Synthesis, Molecular Docking, Druglikeness Analysis, and ADMET Prediction of the Chlorinated Ethanoanthracene Derivatives as Possible Antidepressant Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of 4,5-Dichloro-9,10-dihydro-9,10-ethanoanthracene-12-carbonitrile 5 anti, 1,8-Dichloro-9,10-dihydro-9,10-ethanoanthracene-12-carbonitrile 5 syn, and 9,10-Dihydro-9,10-ethanoanthracene-12-carbonitrile 5 dec
2.1.2. Synthesis of 4,5-Dichloro-12-cyano-9,10-dihydro-9,10-ethanoanthracen-12-yl Acetate 6 anti, 1,8-Dichloro-12-cyano-9,10-dihydro-9,10-ethanoanthracen-12-yl Acetate 6 syn, and 12-Cyano-9,10-dihydro-9,10-ethanoanthracen-12-yl Acetate 6 dec
2.1.3. Synthesis of 4,5-Dichloro-12-(phenylsulfonyl)-9,10-dihydro-9,10-ethanoanthracene 7 anti, 1,8-Dichloro-12-(phenylsulfonyl)-9,10-dihydro-9,10-ethanoanthracene 7 syn, and 12-(Phenylsulfonyl)-9,10-dihydro-9,10-ethanoanthracene 7 dec
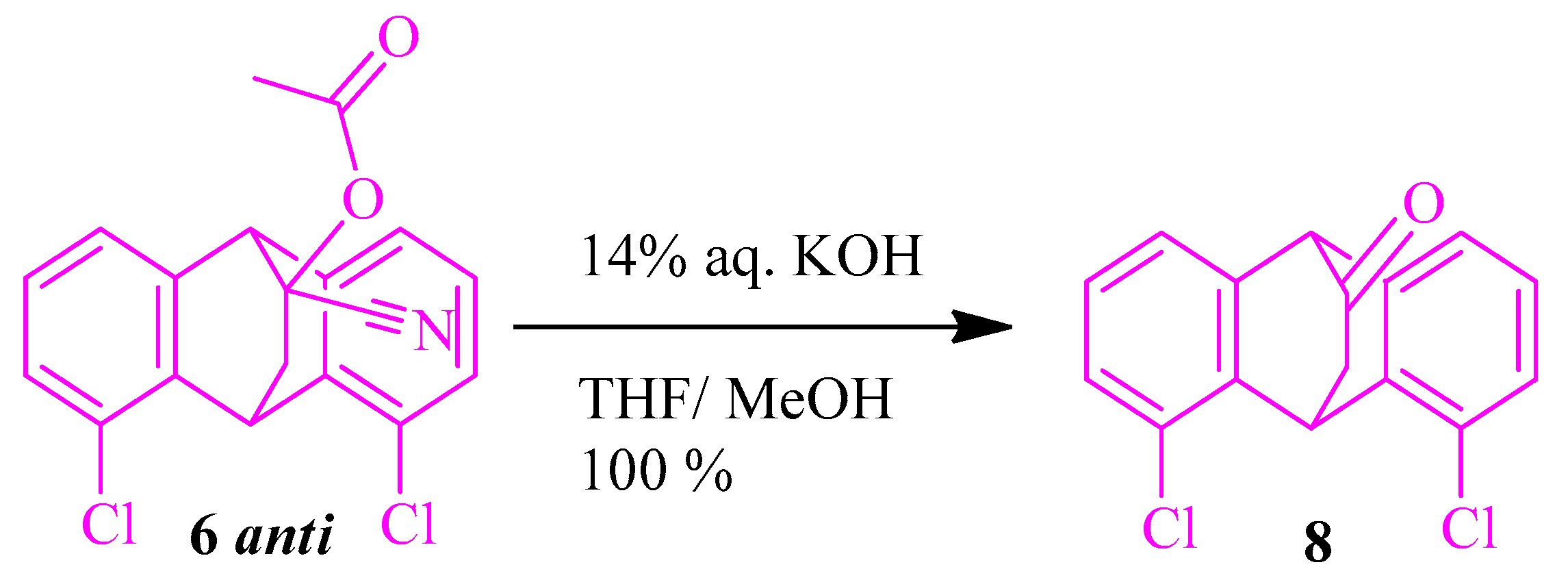
2.1.4. Synthesis of 1,8-Dichloro-9,10-dihydro-9,10-ethanoanthracen-11-one 8
2.2. Cheminformatics Prediction
2.2.1. PASS Online
2.2.2. Molecular Docking
Preparation of the Compounds and Maprotiline
Preparation of the Protein Models
Docking of the Compounds into Protein Models
2.2.3. ADMET Prediction
3. Results and Discussion
3.1. Synthesis
3.2. Cheminformatics Prediction
3.2.1. PASS Online
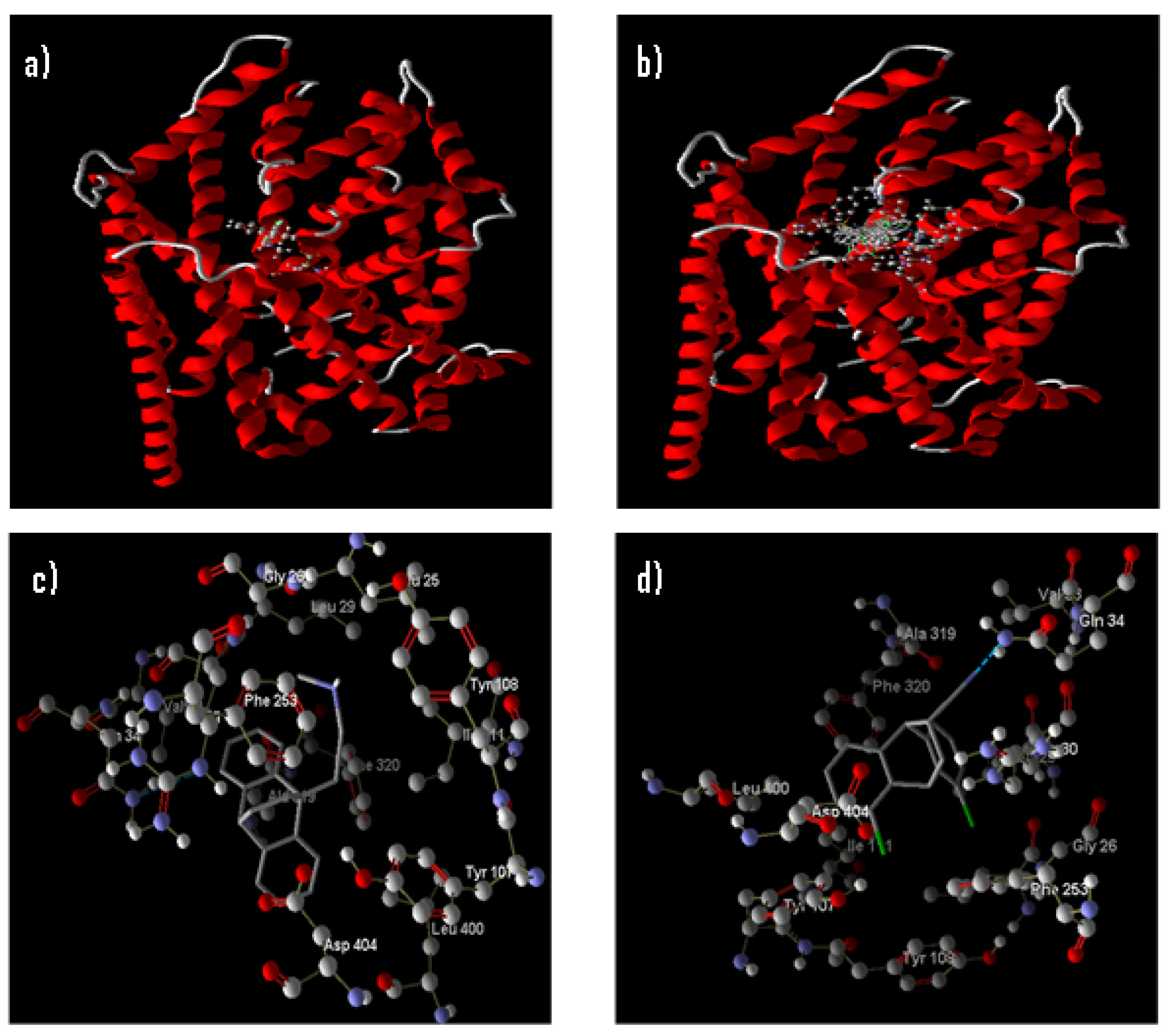
3.2.2. Molecular Docking
Docking of the Compounds (5–7) anti, (5–7) syn, and Maprotiline 9 into 4xnx Model
Docking of the Compounds (5–7) anti, (5–7) syn, and Maprotiline 9 into 2QJU Model
Docking of the Compounds (5–7) anti, (5–7) syn, and Maprotiline 9 into 3GWU Model
3.2.3. Drug-Likeness Prediction
3.2.4. ADMET Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, A.H.; Jang, Y.; Kim, G.H.; Kim, J.J.; Lee, S.S.; Ahn, B.J. Decolorizing an anthraquinone dye by Phlebia brevispora: Intra-species characterization. Eng. Life Sci. 2017, 17, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Niu, G.; Wei, X.; Lan, M.; Zeng, L.; Kinsella, J.M.; Sheng, R. A family of multi-color anthracene carboxyimides: Synthesis, spectroscopic properties, solvatochromic fluorescence and bio-imaging application. Dyes Pigment. 2017, 139, 166–173. [Google Scholar] [CrossRef]
- Wadler, S.; Fuks, J.Z.; Wiernik, P.H. Phase I and II agents in cancer therapy: I. Anthracyclines and related compounds. J. Clin. Pharmacol. 1986, 26, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Sunmonu, T.O.; Owolabi, O.D.; Oloyede, O.B. Anthracene-induced enzymatic changes as stress indicators in African catfish, Heterobranchus bidorsalis Geoffroy Saint Hilaire, 1809. Res. J. Environ. Sci. 2009, 3, 677–686. [Google Scholar]
- McNamara, Y.; Bright, S.; Byrne, A.; Cloonan, S.; McCabe, T.; Williams, D.; Meegan, M. Synthesis and antiproliferative action of a novel series of maprotiline analogues. Eur. J. Med. Chem. 2014, 71, 333–353. [Google Scholar] [CrossRef]
- Cloonan, S.M.; Drozgowska, A.; Fayne, D.; Williams, D.C. The antidepressants maprotiline and fluoxetine have potent selective antiproliferative effects against Burkitt lymphoma independently of the norepinephrine and serotonin transporters. Leuk. Lymphoma 2010, 51, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Cloonan, S.M.; Williams, D.C. The antidepressants maprotiline and fluoxetine induce Type II autophagic cell death in drug-resistant Burkitt’s lymphoma. Int. J. Cancer 2011, 128, 1712–1723. [Google Scholar] [CrossRef]
- Huang, H.-S.; Chiu, H.-F.; Hwang, J.-M.; JEN, Y.-M.; TAO, C.-W.; LEE, K.-Y.; LAI, Y.-L. Studies on anthracenes. 2. Synthesis and cytotoxic evaluation of 9-acyloxy 1, 8-dichloroanthracene derivatives. Chem. Pharm. Bull. 2001, 49, 1346–1348. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-S.; Lin, P.-Y.; Hwang, J.-M.; TAO, C.-W.; Hsu, H.-C.; LAI, Y.-L. Studies on anthracenes. 3. Synthesis, lipid peroxidation and cytotoxic evaluation of 10-substituted 1, 5-dichloro-9 (10H)-anthracenone derivatives. Chem. Pharm. Bull. 2001, 49, 1288–1291. [Google Scholar] [CrossRef] [Green Version]
- Bar, A.K.; Gole, B.; Ghosh, S.; Mukherjee, P.S. Self-assembly of a PdII neutral molecular rectangle via a new organometallic Pd II 2 molecular clip and oxygen donor linker. Dalton Trans. 2009, 6701–6704. [Google Scholar] [CrossRef]
- Phutdhawong, W.; Buddhasukh, D. Facile microwave-assisted synthesis of 9, 10-dihydro-9, 10-ethanoanthracene-11-carboxylic acid methyl ester. Molecules 2005, 10, 1409–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atherton, J.; Jones, S. Diels–Alder reactions of anthracene, 9-substituted anthracenes and 9, 10-disubstituted anthracenes. Tetrahedron 2003, 59, 9039–9057. [Google Scholar] [CrossRef]
- Wise, K.E.; Wheeler, R.A. Donor-acceptor-assisted Diels-Alder reaction of anthracene and tetracyanoethylene. J. Phys. Chem. A 1999, 103, 8279–8287. [Google Scholar] [CrossRef]
- Hiruta, K.; Tokita, S.; Tachikawa, T.; Noguchi, F.; Nishimoto, K. Precise PPP molecular orbital calculations of excitation energies of polycyclic aromatic hydrocarbons. Part 6. 1 Spectrochemical atomic softness parameter. J. Chem. Soc. Perkin Trans. 2 2001, 975–980. [Google Scholar] [CrossRef]
- Bitonti, A.J.; Sjoerdsma, A.; McCann, P.P.; Kyle, D.E.; Oduola, A.; Rossan, R.N.; Milhous, W.K.; Davidson, D.E. Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science 1988, 242, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Alibert, S.; Santelli-Rouvier, C.; Pradines, B.; Houdoin, C.; Parzy, D.; Karolak-Wojciechowska, J.; Barbe, J. Synthesis and Effects on Chloroquine Susceptibility in Plasmodium f alciparum of a Series of New Dihydroanthracene Derivatives. J. Med. Chem. 2002, 45, 3195–3209. [Google Scholar] [CrossRef] [PubMed]
- Szabó, D.; Szabó, G.; Ocsovszki, I.; Aszalos, A.; Molnár, J. Anti-psychotic drugs reverse multidrug resistance of tumor cell lines and human AML cells ex-vivo. Cancer Lett. 1999, 139, 115–119. [Google Scholar] [CrossRef]
- Karama, U.; Sultan, M.A.; Almansour, A.I.; El-Taher, K.E. Synthesis of chlorinated tetracyclic compounds and testing for their potential antidepressant effect in mice. Molecules 2016, 21, 61. [Google Scholar] [CrossRef] [Green Version]
- Sultan, M.A.; Almansour, A.I.; Pillai, R.R.; Kumar, R.S.; Arumugam, N.; Armaković, S.; Armaković, S.J.; Soliman, S.M. Synthesis, theoretical studies and molecular docking of a novel chlorinated tetracyclic:(Z/E)-3-(1, 8-dichloro-9, 10-dihydro-9, 10-ethanoanthracen-11-yl) acrylaldehyde. J. Mol. Struct. 2017, 1150, 358–365. [Google Scholar] [CrossRef]
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe. Justus Liebigs Ann. Chem. 1928, 460, 98–122. [Google Scholar] [CrossRef]
- Brocksom, T.J.; Nakamura, J.; Ferreira, M.L.; Brocksom, U. The Diels-Alder reaction: An update. J. Braz. Chem. Soc. 2001, 12, 597–622. [Google Scholar] [CrossRef]
- Mehta, G.; Uma, R. Stereoelectronic Control in Diels− Alder Reaction of Dissymmetric 1, 3-Dienes. Acc. Chem. Res. 2000, 33, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Kanishchev, O.S.; Sanselme, M.; Bouillon, J.-P. Hetero-Diels–Alder reactions of perfluoroalkyl thioamides with electron-rich 1, 3-dienes: Synthesis of new 2-aminosubstituted-3, 6-dihydro-2H-thiopyrans and related compounds. Tetrahedron 2013, 69, 1322–1336. [Google Scholar] [CrossRef]
- Wilhelm, M.; Schmidt, P. Synthese und Eigenschaften von 1-Aminoalkyl-dibenzo [b, e] bicyclo [2.2. 2] octadienen. Helv. Chim. Acta 1969, 52, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.A.; Karama, U. Substituent effects on regioselectivity of the Diels-Alder reactions: Reactions of 10-allyl-1, 8-dichloroanthracene with 2-chloroacrylonitrile, 1-cyanovinyl acetate and phenyl vinyl sulfone. J. Chem. 2016, 34, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Carr, R.V.; Williams, R.V.; Paquette, L.A. Dienophilic properties of phenyl vinyl sulfone and trans-1-(phenylsulfonyl)-2-(trimethylsilyl) ethylene. Their utilization as synthons for ethylene, 1-alkenes, acetylene, and monosubstituted alkynes in the construction of functionalized six-membered rings via [4+ 2]. pi. cycloaddition methodology. J. Org. Chem. 1983, 48, 4976–4986. [Google Scholar]
- Dalai, S.; Belov, V.N.; Nizamov, S.; Rauch, K.; Finsinger, D.; de Meijere, A. Access to Variously Substituted 5, 6, 7, 8-Tetrahydro-3H-quinazolin-4-ones via Diels–Alder Adducts of Phenyl Vinyl Sulfone to Cyclobutene-Annelated Pyrimidinones. Eur. J. Org. Chem. 2006, 2006, 2753–2765. [Google Scholar] [CrossRef]
- Omodani, T.; Shishido, K. Total enantioselective synthesis of the marine sesquiterpene nanaimoal. J. Chem. Soc. Chem. Commun. 1994, 2781–2782. [Google Scholar] [CrossRef]
- Bull, J.R.; Bischofberger, K. Cycloaddition of phenyl vinyl sulphone to 3-methoxy-16-methylestra-1, 3, 5 (10), 14, 16-pentaen-17-yl acetate: Synthesis of 14-functional ised 19-norpregnane derivatives. J. Chem. Soc. Perkin Trans. 1 1991, 2859–2865. [Google Scholar] [CrossRef]
- Taarning, E.; Madsen, R. Unsaturated aldehydes as alkene equivalents in the Diels–Alder reaction. Chem. -A Eur. J. 2008, 14, 5638–5644. [Google Scholar] [CrossRef] [Green Version]
- Ono, N.; Miyake, H.; Kamimura, A.; Kaji, A. Regioselective Diels–Alder reactions. The nitro group as a regiochemical control element. J. Chem. Soc. Perkin Trans. 1 1987, 1929–1935. [Google Scholar] [CrossRef]
- Wade, P.A.; Murray, J.K.; Shah-Patel, S.; Carroll, P.J. Generation and in situ Diels–Alder reactions of activated nitroethylene derivatives. Tetrahedron Lett. 2002, 43, 2585–2588. [Google Scholar] [CrossRef]
- Zaidlewicz, M.; Binkul, J.R.; Sokół, W. Syntheses with organoboranes. IX. Vinyl-and 1-alkenyldichloroboranes as ethylene and 1-alkene equivalents for the Diels–Alder reaction. J. Organomet. Chem. 1999, 580, 354–362. [Google Scholar] [CrossRef]
- Paquette, L.A.; Moerck, R.E.; Harirchian, B.; Magnus, P.D. Use of phenyl vinyl sulfoxide as an acetylene equivalent in Diels-Alder cycloadditions. J. Am. Chem. Soc. 1978, 100, 1597–1599. [Google Scholar] [CrossRef]
- Alavijeh, M.S.; Chishty, M.; Qaiser, M.Z.; Palmer, A.M. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx 2005, 2, 554–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filimonov, D.; Lagunin, A.; Gloriozova, T.; Rudik, A.; Druzhilovskii, D.; Pogodin, P.; Poroikov, V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Baldi, A. Computational approaches for drug design and discovery: An overview. Syst. Rev. Pharm. 2010, 1, 99. [Google Scholar] [CrossRef]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Z.; Yang, Z.; Chen, K.; Zhu, W. A knowledge-based halogen bonding scoring function for predicting protein-ligand interactions. J. Mol. Model. 2013, 19, 5015–5030. [Google Scholar] [CrossRef]
- Wang, H.; Goehring, A.; Wang, K.H.; Penmatsa, A.; Ressler, R.; Gouaux, E. Structural basis for action by diverse antidepressants on biogenic amine transporters. Nature 2013, 503, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, A.; Singh, S.K.; Kawate, T.; Jin, Y.; Gouaux, E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature 2005, 437, 215–223. [Google Scholar]
- Zheng, G.; Xue, W.; Wang, P.; Yang, F.; Li, B.; Li, X.; Li, Y.; Yao, X.; Zhu, F. Exploring the inhibitory mechanism of approved selective norepinephrine reuptake inhibitors and reboxetine enantiomers by molecular dynamics study. Sci. Rep. 2016, 6, 26883. [Google Scholar] [CrossRef]
- Sultan, M.A.; Karama, U.; Almansour, A.I.; Soliman, S.M.; Ghabbour, H.A.; Mabkhot, Y.N. Synthesis, Characterization and DFT Calculations of 4, 5, 12-and 1, 8, 12-trichloro-9, 10-dihydro-9, 10-ethanoanthracene-12-carbonitriles. Crystals 2017, 7, 259. [Google Scholar] [CrossRef] [Green Version]
- Penmatsa, A.; Wang, K.H.; Gouaux, E. X-ray structures of Drosophila dopamine transporter in complex with nisoxetine and reboxetine. Nat. Struct. Mol. Biol. 2015, 22, 506. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Zhen, J.; Karpowich, N.K.; Goetz, R.M.; Law, C.J.; Reith, M.E.; Wang, D.-N. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science 2007, 317, 1390–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Zhen, J.; Karpowich, N.K.; Law, C.J.; Reith, M.E.; Wang, D.-N. Antidepressant specificity of serotonin transporter suggested by three LeuT–SSRI structures. Nat. Struct. Mol. Biol. 2009, 16, 652–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- House, H.O.; Koepsell, D.; Jaeger, W. Derivatives of 1, 8-diphenylanthracene. J. Org. Chem. 1973, 38, 1167–1173. [Google Scholar] [CrossRef]
- del Rosario Benites, M.a.; Fronczek, F.R.; Maverick, A.W. Intermolecular hydrogenation of a C C bond during π-cyclopentadienyliron complexation of 1, 8-dichloro-9, 10-dihydro-9, 10-ethenoanthracene. J. Organomet. Chem. 1999, 577, 24–30. [Google Scholar] [CrossRef]
- Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis—a review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Wathey, B.; Tierney, J.; Lidström, P.; Westman, J. The impact of microwave-assisted organic chemistry on drug discovery. Drug Discov. Today 2002, 7, 373–380. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-assisted chemistry: Synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef]
- Sasaki, S.; Ishibashi, N.; Kuwamura, T.; Sano, H.; Matoba, M.; Nisikawa, T.; Maeda, M. Excellent acceleration of the Diels-Alder reaction by microwave irradiation for the synthesis of new fluorine-substituted ligands of NMDA receptor. Bioorganic Med. Chem. Lett. 1998, 8, 2983–2986. [Google Scholar] [CrossRef]
- Díaz-Ortiz, A.; Carrillo, J.; Gómez-Escalonilla, M.J.; de la Hoz, A.; Moreno, A.; Prieto, P. First Diels-Alder reaction of pyrazolyl imines under microwave irradiation. Synlett 1998, 1998, 1069–1070. [Google Scholar] [CrossRef]
- Kaplan, F.; Conroy, H. Electronic Effects on the Stereochemistry of the Diels—Alder Reaction1. J. Org. Chem. 1963, 28, 1593–1596. [Google Scholar] [CrossRef]
- Verma, S.M.; Singh, M.D. Structural elucidation with nuclear magnetic resonance spectroscopy. Diels-Alder adducts of 1-aminoanthracene and maleic anhydride: Restricted rotation about the aryl C (1)-N bond and intrinsic asymmetry about the imide (Nsp2-Csp3) system. J. Org. Chem. 1977, 42, 3736–3740. [Google Scholar] [CrossRef]
- Singh, M.D.; Ningombam, A. High stereoselectivity in the Diels-Alder reaction of substituted anthracenes: Reactions of 1-succinimidoanthracene and 1-phthalimidoanthracene with maleic anhydride. Indian J. Chem. 2010, 49, 789–794. [Google Scholar] [CrossRef]
- Schmidt, C.; Sabnis, S.; Schmidt, E.; Taylor, D. Substituent Effects on the Orientation of Diels-Alder Reactions. II. Can. J. Chem. 1971, 49, 371–374. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Ningombam, A.; Singh, K.B.; Singh, M.D. Stereoelectronic effects in the Stereoselectivity of the Diels-Alder Reactions: Reactions of Aminoanthracenes with N-phenylmaleimide. J. Chem. Pharm. Res. 2012, 4, 1532–1538. [Google Scholar]
- Chung, Y.; Duerr, B.F.; McKelvey, T.A.; Nanjappan, P.; Czarnik, A.W. Structural effects controlling the rate of the retro-Diels-Alder reaction in anthracene cycloadducts. J. Org. Chem. 1989, 54, 1018–1032. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Brenk, R.; Schipani, A.; James, D.; Krasowski, A.; Gilbert, I.H.; Frearson, J.; Wyatt, P.G. Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. ChemMedChem 2008, 3, 435. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, R.C.; Mitchell, R.C.; Brown, T.H.; Ganellin, C.R.; Griffiths, R.; Jones, M.; Rana, K.K.; Saunders, D.; Smith, I.R. Development of a new physicochemical model for brain penetration and its application to the design of centrally acting H2 receptor histamine antagonists. J. Med. Chem. 1988, 31, 656–671. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cheng, F.; Chen, L.; Du, Z.; Li, W.; Liu, G.; Lee, P.W.; Tang, Y. In silico prediction of chemical Ames mutagenicity. J. Chem. Inf. Modeling 2012, 52, 2840–2847. [Google Scholar] [CrossRef]
- Mulliner, D.; Schmidt, F.; Stolte, M.; Spirkl, H.-P.; Czich, A.; Amberg, A. Computational models for human and animal hepatotoxicity with a global application scope. Chem. Res. Toxicol. 2016, 29, 757–767. [Google Scholar] [CrossRef] [Green Version]
- Kalyaanamoorthy, S.; Barakat, K.H. Development of safe drugs: The hERG challenge. Med. Res. Rev. 2018, 38, 525–555. [Google Scholar] [CrossRef]
- Saxena, P.; Zangerl-Plessl, E.-M.; Linder, T.; Windisch, A.; Hohaus, A.; Timin, E.; Hering, S.; Stary-Weinzinger, A. New potential binding determinant for hERG channel inhibitors. Sci. Rep. 2016, 6, 24182. [Google Scholar] [CrossRef] [Green Version]
- Ponte, M.L.; Keller, G.A.; Girolamo, G.D. Mechanisms of drug induced QT interval prolongation. Curr. Drug Saf. 2010, 5, 44–53. [Google Scholar] [CrossRef]
- Siramshetty, V.B.; Nickel, J.; Omieczynski, C.; Gohlke, B.-O.; Drwal, M.N.; Preissner, R. WITHDRAWN—a resource for withdrawn and discontinued drugs. Nucleic Acids Res. 2016, 44, D1080–D1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Ramos, L.G. Drug-induced QT prolongation and torsades de pointes. Pharm. Ther. 2017, 42, 473. [Google Scholar]
- Low, Y.; Uehara, T.; Minowa, Y.; Yamada, H.; Ohno, Y.; Urushidani, T.; Sedykh, A.; Muratov, E.; Kuz’min, V.; Fourches, D. Predicting drug-induced hepatotoxicity using QSAR and toxicogenomics approaches. Chem. Res. Toxicol. 2011, 24, 1251–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, D.; Laggner, C.; Langer, T. Why drugs fail-a study on side effects in new chemical entities. Curr. Pharm. Des. 2005, 11, 3545–3559. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; McCann, J.; Yamasaki, E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res./Environ. Mutagenesis Relat. Subj. 1975, 31, 347–363. [Google Scholar] [CrossRef]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res./Environ. Mutagenesis Relat. Subj. 2000, 455, 29–60. [Google Scholar] [CrossRef]
- Lei, T.; Li, Y.; Song, Y.; Li, D.; Sun, H.; Hou, T. ADMET evaluation in drug discovery: 15. Accurate prediction of rat oral acute toxicity using relevance vector machine and consensus modeling. J. Cheminformatics 2016, 8, 6. [Google Scholar] [CrossRef] [Green Version]



| Isomer | Chemical Shift | Chemical Shift | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H-9 | H-9′ | H-9″ | H-10 | H-10′ | H-10″ | |||||||
| δ | J | δ | J | δ | J | δ | J | δ | J | δ | J | |
| 5 anti | 5.46 | t; 2.4 | 4.60 | d; 3.2 | ||||||||
| 5 syn | 4.44 | t; 2.8 | 5.71 | t; 2.8 | ||||||||
| 5 dec | 4.39 | t; 3.2 | 4.57 | d; 2.4 | ||||||||
| Isomer | Chemical Shift | Chemical Shift | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H-9 | H-9′ | H-9″ | H-10 | H-10′ | H-10″ | |||||||
| δ | J | δ | J | δ | J | δ | J | δ | J | δ | J | |
| 6 anti | 5.38 | t; 2.8 | 5.12 | s | ||||||||
| 6 syn | 4.41 | t; 2.8 | 6.09 | s | ||||||||
| 6 dec | 4.36 | t; 2 | 5.07 | s | ||||||||
| Isomer | Chemical Shift | Chemical Shift | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H-9 | H-9′ | H-9″ | H-10 | H-10′ | H-10″ | |||||||
| δ | J | δ | J | δ | J | δ | J | δ | J | δ | J | |
| 7 anti | 5.31 | t; 2.6 | 4.82 | d; 2 | ||||||||
| 7 syn | 4.31 | t; 2.6 | 5.56 | broad s | ||||||||
| 7 dec | 4.25 | t; 2.4 | 4.71 | d; 2 | ||||||||
| Compound | Activity/Pa | |
|---|---|---|
| Antidepressant | Phobic Disorders Treatment | |
| 5 anti | 0.226 | 0.877 |
| 5 syn | 0.226 | 0.877 |
| 6 anti | 0.142 | 0.875 |
| 6 syn | 0.142 | 0.875 |
| 7 anti | 0.169 | 0.842 |
| 7 syn | 0.169 | 0.842 |
| Maprotiline 9 | 0.626 | 0.897 |
| 4xnx Model | 2QJU Model | 3GWU Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | H- Bond (Length A) | Steric Interaction | MolDock Score | H- Bond (Length A) | Steric Interaction | MolDock Score | H- Bond (Length A) | Steric Interaction | MolDock Score |
| 5 anti | - | Phe 325, Val 327, Asp 121, Val 120, Phe 43, Gly 425 | −95.1957 | - | Ile 111, Tyr 108, Phe 253, Leu 25, Arg 30, Leu 29, phe 320, Gly 26 | −91.7484 | Gln 34, (2.79245) | Leu 400, Tyr 107, Arg 30, Ala 319, Leu 29 | −79.4448 |
| 5 syn | - | Tyr 123, Tyr 124 | −89.0117 | - | Arg 30, Phe 320, Leu 29, Phe 253, Gly 26 | −81.0802 | - | Ala 319, Ile 111, Leu 400, Phe 320, Phe 253, Gln 34, Val 33 | −82.7372 |
| 6 anti | Tyr 124 (3.09982) Ser 421 (2.92323) | Ser 421, Gly 425, Phe 43, Phe 325 | −103.964 | Arg 30, (3.0999) | Arg 30, Tyr 107, Leu 400, Ala 319, Leu 29, Phe 320, Leu 25, Gly 26 | - | Leu 400, Phe 320, Ala 319, Leu 29, Tyr 107, Tyr 108, Arg 30 | −102.183 | |
| 6 syn | - | Asp 46, Phe 43, Phe 325 | −102.65 | - | Arg 30, Ala 319, Leu 400, Tyr 107, Phe 320, Asp 404, Phe 253, Val 33 | −96.2236 | - | Leu 400, Tyr 107, Ph 253, Gln 34, Ala 319, Phe 320 | −93.9842 |
| 7 anti | - | Tyr 124, Asp 46, Ala 44, Ser 320, Gly 322, Phe 43, Phe 325 | −113.764 | Arg 30, (2.74666) | Ala 319, Val 33, Phe 253, Tyr 108, Ile 111, Tyr 107, Arg 30, Asp 404, Phe 320, Leu 25, Leu 29 | −103.8 | - | Leu 25, Phe 320, Ala 319, Arg 30, Asp 404, Phe 253 | −100.919 |
| 7 syn | - | Phe 43, Phe 325, Tyr 124, Tyr 123, Gly 425, Phe 319 | −117.691 | Arg 30, (2.59754) | Phe 320, Ala 319, Tyr 107, Tyr 108, Ile 111, Phe 324, Leu 29, Leu 25, Arg 30, Phe 253, Gly 26 | −96.6206 | - | Ala 319, Leu 400, Asp 404, Arg 30 | −104.221 |
| Maprotiline 9 | Asp 121, (3.24966) | Tyr 124, Asn 125, Ser 421, Asp 121 | −95.561 | Asp 404, (2.75112) | Arg 30, Asp 401, Asp 404, Phe 320 | −99.2454 | - | Arg 30, Ala 319 | −91.4932 |
| Compound | MW (g/mol) | HBA | HBD | TPSA (Ų) | Consensus Log Po/w * | MR | GI Absorption | BBB Permeant | P-gp Substrate | Lipinski | Bioavailability Score | PAINS (alert) | Brenk (alert) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 anti | 300.18 | 1 | 0 | 23.79 | 4.43 | 80.98 | High | Yes | Yes | Yes ** | 0.55 | 0 | 0 |
| 5 syn | 300.18 | 1 | 0 | 23.79 | 4.42 | 80.98 | High | Yes | Yes | Yes ** | 0.55 | 0 | 0 |
| 6 anti | 358.22 | 3 | 0 | 50.09 | 4.25 | 91.92 | High | Yes | Yes | Yes | 0.55 | 0 | 0 |
| 6 syn | 358.22 | 3 | 0 | 50.09 | 4.22 | 91.92 | High | Yes | Yes | Yes | 0.55 | 0 | 0 |
| 7 anti | 415.33 | 2 | 0 | 42.52 | 5.40 | 109.04 | Low | No | No | Yes ** | 0.55 | 0 | 0 |
| 7 syn | 415.33 | 2 | 0 | 42.52 | 5.38 | 109.04 | Low | No | No | Yes ** | 0.55 | 0 | 0 |
| Maprotiline 9 | 277.40 | 1 | 1 | 12.03 | 4.30 | 88.32 | High | Yes | Yes | Yes ** | 0.55 | 0 | 0 |
| Compound | Absorption | Distribution | Metabolism; P450 CYP | Elimination | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caco-2 p (cm/s) | Pgp (I) | Pgp (S) | HIA | PPB% | VD (L/Kg) | BBB | 1A2 | 74 | 119 | T½ h | CL mL/min/kg | ||||
| I | S | I | S | I | S | ||||||||||
| 5 anti | −4.402 | −0.481 | −0.036 | ++0.866 | 78.271 | 0.581 | +++0.983 | +0.568 | +0.69 | −0.243 | +0.616 | −0.331 | −0.49 | 2.174 | 1.424 |
| 5 syn | −4.402 | +0.521 | −0.048 | ++0.866 | 77.559 | 0.604 | +++0.983 | ++0.708 | ++0.754 | −0.333 | +0.648 | −0.348 | −0.452 | 2.34 | 1.425 |
| 6 anti | −4.479 | ++0.814 | −0.008 | ++0.801 | 93.528 | −0.331 | +++0.915 | −0.312 | +0.576 | −0.182 | ++0.7 | −0.448 | −0.444 | 2.059 | 0.647 |
| 6 syn | −4.472 | ++0.839 | −0.004 | ++0.801 | 93.448 | −0.323 | +++0.915 | −0.328 | +0.674 | −0.334 | +0.684 | −0.369 | −0.473 | 2.065 | 0.613 |
| 7 anti | −4.496 | −0.364 | −0.04 | ++0.751 | 82.876 | 0.022 | +++0.985 | −0.141 | +0.624 | −0.258 | +0.568 | +0.613 | +0.583 | 2.02 | 0.681 |
| 7 syn | −4.493 | −0.384 | −0.039 | ++0.751 | 82.776 | 0.042 | +++0.985 | −0.198 | +0.68 | −0.329 | +0.6 | +0.66 | +0.597 | 2.112 | 0.659 |
| Maprotiline 9 | −4.387 | +0.558 | +0.511 | ++0.895 | 87.029 | 1.603 | +++0.984 | −0.086 | +++0.938 | ++0.885 | +0.612 | −0.036 | −0.281 | 3.401 | 2.599 |
| Compound | Mutagenicity | Tumorigenicity | Reproductive Effect | Irritating Effect | Toxicity * | |||
|---|---|---|---|---|---|---|---|---|
| hERG | H-HT | AMES | LD50 mg/kg | |||||
| 5 anti | None | None | None | None | −0.282 | +0.564 | +0.676 | 802.40 |
| 5 syn | None | None | None | None | −0.261 | −0.414 | +0.676 | 811.69 |
| 6 anti | High | None | High | High | ++0.761 | ++0.756 | −0.31 | 388.28 |
| 6 syn | High | None | High | High | ++0.758 | ++0.774 | −0.31 | 412.24 |
| 7 anti | None | None | None | None | +0.66 | −0.484 | −0.276 | 262.66 |
| 7 syn | None | None | None | None | +0.672 | +0.508 | −0.276 | 249.11 |
| Maprotiline 9 | None | None | High | None | ++0.879 | ++0.878 | −0.282 | 912.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultan, M.A.; Galil, M.S.A.; Al-Qubati, M.; Omar, M.M.; Barakat, A. Synthesis, Molecular Docking, Druglikeness Analysis, and ADMET Prediction of the Chlorinated Ethanoanthracene Derivatives as Possible Antidepressant Agents. Appl. Sci. 2020, 10, 7727. https://doi.org/10.3390/app10217727
Sultan MA, Galil MSA, Al-Qubati M, Omar MM, Barakat A. Synthesis, Molecular Docking, Druglikeness Analysis, and ADMET Prediction of the Chlorinated Ethanoanthracene Derivatives as Possible Antidepressant Agents. Applied Sciences. 2020; 10(21):7727. https://doi.org/10.3390/app10217727
Chicago/Turabian StyleSultan, Mujeeb A., Mansour S. A. Galil, Mohyeddine Al-Qubati, Mufeed M. Omar, and Assem Barakat. 2020. "Synthesis, Molecular Docking, Druglikeness Analysis, and ADMET Prediction of the Chlorinated Ethanoanthracene Derivatives as Possible Antidepressant Agents" Applied Sciences 10, no. 21: 7727. https://doi.org/10.3390/app10217727
APA StyleSultan, M. A., Galil, M. S. A., Al-Qubati, M., Omar, M. M., & Barakat, A. (2020). Synthesis, Molecular Docking, Druglikeness Analysis, and ADMET Prediction of the Chlorinated Ethanoanthracene Derivatives as Possible Antidepressant Agents. Applied Sciences, 10(21), 7727. https://doi.org/10.3390/app10217727






