Comparative Analysis of Wheat Hay and Silage in Methane Production, Fermentation Characteristics and Microbiota Using In Vitro Rumen Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Inoculum and Incubation
2.3. Sampling and Chemical Analyses
2.4. DNA Extraction and 16S rRNA Pyrosequencing
2.5. Sequence Analyses
2.6. Statistical Analysis
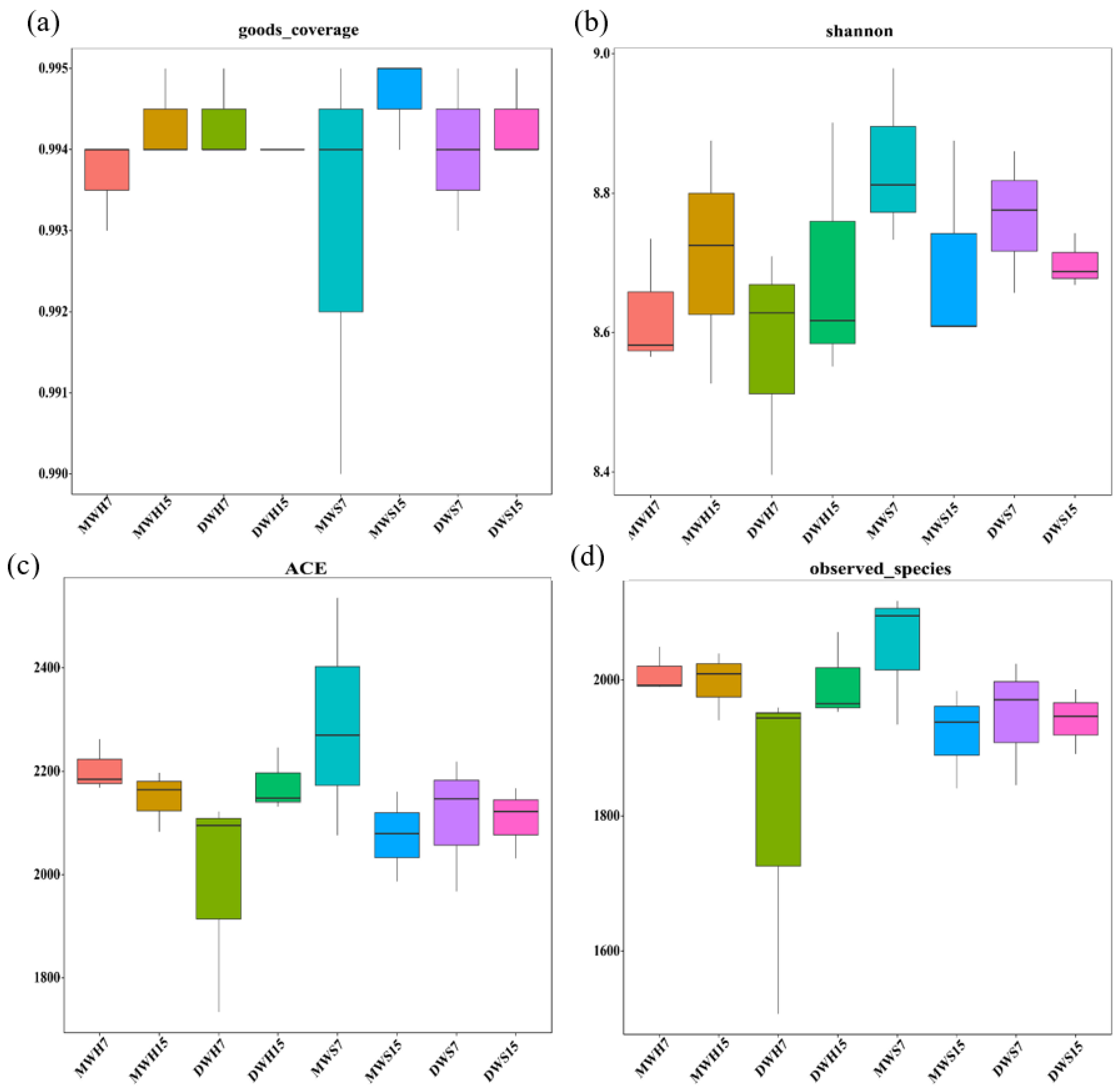
3. Results
3.1. Differences in Feed Degradability and Rumen Fermentation
3.2. Differences in Rumen VFA Profile
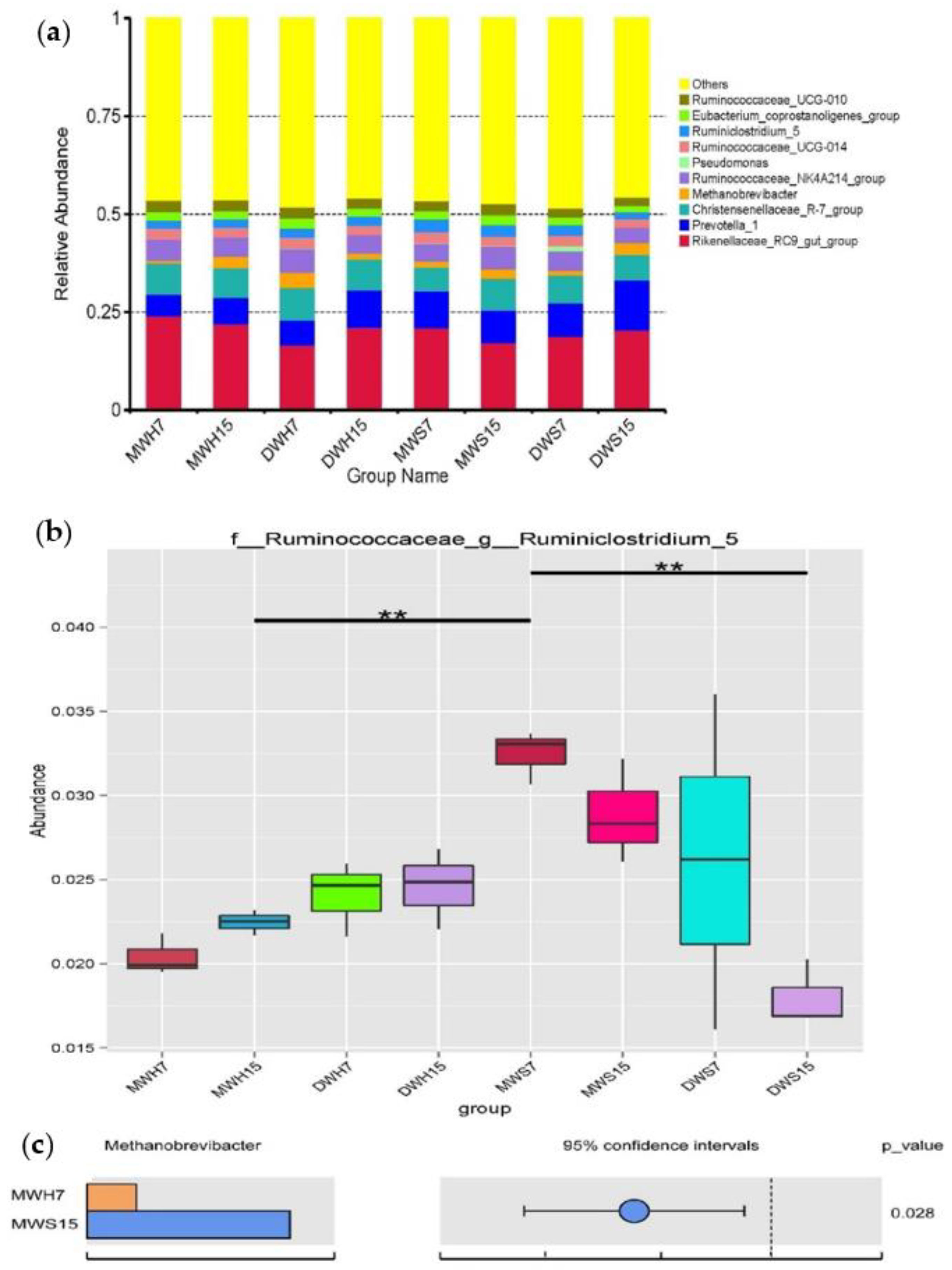
3.3. Changes in the Microbiota Community during Anaerobic Digestion
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Zhang, Q.; Li, J.; Yang, X.; Jin, J. Wheat straw burning and its associated impacts on Beijing air quality. Sci. China Ser. D Earth Sci. 2008, 51, 403–414. [Google Scholar] [CrossRef]
- Walsh, K.; O’Kiely, P.; Taweel, H.Z.; McGee, M.; Moloney, A.P.; Boland, T.M. Intake, digestibility and rumen characteristics in cattle offered whole-crop wheat or barley silages of contrasting grain to straw ratios. Anim. Feed Sci. Technol. 2009, 148, 192–213. [Google Scholar] [CrossRef]
- Keady, T.W.J.; Lively, F.O.; Kilpatrick, D.J.; Moss, B.W. Effects of replacing grass silage with either maize or whole-crop wheat silages on the performance and meat quality of beef cattle offered two levels of concentrates. Animal 2007, 1, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, D.; McGee, M.; Boland, T.; O’Kiely, P. Rumen fermentation, microbial protein synthesis, and nutrient flow to the omasum in cattle offered corn silage, grass silage, or whole-crop wheat. J. Anim. Sci. 2009, 87, 658–668. [Google Scholar] [CrossRef] [Green Version]
- Beauchemin, K.A.; Kreuzer, M.; O’Mara, F.; McAllister, T.A. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agric. 2008, 48, 21. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef] [Green Version]
- Geough, E.J.M.; O’Kiely, P.; Hart, K.J.; Moloney, A.P.; Boland, T.M.; Kenny, D.A. Methane emissions, feed intake, performance, digestibility, and rumen fermentation of finishing beef cattle offered whole-crop wheat silages differing in grain content1. J. Anim. Sci. 2010, 88, 2703. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Morrison, M.; Yu, Z. Status of the phylogenetic diversity census of ruminal microbiomes. FEMS Microbiol. Ecol. 2011, 76, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Ziemer, C.J.; Sharp, R.; Stern, M.D.; Cotta, M.A.; Whitehead, T.R.; Stahl, D.A. Comparison of microbial populations in model and natural rumens using 16S ribosomal RNA-targeted probes. Environ. Microbiol. 2000, 2, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Morgavi, D.P.; Martin, C. Methanogens and Methanogenesis in the Rumens and Ceca of Lambs Fed Two Different High-Grain-Content Diets. Appl. Environ. Microb. 2013, 79, 1777–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, W.; He, Y.; Xia, C.; Rahman, M.A.U.; Qiu, Q.; Shao, T.; Liang, Y.; Ji, L.; Wang, H.; Cao, B. Effects of replacing Leymus chinensis with whole-crop wheat hay on Holstein bull apparent digestibility, plasma parameters, rumen fermentation, and microbiota. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, Z.G.; Khanal, P.; Yildiz, C.; Chen, Y.; Arieli, A. Effects of stage of maturity at harvest, wilting and LAB inoculant on aerobic stability of wheat silages. Anim. Feed Sci. Technol. 2010, 158, 29–35. [Google Scholar] [CrossRef]
- Xia, C.; Liang, Y.; Bai, S.; He, Y.; Muhammad, A.U.R.; Su, H.; Cao, B. Effects of harvest time and added molasses on nutritional content, ensiling characteristics and in vitro degradation of whole-crop wheat. Asian Austral. J. Anim. 2018, 31, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Blümmel, M.; Steingaβ, H.; Becker, K. The relationship between in vitro gas production, in vitro microbial biomass yield and N incorporation and its implications for the prediction of voluntary feed intake of roughages. Br. J. Nutr. 1997, 77, 911. [Google Scholar] [CrossRef] [Green Version]
- Bremner, J.M.; Keeney, D.R. Steam distillation methods for determination of ammonium, nitrate and nitrite. Anal. Chim. Acta 1965, 32, 485–495. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microb. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microb. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouany, J.P. Optimizing rumen functions in the close-up transition period and early lactation to drive dry matter intake and energy balance in cows. Anim. Reprod. Sci. 2006, 96, 250–264. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Combinations of nitrate, saponin, and sulfate additively reduce methane production by rumen cultures in vitro while not adversely affecting feed digestion, fermentation or microbial communities. Bioresour. Technol. 2014, 155, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.; Kebreab, E.; Bannink, A.; France, J.; Lopez, S. Application of the gas production technique to feed evaluation systems for ruminants. Anim. Feed Sci. Technol. 2005, 123–124, 561–578. [Google Scholar] [CrossRef]
- Keim, J.P.; Alvarado-Gilis, C.; Arias, R.A.; Gandarillas, M.; Cabanilla, J. Evaluation of sources of variation on in vitro fermentation kinetics of feedstuffs in a gas production system. Anim. Sci. J. 2017, 88, 1547–1555. [Google Scholar] [CrossRef]
- Del Bianco Benedeti, P.; Paulino, P.V.R.; Marcondes, M.I.; Maciel, I.F.S.; Da Silva, M.C.; Faciola, A.P. Partial Replacement of Ground Corn with Glycerol in Beef Cattle Diets: Intake, Digestibility, Performance, and Carcass Characteristics. PLoS ONE 2016, 11, e148224. [Google Scholar] [CrossRef]
- Finlay, B.J.; Esteban, G.; Clarke, K.J.; Williams, A.G.; Embley, T.M.; Hirt, R.P. Some rumen ciliates have endosymbiotic methanogens. FEMS Microbiol. Lett. 1994, 117, 157–161. [Google Scholar] [CrossRef]
- Holter, J.B.; Young, A.J. Methane prediction in dry and lactating Holstein cows. J. Dairy Sci. 1992, 8, 2165–2175. [Google Scholar] [CrossRef]
- Zhao, G.; Xue, Y.; Zhang, W. Relationship between in vitro gas production and dry matter and organic matter digestibility of rations for sheep. J. Anim. Feed Sci. 2007, 16, 229–234. [Google Scholar] [CrossRef]
- Burke, E.J.; Chadburn, S.E.; Huntingford, C.; Jones, C.D. CO2 loss by permafrost thawing implies additional emissions reductions to limit warming to 1.5 or 2 °C. Environ. Res. Lett. 2018, 13, 24024. [Google Scholar] [CrossRef]
- Friedt, A.D.; McAllister, T.A.; He, M.L.; Penner, G.B.; McKinnon, J.J. Effects of replacing barley grain with graded levels of wheat bran on rumen fermentation, voluntary intake and nutrient digestion in beef cattle. Can. J. Anim. Sci. 2014, 94, 129–137. [Google Scholar] [CrossRef]
- Chuntao, Y.; Qiyu, D.; Peibin, Q.; Bingwen, S.; Junnan, M.; Yucai, Z.; Yan, T. Effects of Candida tropicalis and Mulberry Leaf Flavonoids on Nutrient Metabolism and Rumen Fermentation of Calves. Chin. J. Anim. Nutr. 2016, 28, 224–234. [Google Scholar]
- Andries, J.I.; Buysse, F.X.; De Brabander, D.L.; Cottyn, B.G. Isoacids in ruminant nutrition: Their role in ruminal and intermediary metabolism and possible influences on performances—A review. Anim. Feed Sci. Tech. 1987, 18, 169–180. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, W.; Zhu, W.; Liu, J.; Mao, S. Effect of dietary forage sources on rumen microbiota, rumen fermentation and biogenic amines in dairy cows. J. Sci. Food Agric. 2014, 94, 1886–1895. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Liu, J.; Yang, F.; Zhu, W.; Yuan, X.; Hu, Y.; Cui, Z.; Wang, X. Effect of ensiling and silage additives on biogas production and microbial community dynamics during anaerobic digestion of switchgrass. Bioresour. Technol. 2017, 241, 349–359. [Google Scholar] [CrossRef]
- St-Pierre, B.; Wright, A.D. Comparative metagenomic analysis of bacterial populations in three full-scale mesophilic anaerobic manure digesters. Appl. Microbiol. Biotechnol. 2014, 98, 2709–2717. [Google Scholar] [CrossRef]
- Yue, Z.; Chen, R.; Yang, F.; Maclellan, J.; Marsh, T. Effects of dairy manure and corn stover co-digestion on anaerobic microbes and corresponding digestion performance. Bioresour. Technol. 2013, 128, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, J.H.; Kang, H.J.; Lee, Y.H.; Lee, T.J. Distribution and abundance of Spirochaetes in full-scale anaerobic digesters. Bioresour. Technol. 2013, 145, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Delbes, C.; Moletta, R.; Godon, J.J. Monitoring of activity dynamics of an anaerobic digester bacterial community using 16S rRNA polymerase chain reaction–single-strand conformation polymorphism analysis. Environ. Microbiol. 2000, 2, 506–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zened, A.; Combes, S.; Cauquil, L.; Mariette, J.; Klopp, C. Microbial ecology of the rumen evaluated by 454 GS FLX pyrosequencing is affected by starch and oil supplementation of diets. FEMS Microbiol. Ecol. 2013, 83, 504–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albertsen, M.; Hugenholtz, P.; Skarshewski, A.; Nielsen, K.L.; Tyson, G.W.; Nielsen, P.H. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat. Biotechnol. 2013, 31, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Pitta, D.W.; Pinchak, W.E.; Dowd, S.E.; Osterstock, J.; Gontcharova, V. Rumen Bacterial Diversity Dynamics Associated with Changing from Bermudagrass Hay to Grazed Winter Wheat Diets. Microb. Ecol. 2010, 59, 511–522. [Google Scholar] [CrossRef]
- Danielsson, R.; Ramin, M.; Bertilsson, J.; Lund, P.; Huhtanen, P. Evaluation of a gas in vitro system for predicting methane production in vivo. J. Dairy Sci. 2017, 100, 8881–8894. [Google Scholar] [CrossRef]
- Wallace, R.J. Conference: Altering Ruminal Nitrogen Metabolism to Improve Protein utilization. J. Nutr. 1996, 126, 1326S–1334S. [Google Scholar] [CrossRef] [Green Version]
- Niu, W.; He, Y.; Wang, H.; Xia, C.; Shi, H.; Cao, B.; Su, H. Effects of Leymus chinensis replacement with whole-crop wheat hay on blood parameters, fatty acid composition, and microbiotas of Holstein bulls. J. Dairy Sci. 2018, 101, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Rey, M.; Enjalbert, F.; Combes, S.; Cauquil, L.; Bouchez, O. Establishment of ruminal bacterial community in dairy calves from birth to weaning is sequential. J. Appl. Microbiol. 2013, 116, 245–257. [Google Scholar] [CrossRef]
- Li, Y.; Shi, J.; Nelson, M.C.; Chen, P.; Graf, J.; Li, Y.; Yu, Z. Impact of different ratios of feedstock to liquid anaerobic digestion effluent on the performance and microbiota of solid-state anaerobic digesters digesting corn stover. Bioresour. Technol. 2016, 200, 744–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Item | Treatments | SEM 3 | p-Value 4,5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MWH7 | MWS7 | DWH7 | DWS7 | MWH15 | MWS15 | DWH15 | DWS15 | S | Tr | Ti | ||
| EE | 1.58 b | 2.51 ab | 1.6 b | 3.31 a | 2.42 ab | 2.82 ab | 1.82 ab | 1.81 ab | 0.185 | 0.15 | 0.022 | 0.214 |
| aNDF | 47.88 b | 47.01 bc | 47.13 bc | 43.97 c | 46.52 bc | 47.68 b | 51.9 a | 41.44 c | 0.556 | 0.009 | 0.031 | 0.185 |
| ADF | 28.58 | 29.09 | 25.81 | 28.66 | 27.08 | 28.99 | 26.37 | 25.98 | 0.423 | 0.189 | 0.159 | 0.128 |
| OM | 91.42 b | 90.71 d | 92.18 a | 90.7 d | 92.14 a | 90.89 cd | 92.05 a | 91.33 bc | 0.132 | 0.036 | 0.043 | 0.033 |
| CP | 10.99 b | 12.06 a | 10.44 c | 11.06 b | 10.39 c | 11.23 b | 10.44 c | 11.2 b | 0.1126 | 0.019 | 0.089 | 0.015 |
| Item | Treatments 3 | SEM | p-Value 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MWH7 | MWS7 | DWH7 | DWS7 | MWH15 | MWS15 | DWH15 | DWS15 | S | Tr | Ti | ||
| pH | 6.78 ab | 6.78 ab | 6.83 a | 6.78 ab | 6.77 b | 6.67 c | 6.79 ab | 6.76 b | 0.010 | 0.006 | 0.003 | 0.004 |
| Gas (mL/g) | 257.8 c | 266.1 bc | 262.2 c | 276.1 a | 272.4 ab | 280.9 a | 276.7 a | 278.4 a | 0.407 | 0.077 | 0.002 | <0.001 |
| Ammonia (mmol/L) | 11.1 | 10.6 | 11.5 | 11.4 | 10.4 | 11.1 | 11.6 | 10.5 | 0.225 | 0.373 | 0.599 | 0.615 |
| DM degradation (%) | 37.6 d | 41.5 b | 32.5 e | 40.4 bc | 38.2 cd | 44.2 a | 39.1 bcd | 38.9 bcd | 0.705 | 0.001 | <0.001 | 0.003 |
| aNDF degradation (%) | 43.2 b | 42.3 bc | 46.9 a | 39.6 cd | 40.7 bc | 39.4 cd | 36.7 d | 40.1 c | 0.658 | 0.359 | 0.036 | <0.001 |
| Methane (%) | 19.8 b | 19.6 b | 19.1 b | 22.1 a | 19.9 ab | 21.0 ab | 19.6 b | 21.0 ab | 0.285 | 0.453 | 0.015 | 0.669 |
| CO2 (%) | 71.3 ab | 67.3 b | 67.9 ab | 71.0 ab | 70.5 ab | 71.8 ab | 72.6 ab | 73.5 a | 0.666 | 0.428 | 0.808 | 0.042 |
| VFA (mM) | ||||||||||||
| TVFA | 49.2 | 47.5 | 51.0 | 53.3 | 49.6 | 48.3 | 46.6 | 51.5 | 0.830 | 0.272 | 0.547 | 0.466 |
| Acetate(A) | 34.2 | 32.5 | 34.9 | 36.8 | 34.3 | 33.3 | 31.8 | 35.5 | 0.607 | 0.359 | 0.559 | 0.483 |
| Propionate(P) | 8.26 | 8.81 | 8.78 | 8.97 | 8.44 | 8.40 | 8.17 | 8.69 | 0.129 | 0.534 | 0.289 | 0.326 |
| A: P ratio | 4.14 a | 3.68 c | 3.97 ab | 4.11 a | 4.06 ab | 3.96 ab | 3.89 b | 4.08 a | 0.034 | 0.228 | 0.175 | 0.643 |
| Isobutyrate | 0.56 | 0.60 | 0.60 | 0.64 | 0.59 | 0.61 | 0.55 | 0.60 | 0.009 | 0.522 | 0.056 | 0.522 |
| Butyrate | 4.51 ab | 3.8 c | 4.92 a | 4.75 ab | 4.54 ab | 4.2 bc | 4.46 ab | 4.74 ab | 0.089 | 0.004 | 0.096 | 0.956 |
| Isovalerate | 1.11 c | 1.2 bc | 1.21 bc | 1.44 a | 1.16 bc | 1.22 bc | 1.07 c | 1.29 b | 0.027 | 0.047 | 0.001 | 0.141 |
| Valerate | 0.61 | 0.63 | 0.65 | 0.67 | 0.59 | 0.61 | 0.56 | 0.71 | 0.014 | 0.135 | 0.075 | 0.339 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, W.; Wang, H.; He, Y.; Qiu, Q.; Shao, T.; Cao, B.; Su, H. Comparative Analysis of Wheat Hay and Silage in Methane Production, Fermentation Characteristics and Microbiota Using In Vitro Rumen Cultures. Appl. Sci. 2020, 10, 8456. https://doi.org/10.3390/app10238456
Niu W, Wang H, He Y, Qiu Q, Shao T, Cao B, Su H. Comparative Analysis of Wheat Hay and Silage in Methane Production, Fermentation Characteristics and Microbiota Using In Vitro Rumen Cultures. Applied Sciences. 2020; 10(23):8456. https://doi.org/10.3390/app10238456
Chicago/Turabian StyleNiu, Wenjing, Haibo Wang, Yang He, Qinghua Qiu, Taoqi Shao, Binghai Cao, and Huawei Su. 2020. "Comparative Analysis of Wheat Hay and Silage in Methane Production, Fermentation Characteristics and Microbiota Using In Vitro Rumen Cultures" Applied Sciences 10, no. 23: 8456. https://doi.org/10.3390/app10238456
APA StyleNiu, W., Wang, H., He, Y., Qiu, Q., Shao, T., Cao, B., & Su, H. (2020). Comparative Analysis of Wheat Hay and Silage in Methane Production, Fermentation Characteristics and Microbiota Using In Vitro Rumen Cultures. Applied Sciences, 10(23), 8456. https://doi.org/10.3390/app10238456






