Brazil). Appl. Sci. 2020, 10, 2215. [Google Scholar] [CrossRef] [Green Version]
- Zang, Z.; Zou, X.; Zuo, P.; Song, Q.; Wang, C.; Wang, J. Impact of landscape patterns on ecological vulnerability and ecosystem service values: An empirical analysis of Yancheng Nature Reserve in China. Ecol. Indic. 2017, 72, 142–152. [Google Scholar] [CrossRef]
- Albuquerque, A.G.B.M.; Ferreira, T.O.; Nóbrega, G.N.; Romero, R.E.; Júnior, V.S.D.S.; Meireles, A.J.D.A.; Otero, X.L. Soil genesis on hypersaline tidal flats (apicum ecosystem) in a tropical semi-arid estuary (Ceará, Brazil). Soil Res. 2014, 52, 140–154. [Google Scholar] [CrossRef]
- Fatubarin, A.; Olojugba, M.R. Effect of rainfall season on the chemical properties of the soil of a Southern Guinea Savanna ecosystem in Nigeria. J. Ecol. Nat. Environ. 2014, 6, 182–189. [Google Scholar] [CrossRef]
- De Lacerda, L.D.; De Menezes, M.O.T.; Molisani, M.M. Changes in mangrove extension at the Pacoti River estuary, CE, NE Brazil due to regional environmental changes between 1958 and 2004. Biota Neotrop. 2007, 7, 67–72. [Google Scholar] [CrossRef]
- Nóbrega, G.N.; Ferreira, T.O.; Neto, M.S.; Queiroz, H.M.; Artur, A.G.; Mendonça, E.D.S.; Silva, E.O.; Otero, X.L. Edaphic factors controlling summer (rainy season) greenhouse gas emissions (CO2 and CH4) from semiarid mangrove soils (NE-Brazil). Sci. Total Environ. 2016, 542, 685–693. [Google Scholar] [CrossRef] [Green Version]
- Freire, G. Etude hydrologique et sedimentologique de l’estuaire du rio pacoti (fortaleza-ceare-bresil). Ph.D. Thesis, Université de Nantes, Nantes, France, 1989. [Google Scholar]
- Albuquerque, A. Pedogênese e evolução De Solos De Apicum Em Clima Tropical Semiárido. Ph.D. Thesis, Universidade Federal do Ceará, Fortaleza, Ceará, Brazil, 2015. [Google Scholar]
- Bell, H.L.; O’Leary, J.W. Effects of salinity on growth and cation accumulation of Sporobolus virginicus (Poaceae). Am. J. Bot. 2003, 90, 1416–1424. [Google Scholar] [CrossRef] [Green Version]
- Cordazzo, C.V.; Seeliger, U. Reproduction and vegetative regeneration in Blutaparon portulacoides (Amaranthaceae) on backshores in southern Brazil. J. Coast. Res. 2003, 481–485. [Google Scholar]
- Lonard, R.I.; Judd, F.W.; Stalter, R. The Biological Flora of Coastal Dunes and Wetlands: Batis maritima C. Linnaeus. J. Coast. Res. 2011, 27, 441. [Google Scholar] [CrossRef]
- Álvarez, E.; Fernández-Sanjurjo, M.J.; Otero, X.L.; Macías, F. Aluminum speciation in the bulk and rhizospheric soil solution of the species colonizing an abandoned copper mine in Galicia (NW Spain). J. Soils Sediments 2010, 11, 221–230. [Google Scholar] [CrossRef]
- Chung, J.-B.; Zasoski, R.J. Ammonium-Potassium and Ammonium-Calcium Exchange Equilibria in Bulk and Rhizosphere Soil. Soil Sci. Soc. Am. J. 1994, 58, 1368–1375. [Google Scholar] [CrossRef]
- Otero, X.L.; Huerta-Diaz, M.A.; De La Peña, S.; Ferreira, T.O. Sand as a relevant fraction in geochemical studies in intertidal environments. Environ. Monit. Assess. 2013, 185, 7945–7959. [Google Scholar] [CrossRef] [PubMed]
- Perlatti, F.; Ferreira, T.O.; Sartor, L.R.; Otero, X.L. Copper Biogeochemistry in Response to Rhizosphere Soil Processes Under Four Native Plant Species Growing Spontaneously in an Abandoned Mine Site in NE Brazil. Water Air Soil Pollut. 2016, 227, 1–15. [Google Scholar] [CrossRef]
- Loveland, P.J.; Whalley, W.R.; Smith, K.; Mullins, C. Particle size analysis. Smith Mullins Soil Anal. Phys. Methods 2000, 281–314. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 1201–1229. [Google Scholar]
- EMBRAPA. Manual de Métodos de Análise de Solo; Embrapa Solos: Rio de Janeiro, RJ, Brasil, 1997. [Google Scholar]
- Moore, T.J.; Loeppert, R.H.; West, L.T.; Hallmark, C.T. Routine method for calcium carbonate equivalent of soils. Commun. Soil Sci. Plant Anal. 1987, 18, 265–277. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Kempers, A. Determination of sub-microquantities of ammonium and nitrates in soils with phenol, sodiumnitroprusside and hypochlorite. Geoderma 1974, 12, 201–206. [Google Scholar] [CrossRef]
- Huerta-Diaz, M.A.; Morse, J.W. A quantitative method for determination of trace metal concentrations in sedimentary pyrite. Mar. Chem. 1990, 29, 119–144. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Berner, R.A. Sedimentary pyrite formation. Am. J. Sci. 1970, 268, 1–23. [Google Scholar] [CrossRef]
- Dantas, J.S.; de Souza, A.P.; de Farias, M.F.; Nogueira, V.d.F.B. Interactions among groups of microorganisms with rhizosphere. Appl. Res. Agrotechnol. 2009, 2, 213–224. [Google Scholar]
- Yin, Y.; Yan, Z. Variations of soil bacterial diversity and metabolic function with tidal flat elevation gradient in an artificial mangrove wetland. Sci. Total Environ. 2020, 718, 137385. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Walker, L.R. Competition and facilitation: A synthetic approach to interactions in plant communities. Ecology 1997, 78, 1958–1965. [Google Scholar] [CrossRef]
- Fowler, N. The role of competition in plant communities in arid and semiarid regions. Annu. Rev. Ecol. Syst. 1986, 17, 89–110. [Google Scholar] [CrossRef]
- Meireles, A.J.d.A.; Cassola, R.S.; Tupinambá, S.V.; Queiroz, L.d.S. Impactos Ambientais Decorrentes Das Atividades Da Carcinicultura Ao Longo Do Litoral Cearense, Nordeste Do Brasil. Mercat. Rev. Geogr. UFC 2007, 6, 83–106. [Google Scholar] [CrossRef]
- Van Breemen, N.; Buurman, P. Soil Formation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002; p. 408. [Google Scholar]
- Weidenhamer, J.D.; Callaway, R.M. Direct and Indirect Effects of Invasive Plants on Soil Chemistry and Ecosystem Function. J. Chem. Ecol. 2010, 36, 59–69. [Google Scholar] [CrossRef]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Yin, H.; Xu, Z.; Chen, Z.; Wei, Y.; Liu, Q. Nitrogen transformation in the rhizospheres of two subalpine coniferous species under experimental warming. Appl. Soil Ecol. 2012, 59, 60–67. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, Y.; Shen, Q.; Zhang, F. Effect of Ammonium and Nitrate Nutrition On Some Physiological Processes in Higher Plants—Growth, Photosynthesis, Photorespiration, and Water Relations. Plant Biol. 2007, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Hogh Jensen, H.; Schjoerring, J. Effects of drought and inorganic N form on nitrogen fixation and carbon isotope discrimination in Trifolium repens. Plant Physiol. Biochem. (Paris) 1997, 35, 55–62. [Google Scholar]
- Roiloa, S.R.; Antelo, B.; Retuerto, R. Physiological integration modifies δ15N in the clonal plant Fragaria vesca, suggesting preferential transport of nitrogen to water-stressed offspring. Ann. Bot. 2014, 114, 399–411. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Liu, C.; Wang, Q.; Yao, M.; Rui, J.; Zhang, S.; Li, X. Soil bacterial community structure in Chinese wetlands. Geoderma 2019, 337, 290–299. [Google Scholar] [CrossRef]
- Vogt, J.C.; Abed, R.M.M.; Albach, D.C.; Palinska, K.A. Bacterial and Archaeal Diversity in Hypersaline Cyanobacterial Mats Along a Transect in the Intertidal Flats of the Sultanate of Oman. Microb. Ecol. 2017, 75, 331–347. [Google Scholar] [CrossRef]
- Vera-Gargallo, B.; Ventosa, A. Metagenomic Insights into the Phylogenetic and Metabolic Diversity of the Prokaryotic Community Dwelling in Hypersaline Soils from the Odiel Saltmarshes (SW Spain). Genes 2018, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Abed, R.M.M.; De Beer, D.; Stief, P. Functional-Structural Analysis of Nitrogen-Cycle Bacteria in a Hypersaline Mat from the Omani Desert. Geomicrobiol. J. 2015, 32, 119–129. [Google Scholar] [CrossRef]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef] [Green Version]
- Meyer, R.L.; Allen, D.E.; Schmidt, S. Nitrification and denitrification as sources of sediment nitrous oxide production: A microsensor approach. Mar. Chem. 2008, 110, 68–76. [Google Scholar] [CrossRef]
- Bolhuis, H.; Severin, I.; Confurius-Guns, V.; Wollenzien, U.I.A.; Stal, L.J. Horizontal transfer of the nitrogen fixation gene cluster in the cyanobacterium Microcoleus chthonoplastes. ISME J. 2009, 4, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.; Galal, H.K.; Bromham, L. Evolution of halophytes: Multiple origins of salt tolerance in land plants. Funct. Plant. Biol. 2010, 37, 604–612. [Google Scholar] [CrossRef]
- Saitoh, T.; Seiwa, K.; Nishiwaki, A. Importance of physiological integration of dwarf bamboo to persistence in forest understorey: A field experiment. J. Ecol. 2002, 90, 78–85. [Google Scholar] [CrossRef]
- Slade, A.J.; Hutchings, M.J. An analysis of the costs and benefits of physiological integration between ramets in the clonal perennial herb Glechoma hederacea. Oecologia 1987, 73, 425–431. [Google Scholar] [CrossRef]
- Roiloa, S.R.; Retuerto, R. Development, photosynthetic activity and habitat selection of the clonal plant Fragaria vesca growing in copper-polluted soil. Funct. Plant Biol. 2006, 33, 961. [Google Scholar] [CrossRef]
- Barcellos, D.; Cyle, K.T.; Thompson, A. Faster redox fluctuations can lead to higher iron reduction rates in humid forest soils. Biogeochemistry 2018, 137, 367–378. [Google Scholar] [CrossRef]
- Chen, C.; Meile, C.; Wilmoth, J.L.; Barcellos, D.; Thompson, A. Influence of pO2 on Iron Redox Cycling and Anaerobic Organic Carbon Mineralization in a Humid Tropical Forest Soil. Environ. Sci. Technol. 2018, 52, 7709–7719. [Google Scholar] [CrossRef] [PubMed]
- McKee, K.L.; Cahoon, D.R.; Feller, I.C. Caribbean mangroves adjust to rising sea level through biotic controls on change in soil elevation. Glob. Ecol. Biogeogr. 2007, 16, 545–556. [Google Scholar] [CrossRef]
- Gordon, D.M. Disturbance to mangroves in tropical-arid Western Australia: Hypersalinity and restricted tidal exchange as factors leading to mortality. J. Arid. Environ. 1988, 15, 117–145. [Google Scholar] [CrossRef]
- Xu, C.; Pu, L.; Zhu, M.; Li, J.; Chen, X.; Wang, X.; Xie, X. Ecological Security and Ecosystem Services in Response to Land Use Change in the Coastal Area of Jiangsu, China. Sustainability 2016, 8, 816. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral, R.L.; Ferreira, T.O.; Nóbrega, G.N.; Barcellos, D.; Roiloa, S.R.; Zandavalli, R.B.; Otero, X.L. How Do Plants and Climatic Conditions Control Soil Properties in Hypersaline Tidal Flats? Appl. Sci. 2020, 10, 7624. https://doi.org/10.3390/app10217624
Cabral RL, Ferreira TO, Nóbrega GN, Barcellos D, Roiloa SR, Zandavalli RB, Otero XL. How Do Plants and Climatic Conditions Control Soil Properties in Hypersaline Tidal Flats? Applied Sciences. 2020; 10(21):7624. https://doi.org/10.3390/app10217624
Chicago/Turabian StyleCabral, Raiana L., Tiago O. Ferreira, Gabriel N. Nóbrega, Diego Barcellos, Sergio R. Roiloa, Roberta B. Zandavalli, and Xosé L. Otero. 2020. "How Do Plants and Climatic Conditions Control Soil Properties in Hypersaline Tidal Flats?" Applied Sciences 10, no. 21: 7624. https://doi.org/10.3390/app10217624
APA StyleCabral, R. L., Ferreira, T. O., Nóbrega, G. N., Barcellos, D., Roiloa, S. R., Zandavalli, R. B., & Otero, X. L. (2020). How Do Plants and Climatic Conditions Control Soil Properties in Hypersaline Tidal Flats? Applied Sciences, 10(21), 7624. https://doi.org/10.3390/app10217624








