Sericin for Tissue Engineering
Abstract
:1. Introduction
2. Sericin as a Degumming Product
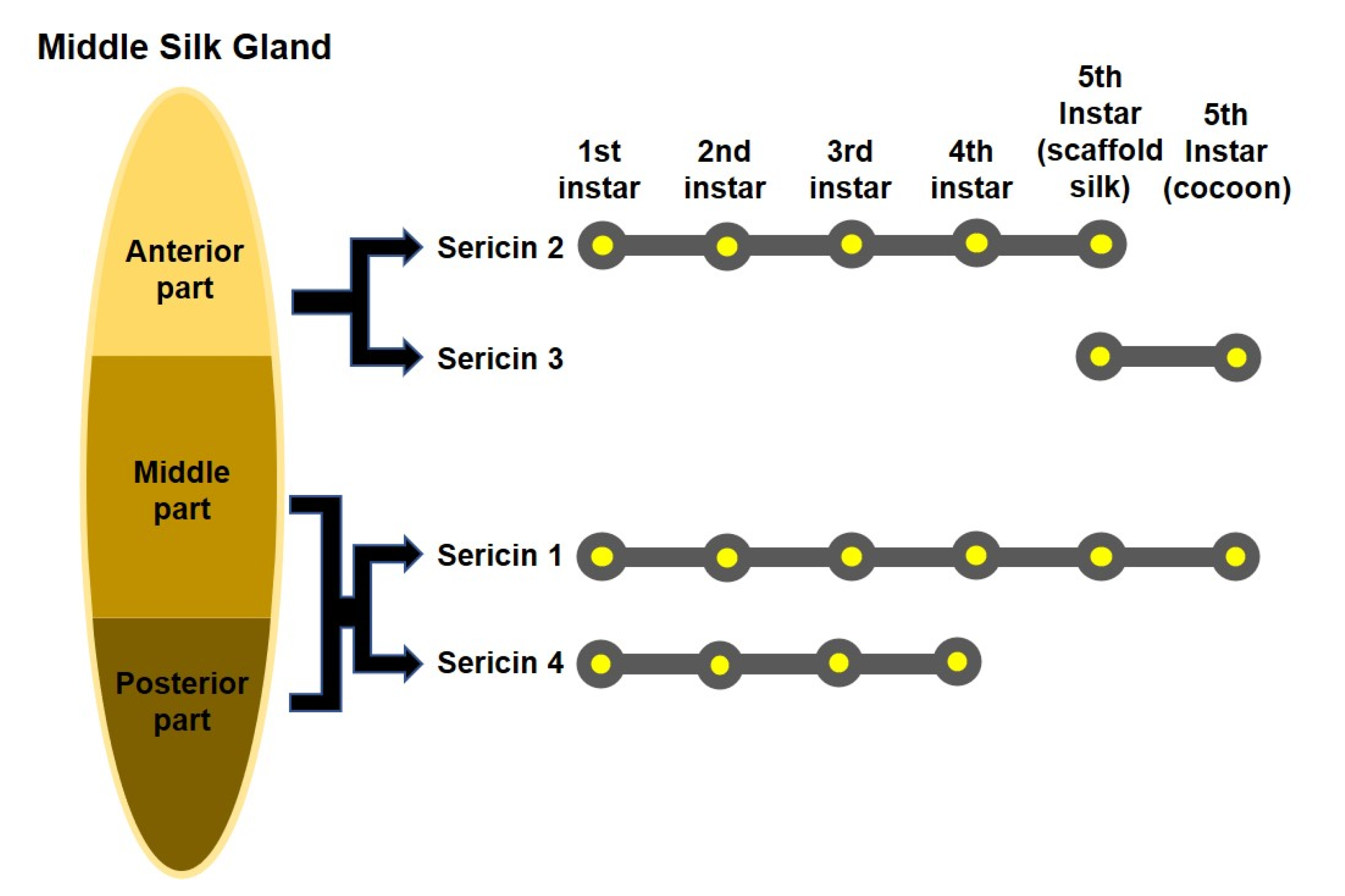
2.1. Sericin Gene
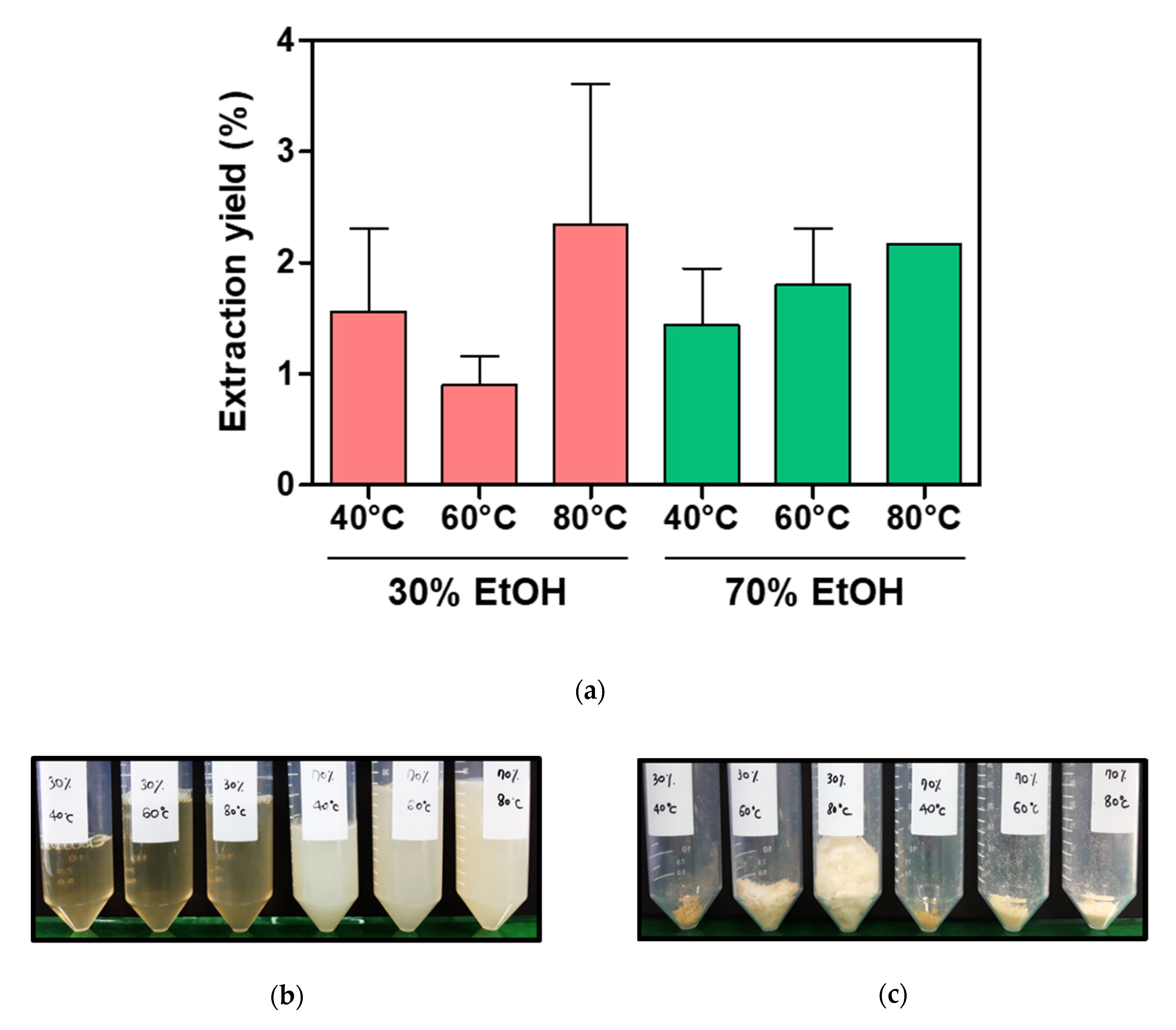
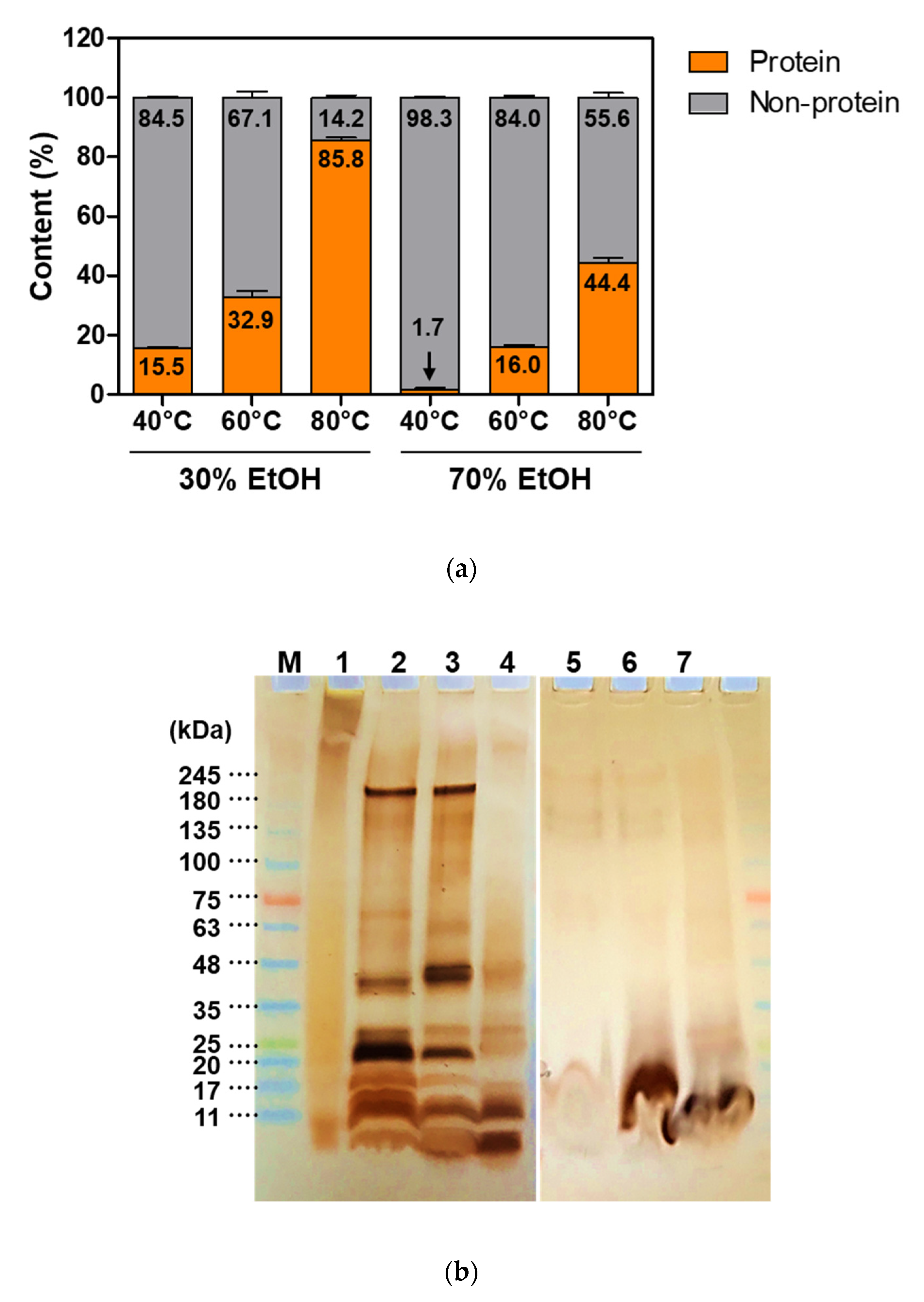
2.2. Sericin Molecular Weight and Degumming Process
2.3. Cellular Response to Sericin and Influence of the Degumming Method on Sericin Biological Activity
3. Sericin and Tissue Engineering
3.1. Sericin for Burn Dressing
3.2. Sericin for Oral Gargling
3.3. Sericin for Drug Delivery
3.4. Sericin for Bone Regeneration
3.5. Sericin for Other Tissues
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Park, C.J.; Ryoo, J.; Ki, C.S.; Kim, J.W.; Kim, I.S.; Bae, D.G.; Um, I.C. Effect of molecular weight on the structure and mechanical properties of silk sericin gel, film, and sponge. Int. J. Biol. Macromol. 2018, 119, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, Y.; Shi, Z.; Jiang, W.; Liu, X.; Ni, Q.-Q. Degumming of raw silk via steam treatment. J. Clean. Prod. 2018, 203, 492–497. [Google Scholar] [CrossRef]
- Craig, C.L. Evolution of arthropod silks. Annu. Rev. Entomol. 1997, 42, 231–267. [Google Scholar] [CrossRef]
- Ude, A.; Eshkoor, R.; Zulkifili, R.; Ariffin, A.; Dzuraidah, A.; Azhari, C. Bombyx mori silk fibre and its composite: A review of contemporary developments. Mater. Des. 2014, 57, 298–305. [Google Scholar] [CrossRef]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Jo, Y.-Y.; Oh, J.-H. New resorbable membrane materials for guided bone regeneration. Appl. Sci. 2018, 8, 2157. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Wang, P.; Zhang, F.; Zhou, X.; Zuo, B.; You, X.; Gao, Y.; Liu, H.; Tang, H. A novel silk fibroin nanofibrous membrane for guided bone regeneration: A study in rat calvarial defects. Am. J. Transl. Res. 2015, 7, 2244–2253. [Google Scholar]
- Song, J.M.; Shin, S.H.; Kim, Y.D.; Lee, J.Y.; Baek, Y.J.; Yoon, S.Y.; Kim, H.S. Comparative study of chitosan/fibroin-hydroxyapatite and collagen membranes for guided bone regeneration in rat calvarial defects: Micro-computed tomography analysis. Int. J. Oral Sci. 2014, 6, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wang, Y.; Tian, C.; Xu, S.; Wang, R.; Hou, K.; Chen, W.; Zhao, P.; Yu, L.; Lu, Z.; et al. Fabrication of the FGF1-functionalized sericin hydrogels with cell proliferation activity for biomedical application using genetically engineered Bombyx mori (B. mori) silk. Acta Biomater. 2018, 79, 239–252. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, M.K.; Lee, K.H.; Nho, S.K.; Han, M.S.; Um, I.C. Effect of degumming methods on structural characteristics and properties of regenerated silk. Int. J. Biol. Macromol. 2017, 104, 294–302. [Google Scholar] [CrossRef]
- Kwon, K.-J.; Seok, H. Silk Protein-Based Membrane for Guided Bone Regeneration. Appl. Sci. 2018, 8, 1214. [Google Scholar] [CrossRef] [Green Version]
- Song, J.-Y.; Kim, S.-G.; Lee, J.-W.; Chae, W.-S.; Kweon, H.; Jo, Y.-Y.; Lee, K.-G.; Lee, Y.-C.; Choi, J.-Y.; Kim, J.-Y. Accelerated healing with the use of a silk fibroin membrane for the guided bone regeneration technique. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 112, e26–e33. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Tao, H.; Kaplan, D.L.; Omenetto, F.G. Silk materials—A road to sustainable high technology. Adv. Mater. 2012, 24, 2824–2837. [Google Scholar] [CrossRef] [PubMed]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Min, B.M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef]
- Jin, H.-J.; Chen, J.; Karageorgiou, V.; Altman, G.H.; Kaplan, D.L. Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials 2004, 25, 1039–1047. [Google Scholar] [CrossRef]
- Yu, K.; Lu, F.; Li, Q.; Chen, H.; Lu, B.; Liu, J.; Li, Z.; Dai, F.; Wu, D.; Lan, G. In situ assembly of Ag nanoparticles (AgNPs) on porous silkworm cocoon-based wound film: Enhanced antimicrobial and wound healing activity. Sci. Rep. 2017, 7, 2107. [Google Scholar] [CrossRef]
- Yu, K.; Lu, F.; Li, Q.; Zou, Y.; Xiao, Y.; Lu, B.; Liu, J.; Dai, F.; Wu, D.; Lan, G. Accelerated wound-healing capabilities of a dressing fabricated from silkworm cocoon. Int. J. Biol. Macromol. 2017, 102, 901–913. [Google Scholar] [CrossRef]
- Kim, M.K.; Kwak, H.W.; Kim, H.H.; Kwon, T.R.; Kim, S.Y.; Kim, B.J.; Park, Y.H.; Lee, K.H. Surface modification of silk fibroin nanofibrous mat with dextran for wound dressing. Fibers Polym. 2014, 15, 1137–1145. [Google Scholar] [CrossRef]
- Lawrence, B.D.; Marchant, J.K.; Pindrus, M.A.; Omenetto, F.G.; Kaplan, D.L. Silk film biomaterials for cornea tissue engineering. Biomaterials 2009, 30, 1299–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kim, C.H.; Park, C.H.; Seo, J.N.; Kweon, H.; Kang, S.W.; Lee, K.G. Comparison of methods for the repair of acute tympanic membrane perforations: Silk patch vs. paper patch. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2010, 18, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Keshav, V.; Wang, X.; Johanas, J.T.; Leisk, G.G.; Kaplan, D.L. Dynamic culture conditions to generate silk-based tissue-engineered vascular grafts. Biomaterials 2009, 30, 3213–3223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, A.R.; Park, Y.-H.; Kim, H.J.; Kim, M.-K.; Kim, S.-G.; Kweon, H.; Kundu, S.C. Tri-layered silk fibroin and poly-ɛ-caprolactone small diameter vascular grafts tested in vitro and in vivo. Macromol. Res. 2015, 23, 924–936. [Google Scholar] [CrossRef]
- Park, S.Y.; Ki, C.S.; Park, Y.H.; Lee, K.G.; Kang, S.W.; Kweon, H.Y.; Kim, H.J. Functional recovery guided by an electrospun silk fibroin conduit after sciatic nerve injury in rats. J. Tissue Eng. Regen. Med. 2015, 9, 66–76. [Google Scholar] [CrossRef]
- Yucel, T.; Lovett, M.L.; Kaplan, D.L. Silk-based biomaterials for sustained drug delivery. J. Control. Release Off. J. Control. Release Soc. 2014, 190, 381–397. [Google Scholar] [CrossRef] [Green Version]
- Panilaitis, B.; Altman, G.H.; Chen, J.; Jin, H.J.; Karageorgiou, V.; Kaplan, D.L. Macrophage responses to silk. Biomaterials 2003, 24, 3079–3085. [Google Scholar] [CrossRef]
- Rocha, L.K.; Favaro, L.I.; Rios, A.C.; Silva, E.C.; Silva, W.F.; Stigliani, T.P.; Guilger, M.; Lima, R.; Oliveira, J.M., Jr.; Aranha, N. Sericin from Bombyx mori cocoons. Part I: Extraction and physicochemical-biological characterization for biopharmaceutical applications. Process Biochem. 2017, 61, 163–177. [Google Scholar] [CrossRef]
- Kumar, J.P.; Mandal, B.B. Antioxidant potential of mulberry and non-mulberry silk sericin and its implications in biomedicine. Free Radic. Biol. Med. 2017, 108, 803–818. [Google Scholar] [CrossRef]
- Bari, E.; Perteghella, S.; Farago, S.; Torre, M.L. Association of silk sericin and platelet lysate: Premises for the formulation of wound healing active medications. Int. J. Biol. Macromol. 2018, 119, 37–47. [Google Scholar] [CrossRef]
- Chlapanidas, T.; Farago, S.; Lucconi, G.; Perteghella, S.; Galuzzi, M.; Mantelli, M.; Avanzini, M.A.; Tosca, M.C.; Marazzi, M.; Vigo, D.; et al. Sericins exhibit ROS-scavenging, anti-tyrosinase, anti-elastase, and in vitro immunomodulatory activities. Int. J. Biol. Macromol. 2013, 58, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kunz, R.I.; Brancalhao, R.M.; Ribeiro, L.F.; Natali, M.R. Silkworm Sericin: Properties and Biomedical Applications. BioMed Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aramwit, P.; Sangcakul, A. The effects of sericin cream on wound healing in rats. Biosci. Biotechnol. Biochem. 2007, 71, 2473–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsubouchi, K. Occlusive dressing consisting essentially of silk fibroin and silk sericin and its production. Japan Patent 11-070160A, 16 March 1999. [Google Scholar]
- Kundu, B.; Kundu, S.C. Silk sericin/polyacrylamide in situ forming hydrogels for dermal reconstruction. Biomaterials 2012, 33, 7456–7467. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Siritienthong, T.; Srichana, T.; Ratanavaraporn, J. Accelerated healing of full-thickness wounds by genipin-crosslinked silk sericin/PVA scaffolds. Cells Tissues Organs 2013, 197, 224–238. [Google Scholar] [CrossRef]
- Nagai, N.; Murao, T.; Ito, Y.; Okamoto, N.; Sasaki, M. Enhancing effects of sericin on corneal wound healing in Otsuka Long-Evans Tokushima fatty rats as a model of human type 2 diabetes. Biol. Pharm. Bull. 2009, 32, 1594–1599. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Murao, T.; Ito, Y.; Okamoto, N.; Sasaki, M. Enhancing effects of sericin on corneal wound healing in rat debrided corneal epithelium. Biol. Pharm. Bull. 2009, 32, 933–936. [Google Scholar] [CrossRef] [Green Version]
- Terada, S.; Nishimura, T.; Sasaki, M.; Yamada, H.; Miki, M. Sericin, a protein derived from silkworms, accelerates the proliferation of several mammalian cell lines including a hybridoma. Cytotechnology 2002, 40, 3–12. [Google Scholar] [CrossRef]
- Terada, S.; Sasaki, M.; Yanagihara, K.; Yamada, H. Preparation of silk protein sericin as mitogenic factor for better mammalian cell culture. J. Biosci. Bioeng. 2005, 100, 667–671. [Google Scholar] [CrossRef]
- Sasaki, M.; Kato, Y.; Yamada, H.; Terada, S. Development of a novel serum-free freezing medium for mammalian cells using the silk protein sericin. Biotechnol. Appl. Biochem. 2005, 42, 183–188. [Google Scholar] [CrossRef]
- Morikawa, M.; Kimura, T.; Murakami, M.; Katayama, K.; Terada, S.; Yamaguchi, A. Rat islet culture in serum-free medium containing silk protein sericin. J. Hepato-Biliary-Pancreat. Surg. 2009, 16, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Terada, S.; Kanayama, T.; Miki, M.; Morikawa, M.; Kimura, T.; Yamaguchi, A.; Sasaki, M.; Yamada, H. Improvement of islet culture with sericin. J. Biosci. Bioeng. 2004, 98, 217–219. [Google Scholar] [CrossRef]
- Kato, N.; Sato, S.; Yamanaka, A.; Yamada, H.; Fuwa, N.; Nomura, M. Silk protein, sericin, inhibits lipid peroxidation and tyrosinase activity. Biosci. Biotechnol. Biochem. 1998, 62, 145–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.B.; Wu, L.P.; Chen, L.S.; Mao, X.Y.; Ren, F.Z. Antioxidant activities of silk sericin from silkworm Bombyx mori. J. Food Biochem. 2009, 33, 74–88. [Google Scholar] [CrossRef]
- Takasu, Y.; Yamada, H.; Tsubouchi, K. Isolation of three main sericin components from the cocoon of the silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2002, 66, 2715–2718. [Google Scholar] [CrossRef]
- Dong, Z.; Guo, K.; Zhang, X.; Zhang, T.; Zhang, Y.; Ma, S.; Chang, H.; Tang, M.; An, L.; Xia, Q.; et al. Identification of Bombyx mori sericin 4 protein as a new biological adhesive. Int. J. Biol. Macromol. 2019, 132, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Ishikawa, E.; Suzuki, Y. Structural analysis of sericin genes. Homologies with fibroin gene in the 5’ flanking nucleotide sequences. J. Biol. Chem. 1982, 257, 15192–15199. [Google Scholar]
- Michaille, J.J.; Couble, P.; Prudhomme, J.C.; Garel, A. A single gene produces multiple sericin messenger RNAs in the silk gland of Bombyx mori. Biochimie 1986, 68, 1165–1173. [Google Scholar] [CrossRef]
- Couble, P.; Michaille, J.J.; Garel, A.; Couble, M.L.; Prudhomme, J.C. Developmental switches of sericin mRNA splicing in individual cells of Bombyx mori silkgland. Dev. Biol. 1987, 124, 431–440. [Google Scholar] [CrossRef]
- Michaille, J.J.; Garel, A.; Prudhomme, J.C. Cloning and characterization of the highly polymorphic Ser2 gene of Bombyx mori. Gene 1990, 86, 177–184. [Google Scholar] [CrossRef]
- Garel, A.; Deleage, G.; Prudhomme, J.C. Structure and organization of the Bombyx mori sericin 1 gene and of the sericins 1 deduced from the sequence of the Ser 1B cDNA. Insect Biochem. Mol. Biol. 1997, 27, 469–477. [Google Scholar] [CrossRef]
- Kludkiewicz, B.; Takasu, Y.; Fedic, R.; Tamura, T.; Sehnal, F.; Zurovec, M. Structure and expression of the silk adhesive protein Ser2 in Bombyx mori. Insect Biochem. Mol. Biol. 2009, 39, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Takasu, Y.; Yamada, H.; Tamura, T.; Sezutsu, H.; Mita, K.; Tsubouchi, K. Identification and characterization of a novel sericin gene expressed in the anterior middle silk gland of the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 2007, 37, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Takasu, Y.; Hata, T.; Uchino, K.; Zhang, Q. Identification of Ser2 proteins as major sericin components in the non-cocoon silk of Bombyx mori. Insect Biochem. Mol. Biol. 2010, 40, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhao, P.; Zhang, Y.; Song, Q.; Zhang, X.; Guo, P.; Wang, D.; Xia, Q. Analysis of proteome dynamics inside the silk gland lumen of Bombyx mori. Sci. Rep. 2016, 6, 21158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Z.; Zhao, P.; Wang, C.; Zhang, Y.; Chen, J.; Wang, X.; Lin, Y.; Xia, Q. Comparative proteomics reveal diverse functions and dynamic changes of Bombyx mori silk proteins spun from different development stages. J. Proteome Res. 2013, 12, 5213–5222. [Google Scholar] [CrossRef]
- Rajput, S.; Singh, M.K. Sericin-a unique biomaterial. IOSR J. Polym. Text. Eng. 2015, 2, 29–35. [Google Scholar]
- Padamwar, M.; Pawar, A. Silk sericin and its applications: A review. J. Sci. Ind. Res. 2004, 63, 323–329. [Google Scholar]
- Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk sericin: A versatile material for tissue engineering and drug delivery. Biotechnol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef]
- Kumar, J.P.; Bhardwaj, N.; Mandal, B.B. Cross-linked silk sericin–gelatin 2D and 3D matrices for prospective tissue engineering applications. RSC Adv. 2016, 6, 105125–105136. [Google Scholar] [CrossRef]
- Da Silva, T.; Junior, A.D.S.; Ribani, M.; Vieira, M.; Gimenes, M.; Da Silva, M.G. Evaluation of molecular weight distribution of sericin in solutions concentrated via precipitation by ethanol and precipitation by freezing/thawing. Chem. Eng. Trans. 2014, 38, 103–108. [Google Scholar]
- Aramwit, P.; Damrongsakkul, S.; Kanokpanont, S.; Srichana, T. Properties and antityrosinase activity of sericin from various extraction methods. Biotechnol. Appl. Biochem. 2010, 55, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The effect of sericin from various extraction methods on cell viability and collagen production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wray, L.S.; Hu, X.; Gallego, J.; Georgakoudi, I.; Omenetto, F.G.; Schmidt, D.; Kaplan, D.L. Effect of processing on silk-based biomaterials: Reproducibility and biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99, 89–101. [Google Scholar] [CrossRef]
- Kaur, J.; Rajkhowa, R.; Afrin, T.; Tsuzuki, T.; Wang, X. Facts and myths of antibacterial properties of silk. Biopolymers 2014, 101, 237–245. [Google Scholar] [CrossRef]
- Mason, R. Fabrics for atopic dermatitis. J. Fam. Health Care 2008, 18, 63–65. [Google Scholar]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Srichana, T. Monitoring of inflammatory mediators induced by silk sericin. J. Biosci. Bioeng. 2009, 107, 556–561. [Google Scholar] [CrossRef]
- Jo, Y.-Y.; Kweon, H.; Kim, D.-W.; Baek, K.; Kim, M.-K.; Kim, S.-G.; Chae, W.-S.; Choi, J.-Y.; Rotaru, H. Bone regeneration is associated with the concentration of tumour necrosis factor-α induced by sericin released from a silk mat. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Eidet, J.R.; Reppe, S.; Pasovic, L.; Olstad, O.K.; Lyberg, T.; Khan, A.Z.; Fostad, I.G.; Chen, D.F.; Utheim, T.P. The Silk-protein Sericin Induces Rapid Melanization of Cultured Primary Human Retinal Pigment Epithelial Cells by Activating the NF-kappaB Pathway. Sci. Rep. 2016, 6, 22671. [Google Scholar] [CrossRef] [Green Version]
- Jo, Y.Y.; Kim, D.W.; Choi, J.Y.; Kim, S.G. 4-Hexylresorcinol and silk sericin increase the expression of vascular endothelial growth factor via different pathways. Sci. Rep. 2019, 9, 3448. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.; Jo, Y.Y.; Garagiola, U.; Choi, J.Y.; Kang, Y.J.; Oh, J.H.; Kim, S.G. Increased Level of Vascular Endothelial Growth Factors by 4-hexylresorcinol is Mediated by Transforming Growth Factor-beta1 and Accelerates Capillary Regeneration in the Burns in Diabetic Animals. Int. J. Mol. Sci. 2020, 21, 3473. [Google Scholar] [CrossRef]
- Kim, J.W.; Jo, Y.Y.; Kim, J.Y.; Oh, J.H.; Yang, B.E.; Kim, S.G. Retrospective comparative clinical study for silk mat application into extraction socket. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 16. [Google Scholar] [CrossRef]
- Kim, J.W.; Jo, Y.Y.; Kweon, H.Y.; Kim, D.W.; Kim, S.G. The effects of proteins released from silk mat layers on macrophages. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 10. [Google Scholar] [CrossRef]
- Kim, D.W.; Jo, Y.Y.; Kweon, H.Y.; Kim, S.G. Different level of tumor necrosis factor-α expression after administration of silk sericin fraction in RAW264.7 cells. Int. J. Ind. Entomol. 2020, 41, 1–5. [Google Scholar]
- Aramwit, P.; Towiwat, P.; Srichana, T. Anti-inflammatory potential of silk sericin. Nat. Prod. Commun. 2013, 8, 501–504. [Google Scholar] [CrossRef] [Green Version]
- Chlapanidas, T.; Perteghella, S.; Leoni, F.; Farago, S.; Marazzi, M.; Rossi, D.; Martino, E.; Gaggeri, R.; Collina, S. TNF-alpha blocker effect of naringenin-loaded sericin microparticles that are potentially useful in the treatment of psoriasis. Int. J. Mol. Sci. 2014, 15, 13624–13636. [Google Scholar] [CrossRef] [Green Version]
- Song, C.J.; Fu, X.M.; Li, J.; Chen, Z.H. Effects of sericine on TGF-beta1/Smad3 signal pathway of diabetic mephropathy rats kidney. Zazhi Chin. J. Appl. Physiol. 2011, 27, 102–105. [Google Scholar]
- Dash, R.; Acharya, C.; Bindu, P.C.; Kundu, S.C. Antioxidant potential of silk protein sericin against hydrogen peroxide-induced oxidative stress in skin fibroblasts. BMB Rep. 2008, 41, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Tsubouchi, K.; Igarashi, Y.; Takasu, Y.; Yamada, H. Sericin enhances attachment of cultured human skin fibroblasts. Biosci. Biotechnol. Biochem. 2005, 69, 403–405. [Google Scholar] [CrossRef]
- Martinez-Mora, C.; Mrowiec, A.; Garcia-Vizcaino, E.M.; Alcaraz, A.; Cenis, J.L.; Nicolas, F.J. Fibroin and sericin from Bombyx mori silk stimulate cell migration through upregulation and phosphorylation of c-Jun. PLoS ONE 2012, 7, e42271. [Google Scholar] [CrossRef]
- Nayak, S.; Dey, T.; Naskar, D.; Kundu, S.C. The promotion of osseointegration of titanium surfaces by coating with silk protein sericin. Biomaterials 2013, 34, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Schurink, M.; van Berkel, W.J.; Wichers, H.J.; Boeriu, C.G. Novel peptides with tyrosinase inhibitory activity. Peptides 2007, 28, 485–495. [Google Scholar] [CrossRef]
- Kurioka, A.; Kurioka, F.; Yamazaki, M. Characterization of sericin powder prepared from citric acid-degraded sericin polypeptides of the silkworm, Bombyx Mori. Biosci. Biotechnol. Biochem. 2004, 68, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Elancheran, R.; Devi, R. Phytochemical screening, antioxidant, antityrosinase, and antigenotoxic potential of Amaranthus viridis extract. Indian J. Pharmacol. 2018, 50, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Zuo, A.R.; Dong, H.H.; Yu, Y.Y.; Shu, Q.L.; Zheng, L.X.; Yu, X.Y.; Cao, S.W. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin. Med. 2018, 13, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; Leroux, M.A. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Garg, V.K.; Paliwal, S.K. Wound-healing activity of ethanolic and aqueous extracts of Ficus benghalensis. J. Adv. Pharm. Technol. Res. 2011, 2, 110–114. [Google Scholar] [CrossRef]
- Shah, A.; Ali Buabeid, M.; Arafa, E.A.; Hussain, I.; Li, L.; Murtaza, G. The wound healing and antibacterial potential of triple-component nanocomposite (chitosan-silver-sericin) films loaded with moxifloxacin. Int. J. Pharm. 2019, 564, 22–38. [Google Scholar] [CrossRef]
- Salas Campos, L.; Fernandes Mansilla, M.; Martinez de la Chica, A.M. Topical chemotherapy for the treatment of burns. Rev. Enferm. 2005, 28, 67–70. [Google Scholar]
- Khorasani, G.; Hosseinimehr, S.J.; Azadbakht, M.; Zamani, A.; Mahdavi, M.R. Aloe versus silver sulfadiazine creams for second-degree burns: A randomized controlled study. Surg. Today 2009, 39, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Palapinyo, S.; Srichana, T.; Chottanapund, S.; Muangman, P. Silk sericin ameliorates wound healing and its clinical efficacy in burn wounds. Arch. Dermatol. Res. 2013, 305, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Kamei, K.; Srichana, T. The effect of sericin with variable amino-acid content from different silk strains on the production of collagen and nitric oxide. J. Biomater. Sci. Polym. Ed. 2009, 20, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Kanokpanont, S.; Punyarit, P.; Srichana, T. Effectiveness of inflammatory cytokines induced by sericin compared to sericin in combination with silver sulfadiazine cream on wound healing. Wounds A Compend. Clin. Res. Pract. 2009, 21, 198–206. [Google Scholar]
- Gilotra, S.; Chouhan, D.; Bhardwaj, N.; Nandi, S.K.; Mandal, B.B. Potential of silk sericin based nanofibrous mats for wound dressing applications. Mater. Sci. Eng. CMater. Biol. Appl. 2018, 90, 420–432. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, B.B. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef]
- Padamwar, M.N.; Pawar, A.P.; Daithankar, A.V.; Mahadik, K. Silk sericin as a moisturizer: An in vivo study. J. Cosmet. Dermatol. 2005, 4, 250–257. [Google Scholar] [CrossRef]
- Oka, S. Mulberry (Morus alba L.). Biotechnol. Agric. For. 1986, 1, 384–392. [Google Scholar]
- Kumar, H.A.; Muralidhar, T.; Acharya, S.; Das, M.J. EMS induced morphometric biomass and phytochemical variations in Morus species (Genotype RFS135). J. Exp. Agric. Int. 2013, 3, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.-J.; Ahn, S.-H.; Kim, Y.-W.; Jo, Y.-Y.; Kweon, H.; Kim, S.-G. Non-randomized, one way cross-over, open label preliminary clinical trial for silk protein based oral gargling. Int. J. Ind. Entomol. 2019, 38, 14–17. [Google Scholar]
- Hardy, J.G.; Römer, L.M.; Scheibel, T.R. Polymeric materials based on silk proteins. Polymer 2008, 49, 4309–4327. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhang, J.; Huang, L.; Liu, J.; Li, Y.; Zhang, G.; Kundu, S.C.; Wang, L. Exploring natural silk protein sericin for regenerative medicine: An injectable, photoluminescent, cell-adhesive 3D hydrogel. Sci. Rep. 2014, 4, 7064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, A.; Yamada, M.; Kanazawa, T.; Takashima, Y.; Ouchi, K.; Okada, H. Sustained-release of protein from biodegradable sericin film, gel and sponge. Int. J. Pharm. 2011, 407, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Huang, L.; Wang, Z.; Wang, L. Design and performance of a sericin-alginate interpenetrating network hydrogel for cell and drug delivery. Sci. Rep. 2015, 5, 12374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisi, O.I.; Fiorillo, M.; Scrivano, L.; Sinicropi, M.S.; Dolce, V.; Iacopetta, D.; Puoci, F.; Cappello, A.R. Sericin/Poly(ethylcyanoacrylate) Nanospheres by Interfacial Polymerization for Enhanced Bioefficacy of Fenofibrate: In Vitro and In Vivo Studies. Biomacromolecules 2015, 16, 3126–3133. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Deng, Y.; Xu, L.; Yang, C.; Zhu, Y.; Wang, G.; Wang, Z.; Wang, L. A sericin/ graphene oxide composite scaffold as a biomimetic extracellular matrix for structural and functional repair of calvarial bone. Theranostics 2020, 10, 741–756. [Google Scholar] [CrossRef]
- Yang, M.; Shuai, Y.; Zhang, C.; Chen, Y.; Zhu, L.; Mao, C.; OuYang, H. Biomimetic nucleation of hydroxyapatite crystals mediated by Antheraea pernyi silk sericin promotes osteogenic differentiation of human bone marrow derived mesenchymal stem cells. Biomacromolecules 2014, 15, 1185–1193. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Min, S.; Mandal, M.; Yang, M.; Zhu, L. Hydroxyapatite/sericin composite film prepared through mineralization of flexible ethanol-treated sericin film with simulated body fluids. Ceram. Int. 2014, 40, 985–991. [Google Scholar] [CrossRef]
- Veiga, A.; Castro, F.; Reis, C.C.; Sousa, A.; Oliveira, A.L.; Rocha, F. Hydroxyapatite/sericin composites: A simple synthesis route under near-physiological conditions of temperature and pH and preliminary study of the effect of sericin on the biomineralization process. Mater. Sci. Eng. C 2020, 108, 110400. [Google Scholar] [CrossRef]
- Dinescu, S.; Galateanu, B.; Albu, M.; Lungu, A.; Radu, E.; Hermenean, A.; Costache, M. Biocompatibility assessment of novel collagen-sericin scaffolds improved with hyaluronic Acid and chondroitin sulfate for cartilage regeneration. BioMed Res. Int. 2013, 2013, 598056. [Google Scholar] [CrossRef]
- Qi, C.; Liu, J.; Jin, Y.; Xu, L.; Wang, G.; Wang, Z.; Wang, L. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials 2018, 163, 89–104. [Google Scholar] [CrossRef]
- Xie, H.; Yang, W.; Chen, J.; Zhang, J.; Lu, X.; Zhao, X.; Huang, K.; Li, H.; Chang, P.; Wang, Z.; et al. A silk sericin/silicone nerve guidance conduit promotes regeneration of a transected sciatic nerve. Adv. Healthc. Mater. 2015, 4, 2195–2205. [Google Scholar] [CrossRef]
- Li, X.; Yang, W.; Xie, H.; Wang, J.; Zhang, L.; Wang, Z.; Wang, L. CNT/Sericin Conductive Nerve Guidance Conduit Promotes Functional Recovery of Transected Peripheral Nerve Injury in a Rat Model. ACS Appl. Mater. Interfaces 2020, 12, 36860–36872. [Google Scholar] [CrossRef]

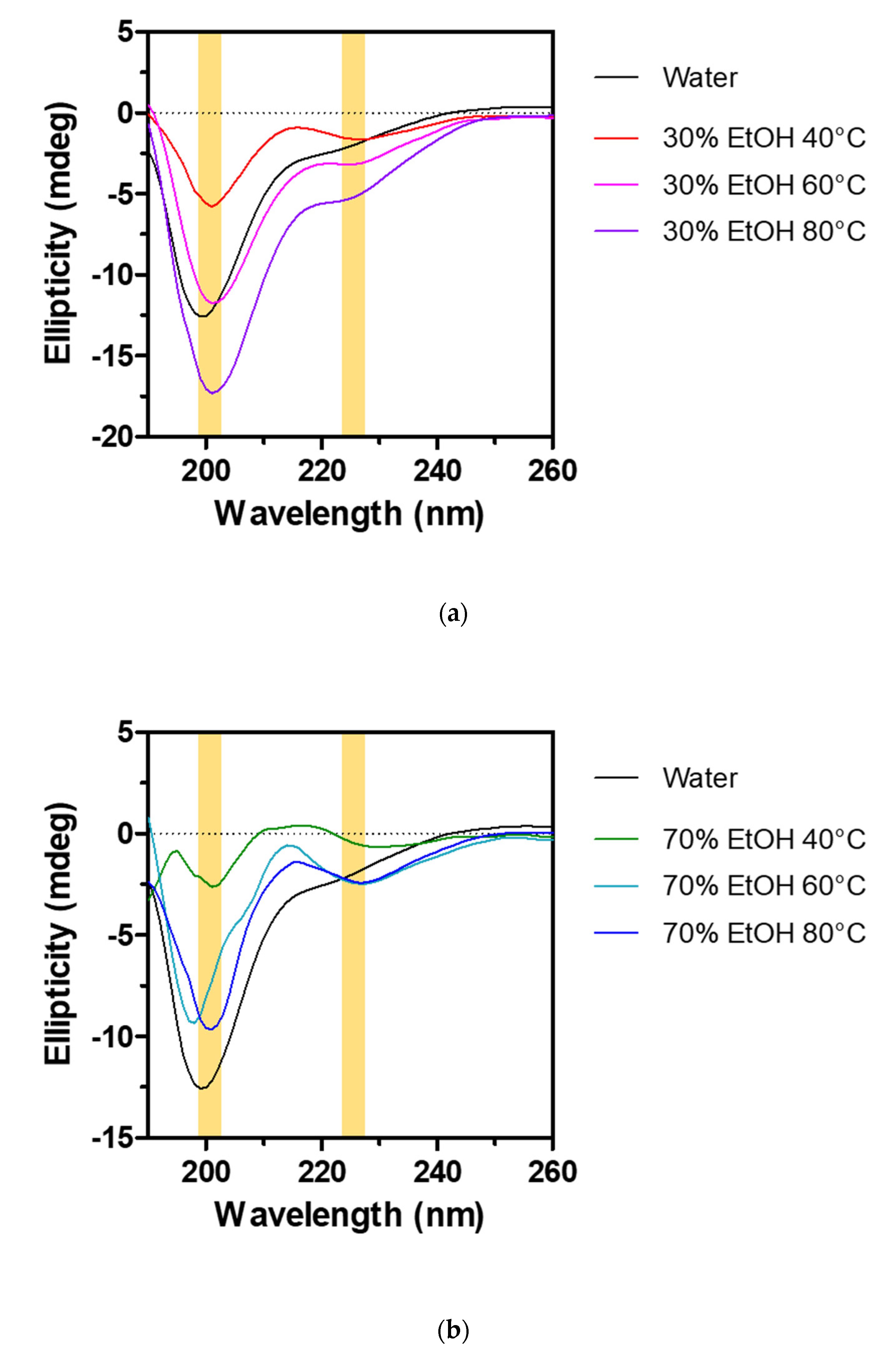

| Sample | Helix | Sheet | Turn | Others | |
|---|---|---|---|---|---|
| Hot water extracts | 3.40% | 34.40% | 14.20% | 48.00% | |
| 30% EtOH extracts | 40 °C | 2.30% | 39.50% | 13.90% | 44.30% |
| 60 °C | 4.30% | 37.10% | 14.10% | 44.50% | |
| 80 °C | 6.90% | 33.90% | 14.90% | 44.30% | |
| 70% EtOH extracts | 40 °C | 0.60% | 41.90% | 13.40% | 44.10% |
| 60 °C | 0.60% | 41.40% | 13.60% | 44.40% | |
| 80 °C | 1.70% | 40.10% | 13.80% | 44.40% | |
| Extraction Technique | Molecular Weight | Biological Activity | Reference |
|---|---|---|---|
| Autoclaving at 120 °C for 60 min | 25–150 kDa | Sericin extracted using urea exhibited the highest cell toxicity and the lowest induction of collagen production, while sericin extracted by the autoclaving method showed the lowest cell toxicity and the highest induction of collagen production. | [64] |
| 1.25% citric acid solution heated for 30 min | 50–150 kDa | ||
| 0.5% Na2CO3 solution heated for 30 min | 15–75 kDa | ||
| 8 M urea for 30 min followed by heating at 85 °C for 30 min | from 10 to >225 kDa | ||
| Autoclaving at 120 °C for 60 min | 35–150 kDa | Compared to other extraction methods, sericin extracted using urea exhibited the highest antityrosinase activity. | [63] |
| 1.25% citric acid solution heated for 30 min | 35–150 kDa | ||
| 0.5% Na2CO3 solution heated for 30 min | 15–75 kDa | ||
| 8 M urea for 30 min followed by heating at 85 °C for 30 min | from 10 to >225 kDa | ||
| Autoclaving at 120 °C for 20 min | smear | Sericin extracted by the alkali-degradation method showed higher efficacy in antioxidant potential than conventional or autoclaving methods. | [29] |
| 1.25% citric acid solution heated for 30 min | smear | ||
| 0.5% Na2CO3 solution heated for 30 min | smear with a clear band at 75 kDa | ||
| 8 M urea for 30 min followed by heating at 85 °C for 30 min | 10–120 kDa | ||
| 0.02 M Na2CO3 solution heated for 30 min | smear |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, Y.-Y.; Kweon, H.; Oh, J.-H. Sericin for Tissue Engineering. Appl. Sci. 2020, 10, 8457. https://doi.org/10.3390/app10238457
Jo Y-Y, Kweon H, Oh J-H. Sericin for Tissue Engineering. Applied Sciences. 2020; 10(23):8457. https://doi.org/10.3390/app10238457
Chicago/Turabian StyleJo, You-Young, HaeYong Kweon, and Ji-Hyeon Oh. 2020. "Sericin for Tissue Engineering" Applied Sciences 10, no. 23: 8457. https://doi.org/10.3390/app10238457





