Effect of Severe Plastic Deformation, through Equal-Channel Angular Press Processing, on the Electrochemical Behavior of Al5083 Alloy
Abstract
:1. Introduction
2. Experimental Procedure
2.1. ECAP Process of the Al5083 Alloy
2.2. Optical Microstructure
2.3. Corrosion Test
2.4. Raman Spectroscopy
2.5. SEM Analysis
3. Results and Discussion
3.1. Microstructure and Grain Size Analysis
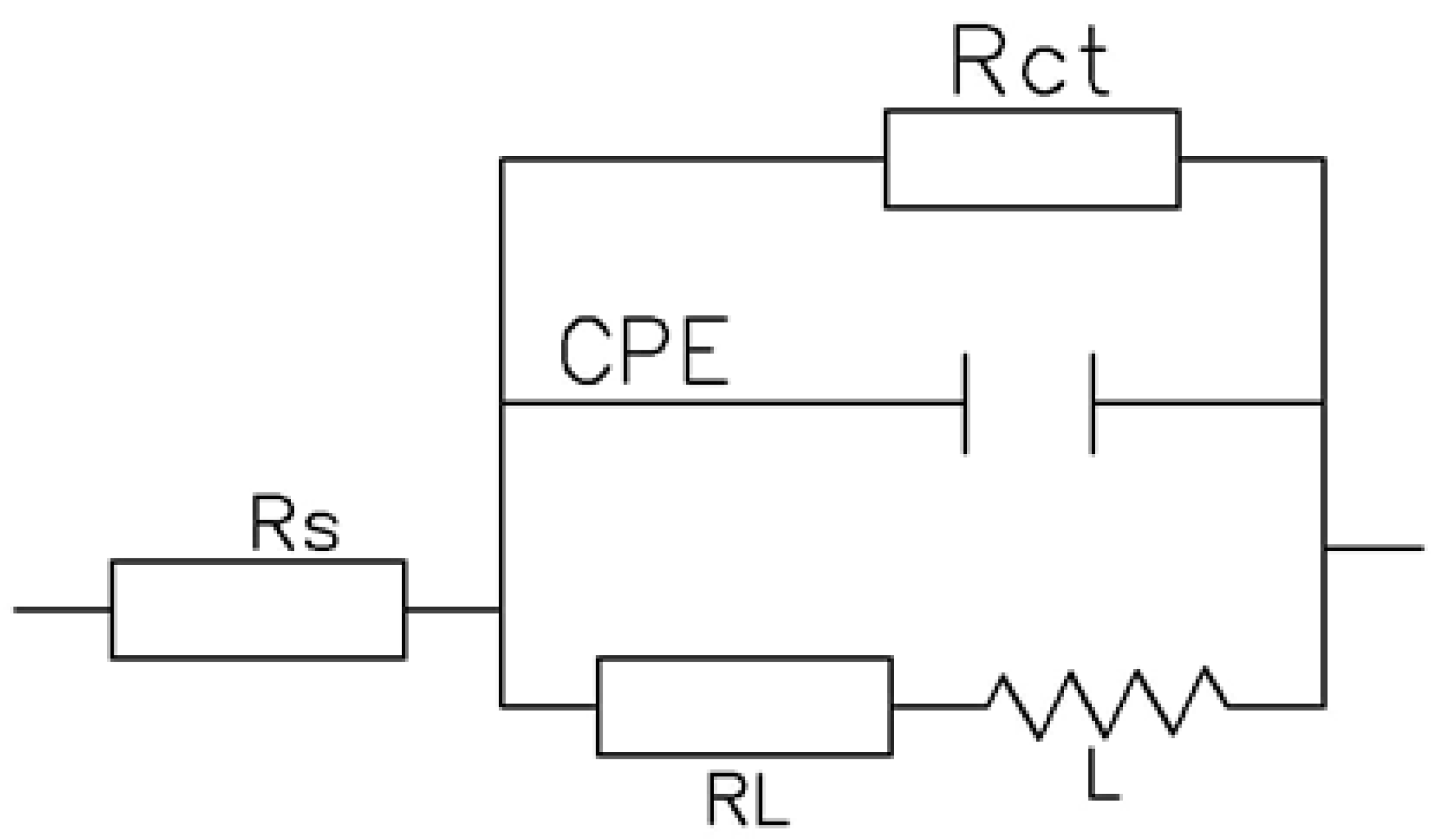
3.2. Electrochemical Behavior
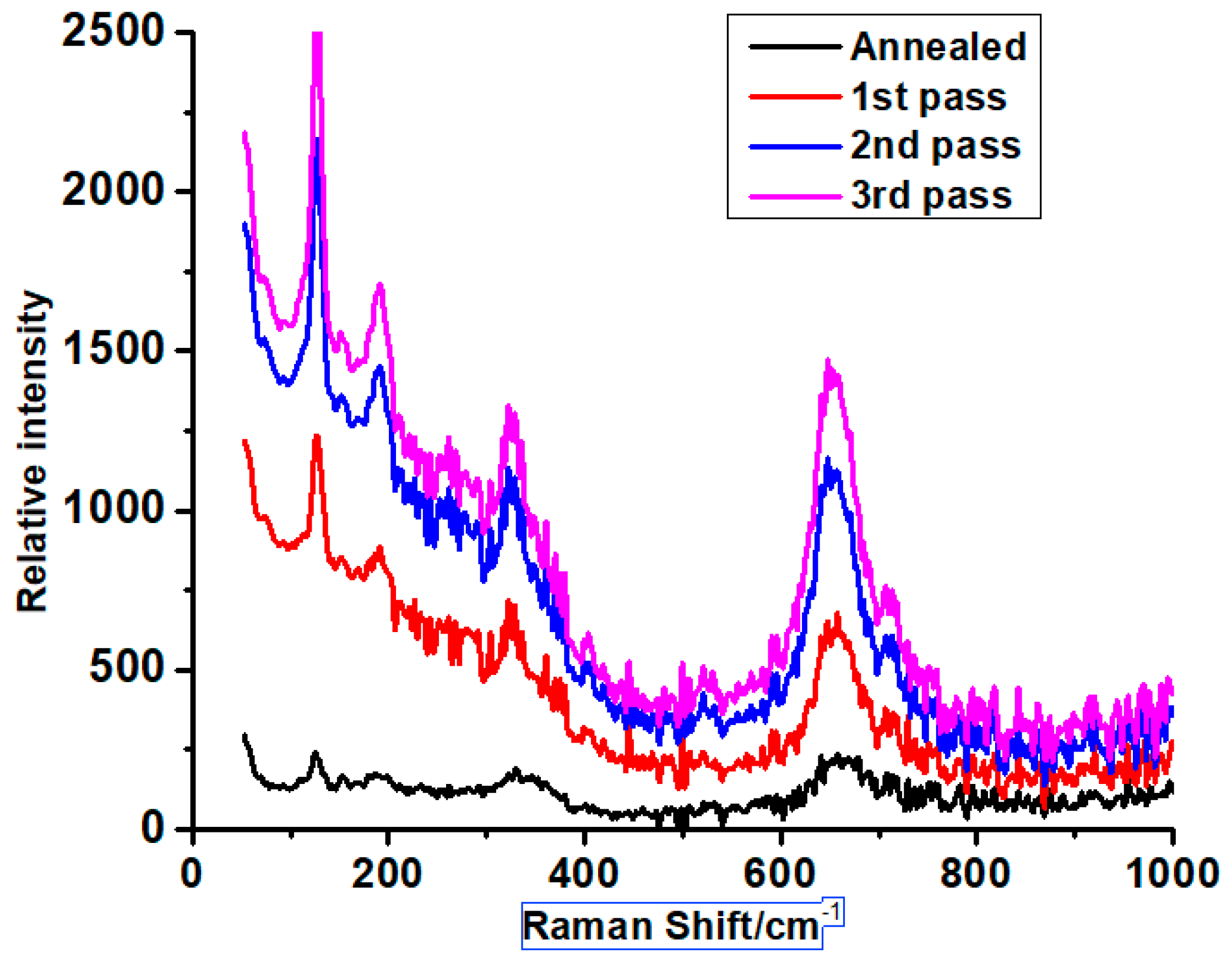
3.3. Raman Spectroscopy Analysis
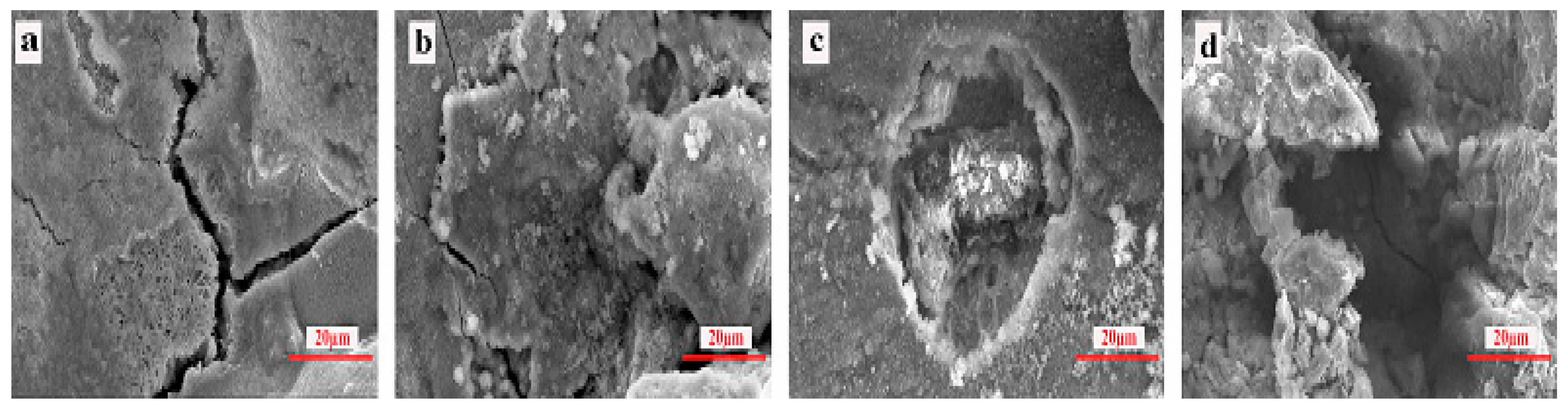
3.4. SEM Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, Y.C.; Li, L.T.; Xia, Y.C.; Jiang, Y.Q. Hot deformation and processing map of a typical Al–Zn–Mg–Cu alloy. J. Alloys Compd. 2013, 550, 438–445. [Google Scholar] [CrossRef]
- Maximov, J.T.; Duncheva, G.V.; Anchev, A.P. A new 3D finite element model of the spherical mandrelling process. Finite Elem. Anal. Des. 2014, 45, 1–14. [Google Scholar] [CrossRef]
- Zhou, M.; Lin, Y.C.; Deng, J.; Jiang, Y.Q. Hot tensile deformation behaviors and constitutive model of an Al–Zn–Mg–Cu alloy. Mater. Des. 2014, 59, 141–150. [Google Scholar] [CrossRef]
- Li, L.T.; Lin, Y.C.; Zhou, H.M.; Xia, Y.C.; Jiang, Y.Q. Modeling the high-temperature creep behaviors of 7075 and 2124 aluminum alloys by continuum damage mechanics model. Comput. Mater. Sci. 2013, 73, 72–78. [Google Scholar] [CrossRef]
- Birbilis, N.; Muster, T.H.; Buchheit, R.G. Corrosion of Aluminum Alloys. In Corrosion Mechanism in Theory and Practice; Marcus, P., Ed.; CRC Press: New York, NY, USA, 2012; pp. 705–736. ISBN 9781420094626. [Google Scholar]
- Frankel, G.S. Pitting Corrosion of Metals. J. Electrochem. Soc. 1998, 145, 2186. [Google Scholar] [CrossRef]
- Chen, G.S.; Gao, M.; Wei, R.P. Microconstituent-Induced Pitting Corrosion in Aluminum Alloy 2024-T3. Corrosion 1996, 52, 8–15. [Google Scholar] [CrossRef]
- Ly, R.; Hartwig, K.T.; Castaneda, H. Effects of strain localization on the corrosion behavior of ultra-fine grained aluminum alloy AA6061. Corros. Sci. 2018, 139, 47–57. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66. [Google Scholar] [CrossRef]
- Yulinova, A.; Nickel, D.; Frint, P.; Lampke, T. Electrochemical Properties of AL-6060 Alloy After Industrial-Scale ECAP. Mater. Sci. 2012, 48, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Sikora, E.; Wei, X.J.; Shaw, B.A. Corrosion Behavior of Nanocrystalline Bulk Al-Mg-Based Alloys. Corrosion 2004, 60, 387–398. [Google Scholar] [CrossRef]
- Son, I.J.; Nakano, H.; Oue, S.; Kobayashi, S.; Fukushima, H.; Horita, Z. Pitting corrosion resistance of anodized aluminum-copper alloy processed by severe plastic deformation. Nippon Kinzoku Gakkaishi/J. Jpn. Inst. Met. 2008, 72, 353–359. [Google Scholar] [CrossRef]
- Hockauf, M.; Meyer, L.W.; Nickel, D.; Alisch, G.; Lampke, T.; Wielage, B.; Krüger, L. Mechanical properties and corrosion behavior of ultrafine-grained AA6082 produced by equal-channel angular pressing. J. Mater. Sci. 2008, 43, 7409–7417. [Google Scholar] [CrossRef]
- Son, I.-J.; Nakano, H.; Oue, S.; Kobayashi, S.; Fukushima, H.; Horita, Z. Pitting Corrosion Resistance of Anodized Aluminum Alloy Processed by Severe Plastic Deformation. Mater. Trans. 2007, 48, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Segal, V.M.; Reznikov, V.I.; Drobyshevskiy, A.E.; Kopylov, V.I. Plastic working of metals by simple shear. Russ. Metall. 1981, 1, 99–105. [Google Scholar]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Progr. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- El-Danaf, E.A. Mechanical properties and microstructure evolution of 1050 aluminum severely deformed by ECAP to 16 passes. Mater. Sci. Eng. A 2008, 487, 189–200. [Google Scholar] [CrossRef]
- Hockauf, M.; Meyer, L.W.; Zillmann, B.; Hietschold, M.; Schulze, S.; Krüger, L. Simultaneous improvement of strength and ductility of Al–Mg–Si alloys by combining equal-channel angular extrusion with subsequent high-temperature short-time aging. Mater. Sci. Eng. A 2009, 503, 167–171. [Google Scholar] [CrossRef]
- Frint, P.; Halle, T.; Wagner, M.F.X.; Hockauf, M.; Lampke, T. Scaling up the equal-channel angular pressing process—A study on a 6000 aluminium alloy. Mat. Sci. Eng. Tech. 2010, 41, 814–821. [Google Scholar]
- Frint, P.; Hockauf, M.; Halle, T.; Strehl, G.; Lampke, T.; Wagner, M.F.X. Microstructural features and mechanical properties after industrial scale ECAP of an Al-6060 alloy. Mater. Sci. Forum 2011, 667–669, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Viceré, A.; Roventi, G.; Paoletti, C.; Cabibbo, M.; Bellezze, T. Corrosion Behavior of AA6012 Aluminum Alloy Processed by ECAP and Cryogenic Treatment. Metals 2019, 9, 408. [Google Scholar] [CrossRef] [Green Version]
- Son, I.J.; Nakano, H.; Oue, S.; Kobayashi, S.; Fukushima, H.; Horita, Z. Effect of equal-channel angular pressing on pitting corrosion resistance of anodized aluminium copper alloy. Trans. Nonferr. Met. Soc. China 2009, 19, 904–908. [Google Scholar] [CrossRef]
- Chung, M.K.; Choi, Y.S.; Kim, J.G.; Kim, Y.; Lee, J. Effect of the number of ECAP pass time on the electrochemical properties of 1050 Al alloys. Mater. Sci. Eng. A 2004, 366, 282–291. [Google Scholar] [CrossRef]
- Jiang, J.H.; Ma, A.B.; Lu, F.M.; Saito, N.; Watazu, A.; Song, D.; Zhang, P.; Nishida, Y. Improving corrosion resistance of Al–11 mass.% Si alloy through a large number of ECAP passes. Mater. Corr. 2011, 62, 848–852. [Google Scholar] [CrossRef]
- Dan, S.; Jiang, J.H.; Lin, P.H.; Yang, D.H. Corrosion behavior of ultrafine-grained industrial pure Al fabricated by ECAP. Trans. Nonferr. Met. Soc. China 2011, 19, 1065–1070. [Google Scholar]
- Akiyama, E.; Zhang, Z.; Watanabe, Y.; Tsuzaki, K. Effects of severe plastic deformation on the corrosion behavior of aluminium alloys. J. Solid State Electrochem. 2009, 13, 277–282. [Google Scholar] [CrossRef]
- Brunner, J.G.; Birbilis, N.; Ralston, K.D.; Virtanen, S. Impact of ultrafine-grained microstructure on the corrosion of aluminium alloy AA2024. Corr. Sci 2012, 57, 209–2014. [Google Scholar] [CrossRef]
- Segal, V.M. Materials processing by simple shear. Mater. Sci. Eng. 1995, 197, 157–164. [Google Scholar] [CrossRef]
- Valiev, R.Z. Structure and mechanical properties of ultrafine-grained metals. Mater. Sci. Eng. A 1997, 234–236, 59–66. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Korznikov, A.V.; Mulyukov, R.R. Structure and properties of ultrafine-grained materials produced by severe plastic deformation. Mater. Sci. Eng. 1993, 168, 141–148. [Google Scholar] [CrossRef]
- Sun, P.L.; Kao, P.W.; Chang, C.P. Effect of deformation route on microstructural development in aluminum processed by equal channel angular extrusion, met. Mater. Trans. A 2004, 35, 1359–1368. [Google Scholar] [CrossRef]
- Bi, J.; Sun, K.; Liu, R.; Fan, R.; Wang, S. Effect of ECAP pass number on mechanical properties of 2A12 al alloy. J. Wuhan Univ. Technol. Mat. Sci. Edit. 2008, 23, 71–73. [Google Scholar] [CrossRef]
- Gao, L.; Cheng, X. Microstructure and mechanical properties of Cu–10%Al–4%Fe alloy produced by equal channel angular extrusion. Mater. Des. 2008, 29, 904–908. [Google Scholar] [CrossRef]
- Khan, Z.A.; Chakkingal, U.; Venugopal, P. Analysis of forming loads, microstructure development and mechanical property evolution during equal channel angular extrusion of a commercial grade aluminum alloy. J. Mater. Process. Technol. 2003, 135, 59–67. [Google Scholar] [CrossRef]
- Kucukomeroglu, T. Effect of equal-channel angular extrusion on mechanical and wear properties of eutectic Al–12Si alloy. Mater. Des. 2010, 31, 782–789. [Google Scholar] [CrossRef]
- Gao, L.L.; Cheng, X.H. Microstructure and dry sliding wear behavior of Cu 10%Al–4%Fe alloy produced by equal channel angular extrusion. Wear 2008, 265, 986–991. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Du, Z.; Qu, J.; Sun, Y.; Luo, S. Enhancing room temperature mechanical properties of Mg–9Al–Zn alloy by multipass equal channel angular extrusion. J. Mater. Process. Technol. 2010, 210, 751–758. [Google Scholar] [CrossRef]
- Mallikarjuna, C.; Shashidhara, S.M.; Mallik, U.S. Evaluation of grain refinement and variation in mechanical properties of equalchannel angular pressed 2014 aluminum alloy. Mater. Des. 2009, 30, 1638–1642. [Google Scholar] [CrossRef]
- Ramu, G.; Bauri, R. Effect of equal channel angular pressing (ECAP) on microstructure and properties of Al–SiCp composites. Mater. Des. 2009, 30, 3554–3559. [Google Scholar] [CrossRef]
- Tham, Y.W.; Fu, M.W.; Hng, H.H.; Yong, M.S.; Lim, K.B. Bulk nanostructured processing of aluminum alloy. J. Mater. Process. Technol. 2007, 192–193, 575–581. [Google Scholar] [CrossRef]
- Pürçek, G. Improvement of mechanical properties for Zn–Al alloys using equal-channel angular pressing. J. Mater. Process. Technol. 2005, 169, 242–248. [Google Scholar] [CrossRef]
- Saray, O.; Purcek, G. Microstructural evolution and mechanical properties of Al–40 wt.%Zn alloy processed by equal-channel angular extrusion. J. Mater. Process. Technol. 2009, 209, 2488–2498. [Google Scholar] [CrossRef]
- Tolaminejad, B.; Dehghani, K. Microstructural characterization and mechanical properties of nanostructured AA1070 aluminum after equal channel angular extrusion. Mater. Des. 2012, 34, 285–292. [Google Scholar] [CrossRef]
- Adedoku, S.T. A review on equal channel angular extrusion as a deformation and grain refinement process. J. Emerg. Trends Eng. Appl. Sci. (JETEAS) 2011, 2, 360–363. [Google Scholar]
- Javidikia, M.; Hashemi, R. Mechanical anisotropy in ultra-fine grained aluminium tubes processed by parallel-tubular-channel angular pressing. Mater. Sci. Technol. 2017, 33, 2265–2273. [Google Scholar] [CrossRef]
- Mohammadtaheri, M. A new metallographic technique for revealing grain boundaries in aluminum alloys. Metallogr. Microstruct. Anal. 2012, 1, 224–226. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.H.; Park, J.W.; Lee, K.H. An analysis of accumulated deformation in the equal channel angular pressing (ECAP) process. Met. Mater. Int. 2006, 12, 289–292. [Google Scholar] [CrossRef]
- Gorman, J.D.; Hughes, A.E.; Jamieson, D.; Paterson, P.J.K. Oxide formation on aluminum alloys in boiling deionised water and NaCl, CeCl3 and CrCl3 solutions. Corros. Sci. 2003, 45, 1103–1124. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting corrosion of aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Lee, J.-C.; Seok, H.-K.; Suh, J.-Y. Microstructural evolutions of the Al strip prepared by ECAP and continuous equal channel angular pressing electrochemical behaviour. Acta Materialia 2002, 50, 4005–4019. [Google Scholar] [CrossRef]
- Nam, C.Y.; Han, J.H.; Chung, Y.H.; Shin, M.C. Effect of precipitates on microstructural evolution of 7050 Al alloy sheet during equal channel angular pressing effect in corrosion. Mater. Sci. Eng. A 2003, 347, 253–257. [Google Scholar] [CrossRef]
- Kubásek, J.; Dvorský, D.; Čavojský, M.; Vojtěch, D.; Beronská, N.; Fousová, M. Superior properties of Mg–4Y–3RE–Zr alloy prepared by powder metallurgy. J. Mater. Sci. Technol. 2017, 33, 652–660. [Google Scholar] [CrossRef]
- Shuzhen, Z.; Lining, X.; Juanjuan, D.; Wei, C.; Minxu, L. Influence of acetic acid on top localized corrosion of x70 steel pipeline in CO2 containing wet gas. J. Chin. Soc. Corros. Prot. 2016, 36, 231–237. [Google Scholar]
- Farelas, F.; Ramirez, A. Carbon dioxide corrosion inhibition of carbon steels Through bis-imidazoline and imidazoline compounds studied by EIS. Int. J. Electrochem. Sci. 2010, 5, 797–814. [Google Scholar]
- Seikh, A.H.; Baig, M.; Ammar, H.R.; Alam, M.A. The influence of transition metals addition on the corrosion resistance of nanocrystalline al alloys produced by mechanical alloying. Metals 2016, 6, 140. [Google Scholar] [CrossRef] [Green Version]
- Khadiri, A.; Ousslim, A.; Bekkouche, K.; Aouniti, A.; Elidrissi, A.; Hammouti, B. Phenolic and non-phenolic Fractions of the Olive Oil Mill Wastewaters as Corrosion Inhibitor for steel in HCl medium. Port. Electrochim. Acta 2014, 32, 35–50. [Google Scholar] [CrossRef]
- Wu, J.; Xue, W.; Wang, B.; Jin, X.; Du, J.; Li, Y. Characterization of carburized layer on T8 steel fabricated by cathodic plasma electrolysis. Surf. Coat. Technol. 2014, 245, 9–15. [Google Scholar] [CrossRef]
- Guo, X.; Guo, Q.; Du, K.; Wang, Y.; Wang, F. Study of filiform corrosion inhibition by a compact plasma electrolytic oxidation film on a AZ31 Mg alloy. RSC Adv. 2016, 6, 39053–39062. [Google Scholar] [CrossRef]
- Teymoory, P.; Zarei-Hanzaki, A.; Farabi, E.; Monajati, H.; Abedi, H.R. Grain refinement through ECAP in severely plastic deformed A206 aluminum alloy. Adv. Eng. Mater. 2018, 20, 1700502. [Google Scholar] [CrossRef]
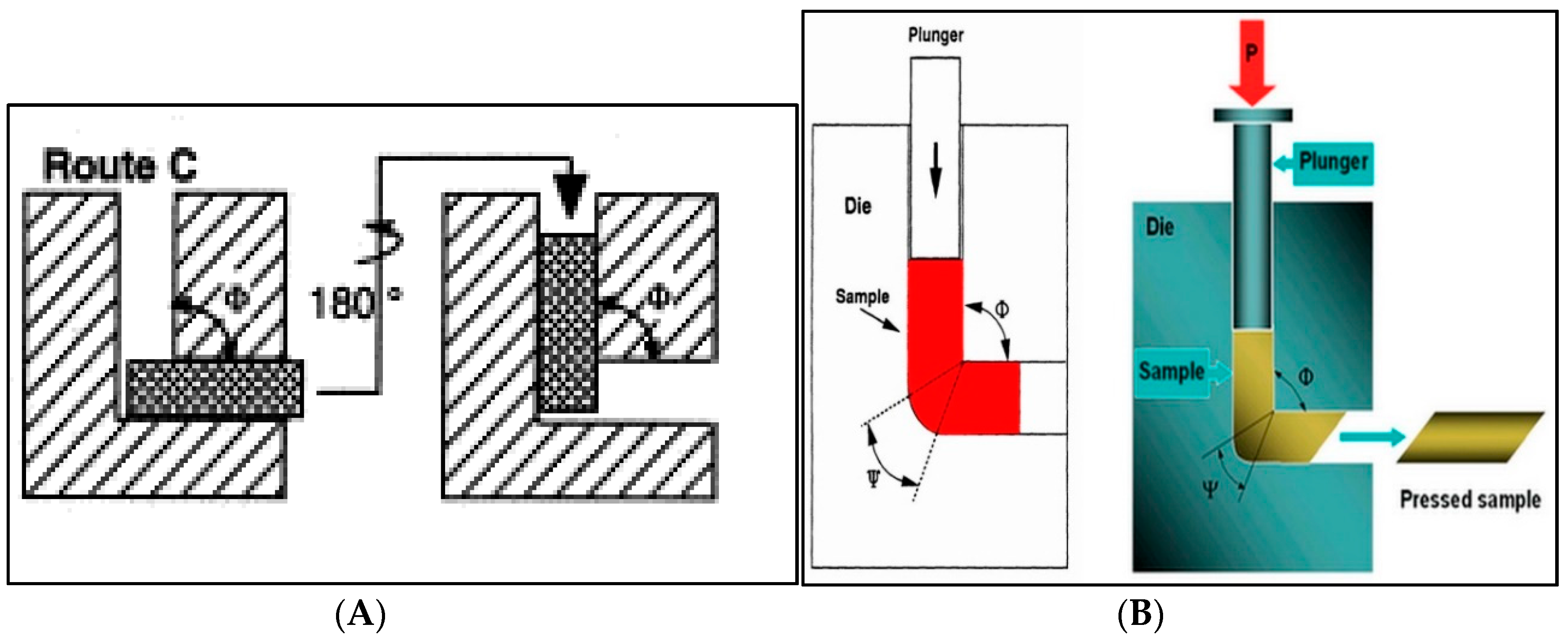

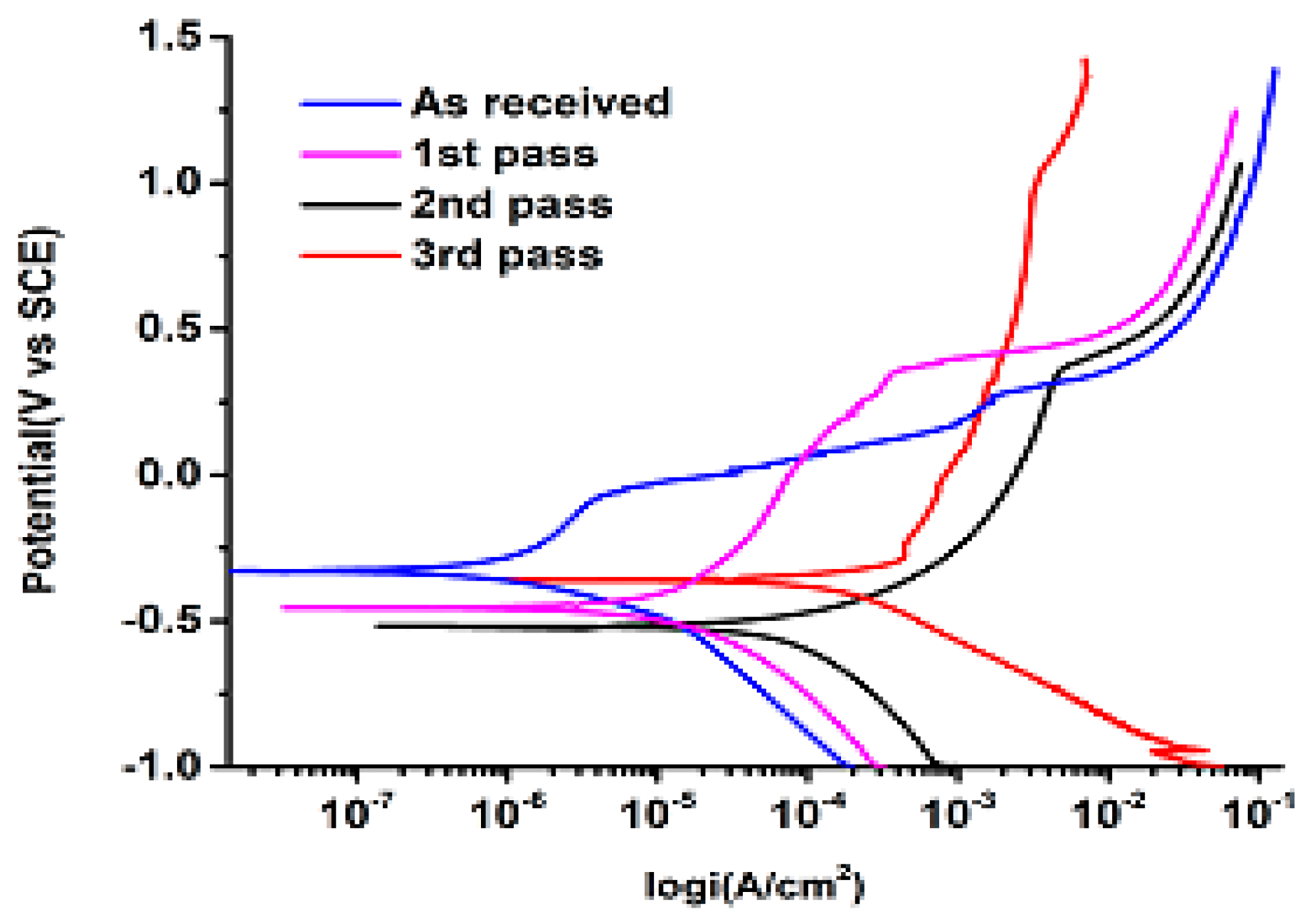
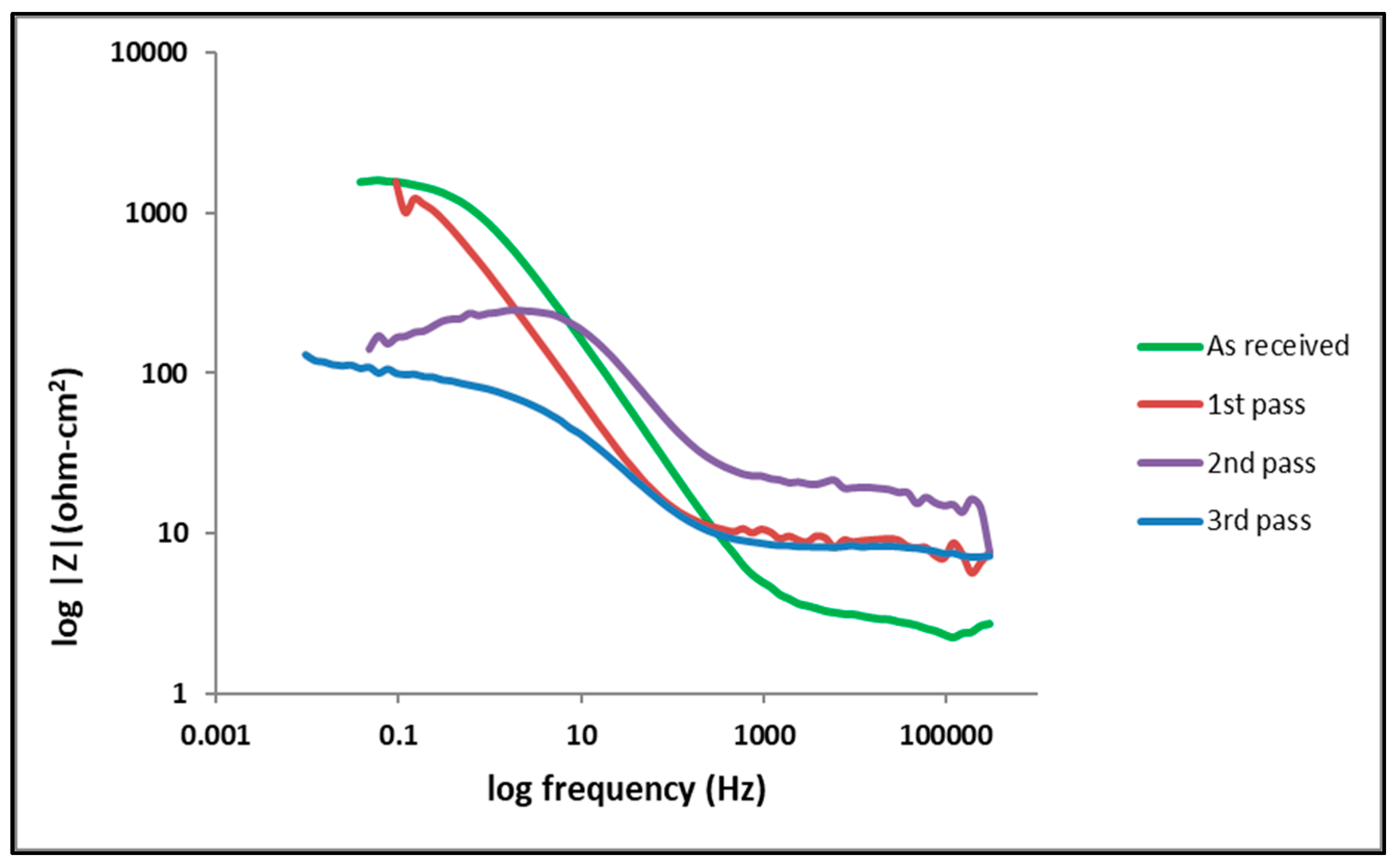

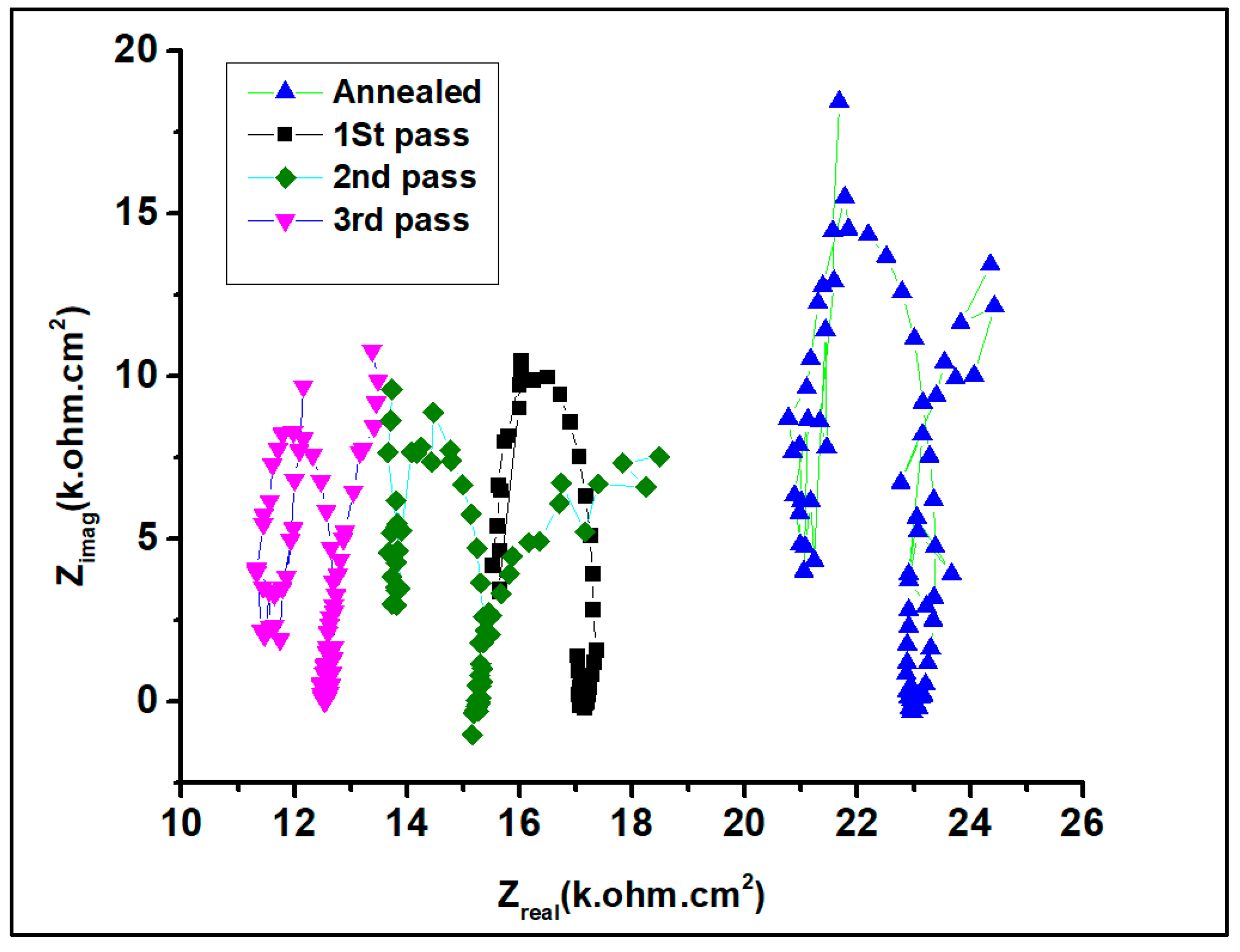
| Sample | icorr (A/cm2) | Ecorr (V) |
|---|---|---|
| Annealed | 1 × 10−6 | −3.29 × 10−1 |
| First pass | 2 × 10−5 | −4.55 × 10−1 |
| Second pass | 1 × 10−4 | −5.18 × 10−1 |
| Third pass | 3 × 10−4 | −3.59 × 10−1 |
| Sample | Rs (Ω cm2) | CPE (µF·Cm−2·S(n−1)) | n | Rct (Ω cm2) | RL (Ω cm2) | L (H cm−2) |
|---|---|---|---|---|---|---|
| As-received | 24.67 | 29.4 | 0.95 | 23.67 × 102 | 2127 | 1873 |
| First pass | 20.74 | 33.3 | 0.90 | 20.84 × 102 | 448 | 365 |
| Second pass | 23.44 | 48.6 | 0.85 | 19.44 × 102 | 302 | 213 |
| Third pass | 22.96 | 50.4 | 0.80 | 15.46 × 102 | 182 | 115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seikh, A.H.; Baig, M.; Ur Rehman, A. Effect of Severe Plastic Deformation, through Equal-Channel Angular Press Processing, on the Electrochemical Behavior of Al5083 Alloy. Appl. Sci. 2020, 10, 7776. https://doi.org/10.3390/app10217776
Seikh AH, Baig M, Ur Rehman A. Effect of Severe Plastic Deformation, through Equal-Channel Angular Press Processing, on the Electrochemical Behavior of Al5083 Alloy. Applied Sciences. 2020; 10(21):7776. https://doi.org/10.3390/app10217776
Chicago/Turabian StyleSeikh, Asiful H., Muneer Baig, and Ateekh Ur Rehman. 2020. "Effect of Severe Plastic Deformation, through Equal-Channel Angular Press Processing, on the Electrochemical Behavior of Al5083 Alloy" Applied Sciences 10, no. 21: 7776. https://doi.org/10.3390/app10217776





