1. Introduction
Although the function of a muscle is determined by its anatomical arrangement, the real effect of muscle contraction during function depends on the state of contraction of other muscles, on the external forces applied to the body, and on the so-called “dynamic coupling” among the anatomical segments. For example, during walking, excessive activity of the ankle plantar flexors at the beginning of the stance phase can lead to knee hyperextension and pelvis internal rotation in cerebral palsy children [
1]. A similar contraction occurring at the late stance phase can produce external rotation of the pelvis and, depending on the level of gastrocnemius contribution, knee flexion instead of knee hyperextension. Quadriceps and hamstring muscle co-contractions can have different effects at different joints configurations [
2], and it appeared that the activity of vasti (VA) associated with hamstrings can enhance the extensor effect of these muscles at the hip, particularly when the knee and the hip are relatively flexed.
To understand the role of the muscles involved in locomotion a study should include an analysis of the muscle activity and also an estimate of what happens if the muscle activity changes.
In the present work, we adopted this approach to investigate the role of rectus femoris (RF) on the execution of the swing phase during walking. In fact, several factors acting at late stance-early swing can affect the swing phase: hip acceleration, ankle plantar flexion, ground reaction forces, inertia, but the ability of RF (a proximal, double joint muscle) to control the distal segments seems to play a relevant role in controlling the movement amplitude and the position of knee and foot at the end of the swing phase. From a clinical point of view, a more thorough understanding of the effects of the RF activity can be useful in planning treatment. Swing phase pathologies are difficult to understand, especially because the knowledge on the effect of RF during swing already in normal conditions is insufficient. If it changes activity, the mechanical effects can be even less well estimated.
Previous studies have shown that the RF activity is present at late stance-early swing to control knee flexion [
3,
4,
5]. Nene et al. [
6] showed that the pattern of this activity depends on gait velocity and also on the angular velocity of the shank. Although some crosstalk of electromyographic (EMG) signals occurs between VA and RF [
7], the same authors have concluded that during the initial swing the recorded activity mostly originated from the RF. Additionally, in a recent publication [
8], the most recurrent pattern of activation was shown to be composed of three phasic activities: at the beginning of the gait cycle, around toe-off, and at the terminal swing. The activity corresponding to the late stance-early swing was considered consistent with the need to control the knee flexion. Muscle-actuated forward dynamic simulations of the swing phase of normal gait further demonstrated that the RF is important to control knee flexion during swing [
9]. Its removal resulted in increased knee flexion during the swing phase which was even more pronounced in pre-swing than in early swing [
10]. According to other authors [
11], the RF was mainly active when a combination of both hip flexion and knee extension moments were required, and not when the knee extension moment was combined with a hip extension moment. This is consistent with the anatomical arrangement of the RF that acts as a hip flexor and knee extensor, and thus it seems particularly suitable when both of these effects are required during movement. However, the following questions remain open and this study was aimed at finding a fundamental answer to them:
Does the RF only control knee flexion in the early swing phase by breaking the movement or does it also actively extend the knee in the second phase?
To what extent are joint kinematics and the segmental position at initial contact altered when the RF is removed?
To what extent are joint kinematics influenced by RF hyper-activity at late stance-early swing?
2. Materials and Methods
RF activity during gait has been described by several authors [
3,
4,
5,
6,
7,
8]. We in contrast, were not interested in activity patterns, but in data of one single normal individual which we modified in a forward modelling study in order to understand the principle of the RF effect. Difference in segment sizes and proportions depending on age may change the values but not the principle as the gait pattern after four years of age changes little [
12].
- (a)
Data acquisition and models.
We performed an inverse dynamics analysis as a first step. Since our study was mostly theoretical, we just used an exemplary collection of data from one normal subject as the input to our model. Kinematic and kinetic data referred to a 23-years-old woman (weight 59.1 kg; height 164 cm) who was walking barefoot at her natural speed in a gait analysis laboratory (motion analyzer Vicon Nexus 1.8.5, 12 cameras, Oxford Metrics plc, Oxford, United Kingdom; 4 dynamometric platforms, Kistler, Winthertur, Switzerland; and a 12 channels Noraxon EMG system, Noraxon, Scottsdale, AZ, USA). Data were processed according to a standard protocol [
13], and were imported into a musculoskeletal model of the AnyBody Software Version 7.0 (AnyBody Technologies, Aalborg, Denmark). An average of six trials was used for the inverse dynamic simulation, which allowed us to obtain joint moments and individual muscle forces and muscle power.
In a second phase, normal gait data were imported into a software platform (SimWise-4D, Design Simulation Technologies, Canton, MI, USA) in which a model of a human subject was constructed for dynamics simulation, with some additional features with respect to a previously developed model [
1,
2]. The model was composed of solid segments representing the trunk, the pelvis, the thigh, the shank, and the foot for both the left and the right sides. The mass and moments of inertia of these solids were tailored on the subject based on anthropometric tables [
14]. The segments were connected to each other by revolute joints, allowing several degrees of freedom: three between trunk and pelvis, three at the hips, two at the knees (flexion/extension, internal/external rotation), and two at the ankle (dorsi/plantar flexion, inversion/eversion). Each revolute joint could be controlled by the corresponding joint angle time course, so that the normal kinematics could be reproduced. In this case the joint moments resulting from the dynamic equilibrium of forces and moments applied to the body segments were obtained (inverse dynamics problem). In a different approach, the revolute joints could be left free to rotate (no internal moment was produced) and the model provided the kinematics resulting from forces and moments applied to the system (forward dynamics problem). Digital anatomical models of pelvis, femur, tibia, and fibula were superimposed to the corresponding solid segments to obtain a reference for the muscle attachment points. Several muscles of the lower limb, represented by linear actuators, were then attached to the model: gluteus maximus, iliopsoas, semimembranosus, semitendinosus, biceps femoris long head and short head, gastrocnemius medialis and lateralis, soleus, tibialis anterior, RF, and VA (intermedius, lateral, medial). The distal tendons of some muscles (gluteus maximus, iliopsoas, semimembranosus, quadriceps femoris, gastrocnemii) were reproduced by a chain of small cylinders that could slide over wrap surfaces representing the bone surfaces. The quadriceps tendon was connected to a parallelepiped representing the patella. This, in turn, was connected to the anterior tuberosity of the tibia by a rope representing the patellar tendon. The patella could slide over a wrap surface representing the femoral trochlea. For the present use of the model, all muscles except the RF were considered inactive (just a 1 N force was imposed to keep the tendons in place). The RF force instead, was input into the model with a predefined amplitude and time course and was modulated, in the subsequent simulations, as to represent a progressive weakening of the muscle or, at the contrary, an exceeding activation. Ground reaction forces and moments obtained from gait analysis were applied to the foot. To analyze the effects of changing the RF force, we first computed the moments produced by its complementary synergistic muscles: the VA at the knee, and the Iliopsoas at the hip. This was done by running the simulation in an inverse dynamics mode, with normal kinematics and RF force as input. Since the RF force produced a moment at hip and knee, the joint moments computed by the model represented the additional contribution required to other muscles than RF. These moments were named the “residual” moments. When the model was used in a forward dynamics mode, the residual moments plus the moments produced by the RF force reproduced exactly the previous kinematics.
To simulate the effect of different RF forces we just changed the RF force in a predetermined time window and we obtained different kinematics. This was the objective of our study.
In this approach, the complementary muscles were supposed to be unaffected by changes of the RF force, so that this condition was considered: “without compensation”. We also considered the possibility of compensation by other muscles to the change of RF force. Of course, it is difficult to imagine what strategy the motor control system can adopt. For the sake of simplicity, we assumed that compensation consisted in reproducing a percentage of the moment originally produced by the RF. For example, 0% compensation means that kinematics will be determined by the residual moments only; 50% compensation means that the residual moments will be incremented by 50% of the moments originally produced by RF; 100% compensation means that the moments originally produced by RF are entirely added to the residual moments so that the resulting kinematics are exactly the original ones.
This criterion of simulating compensations to the altered RF force was applied in two different conditions: hip joint constrained by the predefined, normal kinematics (this represents a full compensation at the hip joint) and both hip and knee free to move under the effect of the forces and moments applied.
According to the purpose of our study, RF force alterations were applied in the time window from 50% to 80% of the gait cycle, which corresponded to the late stance-early swing phase.
- (b)
Simulations
At first, we defined a hypothetical RF force for the time window of interest. The force predicted by the Anybody model, based on static optimization, was deemed to be appropriate. The time course was in line with the activation profiles of the RF muscle described in the literature [
3,
4,
15], although the peak amplitude was relatively higher, and in our simulations produced residual moments close to zero or even negative. We then downscaled the RF force as to have a peak of about 300 N corresponding to the peak value reported in Anderson and Pandy 2001 [
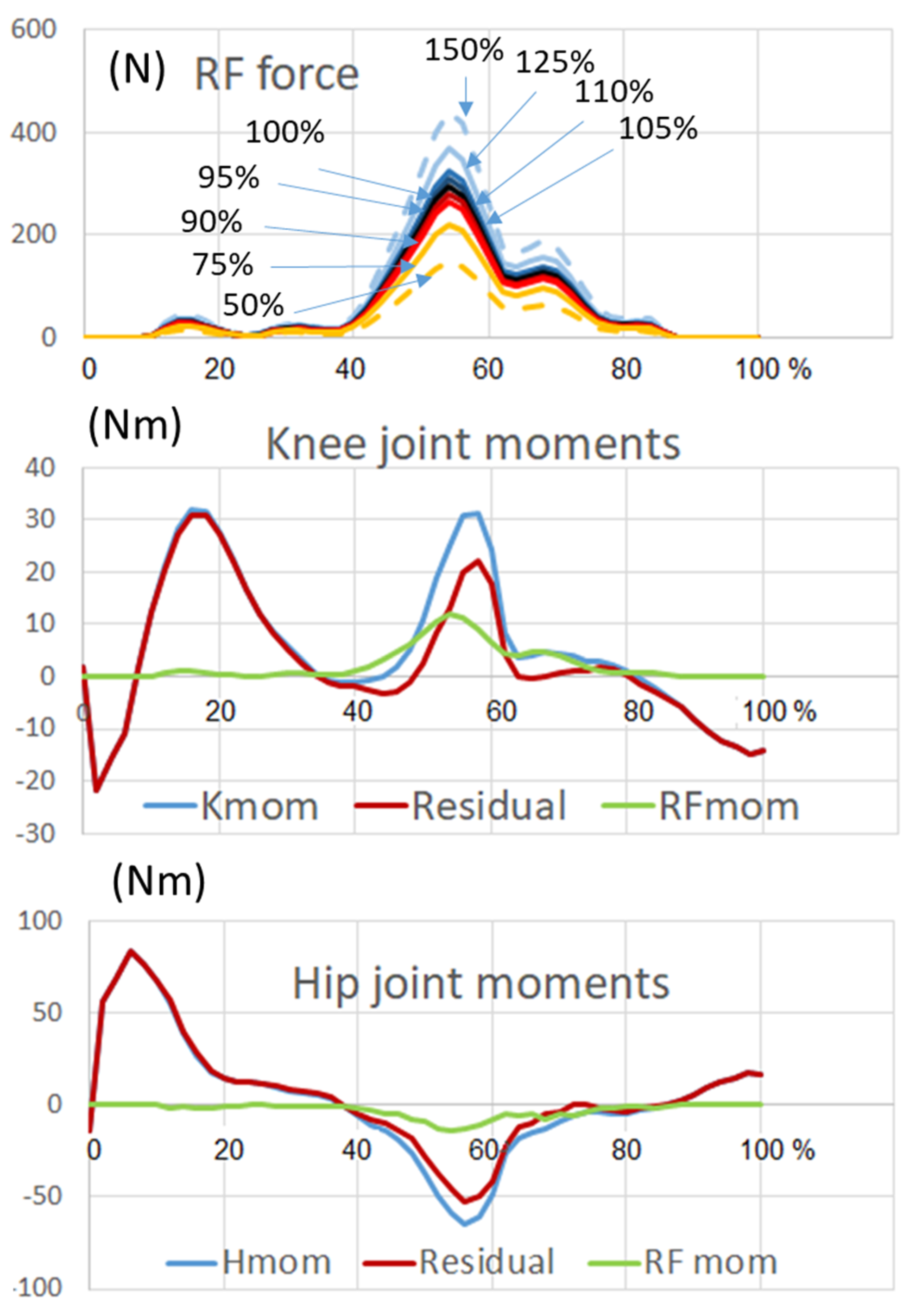
16]. In this way, the moment produced by RF at the knee was about 40% of the total knee extensor moment (
Figure 1), while the residual 60% was produced by the complementary muscles (the VA).
Several simulations were then carried out by imposing the unperturbed kinematics at the hip and letting the knee rotate freely. When the residual knee moment and the RF force (100%) were applied all together, the resulting kinematics were superimposable to the one obtained with imposed kinematics (unperturbed stride movement). Next, in a time window from 50% to 80% of the stride time, we set the RF force to zero in order to simulate a complete elimination of the RF muscle, without compensation from any other knee extensor muscle. Then, we simulated a partial compensation by adding an RF force corresponding to 50%, 75%, 90%, and 95% of the original RF force. To better understand the role of the RF, we also simulated a hyper-activity of this muscle by setting its force to 105%, 110%, 125%, and 150% of the original force. The same sequence of simulations was then repeated by letting both the knee and the hip joints free to rotate.
3. Results
- (a)
Inverse dynamics:
The RF moment at the knee joint obtained from the Anybody computation was in agreement with the EMG activity known from other studies [
4,
6,
8]. The power of RF muscle was also computed and is reported in
Figure 2 (red line). This variable synthetically represents the energetic contribution of RF to the knee joint movement.
Figure 2 shows that in a normal situation the power is negative (energy is absorbed) from 40 to 71% of the gait cycle in correspondence with the knee flexion occurring at the late stance-early swing phase. From 75 to 85% of the gait cycle where the extension of the knee started, the power became positive. This indicates that the RF was transferring energy to the shank and was involved in active knee extension. The power of VA was computed in two different conditions: when RF was contributing as a part of the quadriceps muscle (green line in
Figure 2) and without the contribution of RF (paralyzed muscle, blue line). The sequence of negative and positive power observed at early stance phase (out of interest in this study) was dominated by the VA contribution, so that no difference appears between presence or absence of RF (the green band is completely hidden by the blue band). In the phase of interest (late stance-early swing) the negative power of the VA was very small when RF was present (green line) and became higher, in absolute, than the power required by the RF in normal conditions when RF was paralyzed (blue line).
The missing negative power of the paralyzed RF was replaced by a greater negative power of the VA. Between 75 and 87% of the gait cycle. Further, there was a positive power at the knee joint produced by the VA, which replaced the activity of the paralyzed RF. This indicates that the positive muscle power of the knee extensors was necessary during this phase. It underlines that the initial extension of the knee in swing phase at 75% of the gait cycle (which corresponds to muscular power generation) was not only a passive motion induced by a double pendulum mechanism but also depended on knee extensor action, mainly the one of RF in normal conditions, or of VA in case of a paralyzed RF.
- (b)
Forward modeling
We first considered the condition of hip kinematics unchanged (full compensation at the hip by the hip muscles,
Figure 3). This of course is a hypothetical situation that only can be reproduced in simulation, but it helps understanding the separate effect of RF at the knee joint. With the RF in place (100% RF force and residual knee moments applied), the model performed a normal step as expected. After removal of the RF and without any compensation from other knee extensor muscles (0% RF force and residual moments applied), exaggerated knee flexion and foot rising occurred in swing, which prevented the foot from getting in contact with the ground at the end of the stride cycle (
Figure 3, left panel). The same occurred at 50% of RF force. At 75% of RF force, the step was still incomplete. In clinical terms this would correspond to abnormal foot contact occurring with the toes (initial toe contact). At 90% and 95% of the RF force, the foot recovered a position, at the end of the stride cycle, that was compatible with foot plant initial contact and heel initial contact, respectively.
When the RF was hyperactive, a reduced knee flexion was observed (
Figure 3, right panel) that in some cases prevented the foot clearance (the forefoot moved below the ground level). As shown in
Figure 3, knee extension was faster than at basic condition, so that at the time corresponding to stride duration the knee was hyperextended and the forward displacement of the foot was exaggeratedly enhanced.
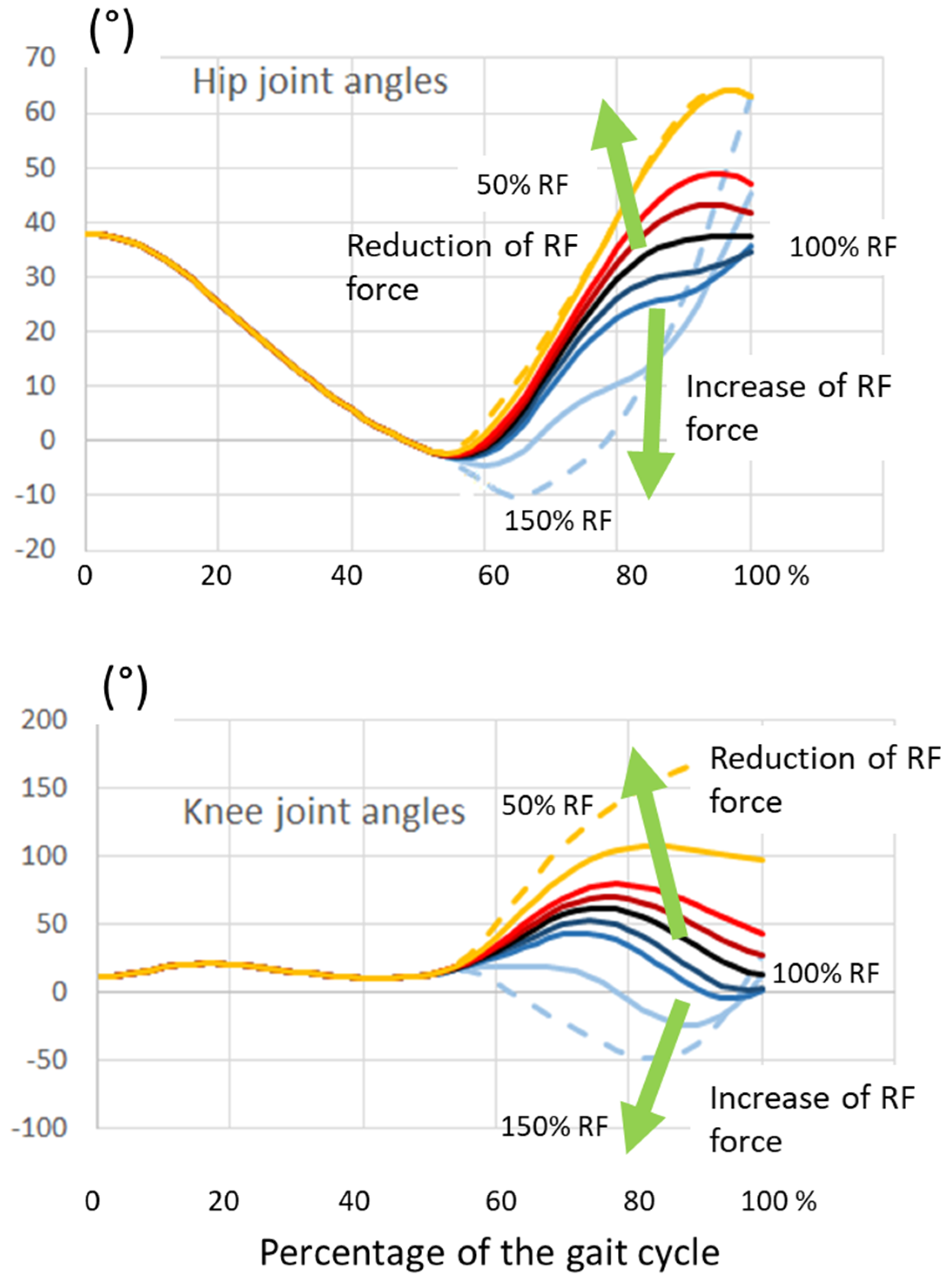
Figure 4 depicts the knee flexion angles for all the simulated percentages of RF force compensation (50%, 75%, 90%, 95%, 100%) and RF hyperactivation (105%, 110% 125%, 150%). It clearly reveals that knee flexion increases as far as the RF force decreases, and, at the contrary, knee flexion decreases while the RF force increases.
Similar effects of RF removal and hyper-activation were obtained when the hip joint as well as the knee joint was left free to rotate under the effect of the RF force and residual hip moment (hip kinematics were not imposed). The entire lower limb kinematics dramatically changed for large reductions in RF force (condition 50% and 75% in
Figure 5).
Less apparent deviations were observed for RF force enhancement. For example, the increase in stride length seemed not too big at 150% RF (
Figure 5, right panel). However, it has to be noted that the foot largely moves below ground level, and this is related to reduced movement at both the hip and knee. In clinical terms, this would correspond to great difficulty to obtain foot clearance.
Figure 6 shows the changes in hip and knee joint angles. Decreasing the RF force produced an increase in both hip and knee flexion; the opposite occurred with increasing RF force. Compared to
Figure 4, the reduction in knee flexion was more pronounced in this case in which the RF also had an effect at the hip.
Figure 3 and
Figure 5 show that when the RF force is decreased, more time would be required for the foot to reach the ground. Therefore, we allowed the simulation to continue beyond the cycle time without any internal joint moment in order to get the position of the foot (angle and step length) at the foot–floor contact time. We also computed the same parameters in the “increased RF force” simulations at the time where the foot was in a proper position to contact the ground.
Figure 7 shows the respective foot positions, and
Figure 8 reports the step parameters.
Note that these figures do not report the results of when the RF force reduced to 50%, since the foot did not reach the ground. As well, it was not possible to calculate the step parameters for the RF force above 120%, when both hip and knee were free to move, because they stayed almost fixed, and the foot soon penetrated the ground.
4. Discussion
The role of the RF as a bi-articular muscle during the gait cycle even in normal human walking is difficult to understand. The effect of this muscle on the mechanics of swing is even more difficult to estimate as the segments of the leg are accelerated in pre- and early swing while muscle activity during the later swing phase is low. The anatomical situation suggesting that the RF acts as a hip flexor and a knee extensor at the same time, and also the possibility that other muscles can compensate for its weakness [
17] makes it difficult to make conclusions about the effects of this muscle on each joint. Several studies based on EMG [
6,
7,
8,
11] and musculoskeletal modelling [
1,
2,
18,
19] have revealed that the RF is important during the transition from stance phase to swing phase to control knee flexion. Its detailed function during this period and its consequence on gait kinematics especially during swing phase were the main focus of this study.
At the knee level, the RF indeed controlled knee flexion from 40 to 75% of the gait cycle. This was achieved, as shown in our inverse dynamics analysis, by absorbing mechanical power and breaking the knee flexion movement. Without knee extensors, the knee would flex more extensively. After the peak of knee flexion, however, RF started to produce a positive power, which resulted in knee extension from 75 to 85% of the gait cycle. After this push, no more muscle power was necessary to further extend the knee because the acceleration of the leg followed a double pendulum movement controlled by negative hamstring power. The importance of the initial push for knee extension during swing was described for stiff knee gait but without considering the effect on foot position at terminal swing [
10].
The forward simulation indeed showed that the RF accelerated the shank segment into an extension movement of the knee. Thus, the RF controls step length and is one factor that determines an adequate foot prepositioning. This has clinical significance, as with reduced knee extensor force (RF removal or weakening), the knee remains flexed at late swing, which results in a short step and initial toe contact. The simultaneous effect of the RF on the hip, however, had little influence when the activity was reduced. In contrast, hyperactivity led to more knee stiffness if the RF had effects on both joint levels, leading to a foot clearance problem. These forward modelling results are of clinical interest, even though our model, of course, can only simulate the mechanical aspects and not the control strategies that a patient would adopt to compensate the RF alterations. We have shown that stiffness at the knee during the swing phase, at least in a healthy person, depends on the increased RF action at both joint levels (although more strongly at the knee). As RF activity during gait has been previously described [
3,
4,
5,
6,
7,
8], we limited our study to its mechanical effect on swing phase kinematics in principle, which required gait data from one normal person only as an example. The amount of the values may change with changing segment sizes and proportions. However, the anatomy is similar and the gait pattern changes little after the age of four [
12]. The effect of bi-articular muscles during gait depends on the anatomy but also on the interplay with other muscles crossing the joints and on physical parameters such as acceleration and inertia. In a clinical situation with pathological gait, the situation is even more complicated. We demonstrated the effects of lower and higher than normal RF activities on gait mechanics, especially with reference to the foot position. RF accelerates the leg segments in late stance-early swing and hence it can affect the whole subsequent movements: an adequate knee extension at late swing and preparation of the leg for the initial heel contact. As shown in our simulations, RF weakness may result in toe walking. This effect may be considered especially for patients who already have problems with knee extension during swing phase and exhibit initial toe contact. Hyperactivity, in contrast, can hinder foot clearance. Both situations occur in clinical practice. The new knowledge may help to identify RF as a possible factor. More sophisticated diagnostic tools are required to facilitate the process of treatment decision-making. Incorporation of musculoskeletal modelling into clinical work may be one promising solution.