Lincomycin-Induced Transcriptional Alterations in the Green Alga Raphidocelis subcapitata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Cultivation Conditions
2.2. Toxicity Test
2.3. RNA Sequencing
2.4. RNA-seq Data Analyses
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Statistical Analyses
3. Results
3.1. Growth Inhibition Test Analysis
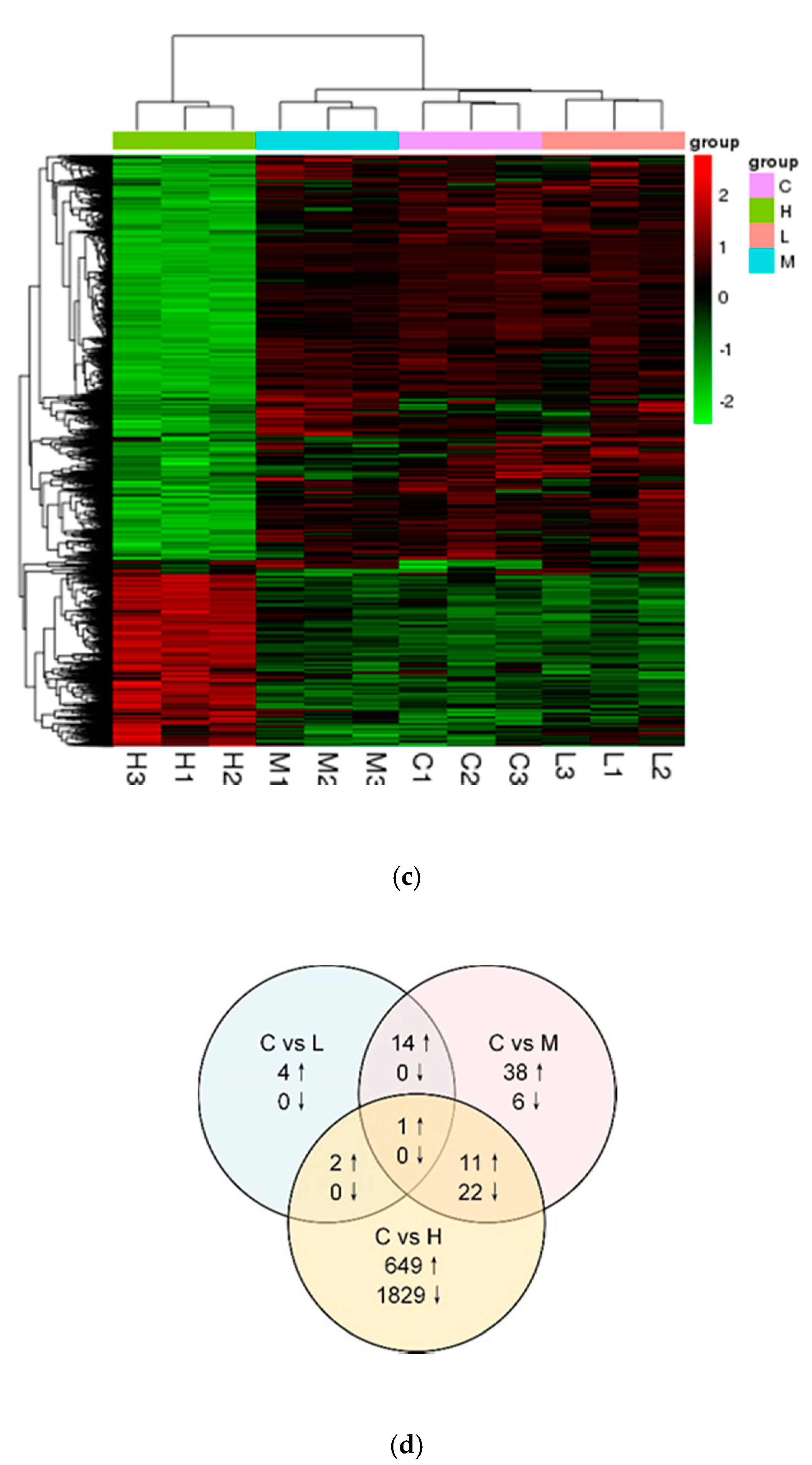
3.2. Analysis of DEGs
3.3. Analyses of GO and Functional Pathway
4. Discussion
4.1. Genes involved in Photosynthesis
4.2. Genes Related to Xenobiotic Metabolism
4.3. Genes Involved in Amino Acids, Nucleotide, and Carbohydrate Metabolism
4.4. Genes Involved in Antioxidant Defense
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ying, G.; Pan, C.; Liu, Y.; Zhao, J. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Canterino, M.; Giudice, R.L.; Marotta, R.; Pinto, G.; Pollio, A. Lincomycin solar photodegradation, algal toxicity and removal from wastewaters by means of ozonation. Water Res. 2006, 40, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Lu, A.; Guo, X.; Zhang, Q.; Wang, Y.; Guo, X.; Wang, L.; Zhang, B. Effects of co-composting of lincomycin mycelia dregs with furfural slag on lincomycin degradation, degradation products, antibiotic resistance genes and bacterial community. Bioresour. Technol. 2019, 272, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, J.; Zhang, Y.; Kong, L.; Jin, M.; Yang, X.; Wu, Q. Trends in the occurrence and risk assessment of antibiotics in shallow lakes in the lower-middle reaches of the Yangtze River basin, China. Ecotoxicol. Environ. Saf. 2019, 183, 109511. [Google Scholar] [CrossRef] [PubMed]
- Kleywegt, S.; Pileggi, V.; Yang, P.; Hao, C.; Zhao, X.; Rocks, C.; Thach, S.; Cheung, P.; Whitehead, B. Pharmaceuticals, hormones and bisphenol A in untreated source and finished drinking water in Ontario, Canada—Occurrence and treatment efficiency. Sci. Total Environ. 2011, 409, 1481–1488. [Google Scholar] [CrossRef]
- Guo, J.; Boxall, A.; Selby, K. Do pharmaceuticals pose a threat to primary producers? Crit. Rev. Environ. Sci. Technol. 2015, 45, 2565–2610. [Google Scholar] [CrossRef]
- OECD. Test No. 201: Freshwater alga and cyanobacteria, growth inhibition test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2011; Volume 2, p. 25. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Bai, Y.; Chen, Z.; Mo, J.; Li, Q.; Sun, H.; Zhang, Q. Transcriptomic analysis suggests the inhibition of DNA damage repair in green alga Raphidocelis subcapitata exposed to roxithromycin. Ecotoxicol. Environ. Saf. 2020, 201, 110737. [Google Scholar] [CrossRef]
- Guo, J.; Ma, Z.; Peng, J.; Mo, J.; Li, Q.; Guo, J.; Yang, F. Transcriptomic analysis of Raphidocelis subcapitata exposed to erythromycin: The role of DNA replication in hormesis and growth inhibition. J. Hazard. Mater. 2021, 402, 123512. [Google Scholar] [CrossRef]
- Brockmeier, E.K.; Hodges, G.; Hutchinson, T.H.; Butler, E.; Hecker, M.; Tollefsen, K.E.; Garcia-Reyero, N.; Kille, P.; Becker, D.; Chipman, K. The role of omics in the application of adverse outcome pathways for chemical risk assessment. Toxicol. Sci. 2017, 158, 252–262. [Google Scholar] [CrossRef]
- Drugbank. Available online: https://go.drugbank.com/drugs/DB01627 (accessed on 15 October 2020).
- Manuell, A.; Beligni, M.; Yamaguchi, K.; Mayfield, S. Regulation of Chloroplast Translation: Interactions of RNA Elements, RNA-Binding Proteins and the Plastid Ribosome; Portland Press Limited: London, UK, 2004. [Google Scholar]
- Brain, R.A.; Hanson, M.L.; Solomon, K.R.; Brooks, B.W. Aquatic plants exposed to pharmaceuticals: Effects and risks. Rev. Environ. Contam. Toxicol. 2008, 192, 67–115. [Google Scholar] [PubMed]
- Guo, J.; Selby, K.; Boxall, A.B. Effects of Antibiotics on the Growth and Physiology of Chlorophytes, Cyanobacteria, and a Diatom. Arch. Environ. Contam. Toxicol. 2016, 71, 589–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Q.; Hu, L.; Liu, Y.; Wang, T.; Ying, G. New insight into the toxic effects of chloramphenicol and roxithromycin to algae using FTIR spectroscopy. Aquat. Toxicol. 2019, 207, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kabra, A.N.; Ji, M.K.; Choi, J.; Kim, J.R.; Govindwar, S.P.; Jeon, B.H. Toxicity of atrazine and its bioaccumulation and biodegradation in a green microalga, Chlamydomonas mexicana. Environ. Sci. Pollut. Res. Int. 2014, 21, 12270–12278. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zheng, Y.; Qi, Q.; Peng, J.; Song, J.; Guo, J.; Guo, J. Involvement of oxidative stress in the sensitivity of two algal species exposed to roxithromycin. Ecotoxicology 2020, 29, 625–633. [Google Scholar] [CrossRef]
- Guo, J.; Peng, J.; Lei, Y.; Kanerva, M.; Li, Q.; Song, J.; Guo, J.; Sun, H. Comparison of oxidative stress induced by clarithromycin in two freshwater microalgae Raphidocelis subcapitata and Chlorella vulgaris. Aquat. Toxicol. 2020, 219, 105376. [Google Scholar] [CrossRef]
- Huang, W.; Ye, J.; Zhang, J.; Lin, Y.; He, M.; Huang, J. Transcriptome analysis of Chlorella zofingiensis to identify genes and their expressions involved in astaxanthin and triacylglycerol biosynthesis. Algal Res. 2016, 17, 236–243. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhou, Q.; Jin, Z.; Jiang, Y.; Xu, L.; Jiang, H.; Zhao, Y. Bioaccumulation, growth performance, and transcriptomic response of Dictyosphaerium sp. after exposure to nonylphenol. Sci. Total Environ. 2019, 687, 416–422. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kolde, R.; Kolde, M.R. Package ‘pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Yan, P.; Mo, Z.; Dong, J.; Chen, F.; Qian, J.; Xia, J.; Xu, L.; Zhang, J.; Li, H. Construction of Mn valence-engineered MnO2/BiOCl heterojunction coupled with carriers-trapping effect for enhanced photoelectrochemical lincomycin aptasensor. Sens. Actuators B Chem. 2020, 320, 128415. [Google Scholar] [CrossRef]
- Pietrzykowska, M.; Suorsa, M.; Semchonok, D.A.; Tikkanen, M.; Boekema, E.J.; Aro, E.M.; Jansson, S. The light-harvesting chlorophyll a/b binding proteins Lhcb1 and Lhcb2 play complementary roles during state transitions in Arabidopsis. Plant Cell 2014, 26, 3646–3660. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Ma, J.; Pan, X.; Zhao, X.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Antenna arrangement and energy transfer pathways of a green algal photosystem-I–LHCI supercomplex. Nat. Plants 2019, 5, 273. [Google Scholar] [CrossRef]
- Qian, H.; Li, J.; Pan, X.; Sun, Z.; Ye, C.; Jin, G.; Fu, Z. Effects of streptomycin on growth of algae Chlorella vulgaris and Microcystis aeruginosa. Environ. Toxicol. 2012, 27, 229–237. [Google Scholar] [CrossRef]
- Ben-Shaul, Y.; Markus, Y. Effects of chloramphenicol on growth, size distribution, chlorophyll synthesis and ultrastructure of Euglena gracilis. J. Cell Sci. 1969, 4, 627–644. [Google Scholar]
- Hankamer, B.; Lehr, F.; Rupprecht, J.; Mussgnug, J.H.; Posten, C.; Kruse, O. Photosynthetic biomass and H2 production by green algae: From bioengineering to bioreactor scale-up. Physiol. Plant. 2007, 131, 10–21. [Google Scholar] [CrossRef]
- van der Grinten, E.; Pikkemaat, M.G.; van den Brandhof, E.J.; Stroomberg, G.J.; Kraak, M.H. Comparing the sensitivity of algal, cyanobacterial and bacterial bioassays to different groups of antibiotics. Chemosphere 2010, 80, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Min, Z.; Li, W.; Xu, L.; Han, J.; Li, P. Interactive effects of roxithromycin and freshwater microalgae, Chlorella pyrenoidosa: Toxicity and removal mechanism. Ecotoxicol. Environ. Saf. 2020, 191, 110156. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xu, H.; Li, Y. Transcriptome-based global analysis of gene expression in response to carbon dioxide deprivation in the green algae Chlorella pyrenoidosa. Algal Res. 2016, 16, 12–19. [Google Scholar] [CrossRef]
- Li, X.; Yuan, Y.; Cheng, D.; Gao, J.; Kong, L.; Zhao, Q.; Wei, W.; Sun, Y. Exploring stress tolerance mechanism of evolved freshwater strain Chlorella sp. S30 under 30 g/L salt. Bioresour. Technol. 2018, 250, 495–504. [Google Scholar] [CrossRef]
- Pan, C.; Peng, F.; Ying, G. Removal, biotransformation and toxicity variations of climbazole by freshwater algae Scenedesmus obliquus. Environ. Pollut. 2018, 240, 534–540. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Wiencke, C.; Sandermann, H. Activity of phase I and phase II detoxication enzymes in Antarctic and Arctic macroalgae. Mar. Environ. Res. 1999, 48, 23–36. [Google Scholar] [CrossRef]
- Ahuja, S.; Jahr, N.; Im, S.C.; Vivekanandan, S.; Popovych, N.; Le Clair, S.V.; Huang, R.; Soong, R.; Xu, J.; Yamamoto, K. A model of the membrane-bound cytochrome b5-cytochrome P450 complex from NMR and mutagenesis data. J. Biol. Chem. 2013, 288, 22080–22095. [Google Scholar] [CrossRef] [Green Version]
- Zanger, U.M.; Raimundo, S.; Eichelbaum, M. Cytochrome P450 2D6: Overview and update on pharmacology, genetics, biochemistry. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 23–37. [Google Scholar] [CrossRef]
- Si, M.; Long, M.; Chaudhry, M.T.; Xu, Y.; Zhang, P.; Zhang, L.; Shen, X. Functional characterization of Corynebacterium glutamicum mycothiol S-conjugate amidase. PLoS ONE 2014, 9, e115075. [Google Scholar] [CrossRef]
- Bolwell, G.P.; Wojtaszek, P. Mechanisms for the generation of reactive oxygen species in plant defence—A broad perspective. Physiol. Mol. Plant Pathol. 1997, 51, 347–366. [Google Scholar] [CrossRef]
- Roy, G.; Sarma, B.K.; Phadnis, P.P.; Mugesh, G. Selenium-containing enzymes in mammals: Chemical perspectives. J. Chem. Sci. 2005, 117, 287–303. [Google Scholar] [CrossRef] [Green Version]
- Vítová, M.; Bišová, K.; Hlavová, M.; Zachleder, V.; Rucki, M.; Čížková, M. Glutathione peroxidase activity in the selenium-treated alga Scenedesmus quadricauda. Aquat. Toxicol. 2011, 102, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Avery, S.V.; Codd, G.A.; Gadd, G.M. Characterisation of Caesium Transport in the Microalga Chlorella salina; Portland Press Ltd.: London, UK, 1995. [Google Scholar]
- Jabusch, T.W.; Swackhamer, D.L. Subcellular accumulation of polychlorinated biphenyls in the green alga Chlamydomonas reinhardtii. Environ. Toxicol. Chem. Int. J. 2004, 23, 2823–2830. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J.; Kretzschmar, T.; Burla, B.; Lee, Y.; Martinoia, E.; Nagy, R. Functions of ABC transporters in plants. Essays Biochem. 2011, 50, 145–160. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Ortiz, C.; Dutta, S.K.; Natarajan, P.; Peña-Garcia, Y.; Abburi, V.; Saminathan, T.; Nimmakayala, P.; Reddy, U.K. Genome-wide identification and gene expression pattern of ABC transporter gene family in Capsicum spp. PLoS ONE 2019, 14, e0215901. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J. Carbohydrate metabolism and respiration in algae. Photosynthesis in Algae. 2003, Volume 1, pp. 205–224. Available online: https://www.researchgate.net/publication/226999701_Carbohydrate_Metabolism_and_Respiration_in_Algae (accessed on 30 November 2020).
- Li, D.W.; Balamurugan, S.; Yang, Y.F.; Zheng, J.W.; Huang, D.; Zou, L.G.; Yang, W.D.; Liu, J.S.; Guan, Y.; Li, H.Y. Transcriptional regulation of microalgae for concurrent lipid overproduction and secretion. Sci. Adv. 2019, 5, eaau3795. [Google Scholar] [CrossRef] [Green Version]
- Yuan, P.; Zhou, Q.; Hu, X. The phases of WS2 nanosheets influence uptake, oxidative stress, lipid peroxidation, membrane damage, and metabolism in algae. Environ. Sci. Technol. 2018, 52, 13543–13552. [Google Scholar] [CrossRef]
- Tevatia, R.; Allen, J.; Rudrappa, D.; White, D.; Clemente, T.E.; Cerutti, H.; Demirel, Y.; Blum, P. The taurine biosynthetic pathway of microalgae. Algal Res. 2015, 9, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, T.; Higuchi, K.; Yoshida, Y.; Sugita-Fujisawa, Y.; Kojima, K.; Sugimoto, M.; Santo, M.; Tomi, M.; Nakashima, E. Hypotaurine is a substrate of GABA transporter family members GAT2/Slc6a13 and TAUT/Slc6a6. Biol. Pharm. Bull. 2018, 41, 1523–1529. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Pang, S.J. Stress tolerance and antioxidant enzymatic activities in the metabolisms of the reactive oxygen species in two intertidal red algae Grateloupia turuturu and Palmaria palmata. J. Exp. Mar. Biol. Ecol. 2010, 382, 82–87. [Google Scholar] [CrossRef]
- Belenky, P.; Jonathan, D.Y.; Porter, C.B.; Cohen, N.R.; Lobritz, M.A.; Ferrante, T.; Jain, S.; Korry, B.J.; Schwarz, E.G.; Walker, G.C. Bactericidal antibiotics induce toxic metabolic perturbations that lead to cellular damage. Cell Rep. 2015, 13, 968–980. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; Kurade, M.B.; Abou-Shanab, R.A.; Ji, M.; Choi, J.; Kim, J.O.; Jeon, B. Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, G.; Ding, J.; Zhang, Z.; Wang, Y. Tissue distribution, bioconcentration, metabolism, and effects of erythromycin in crucian carp (Carassius auratus). Sci. Total Environ. 2014, 490, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, O.; Pinto, E.; Latorre, L.; Bechara, E.; Colepicolo, P. Antioxidant modulation in response to metal-induced oxidative stress in algal chloroplasts. Arch. Environ. Contam. Toxicol. 2001, 40, 18–24. [Google Scholar] [PubMed]
- Heredia-Martínez, L.G.; Andrés-Garrido, A.; Martínez-Force, E.; Pérez-Pérez, M.E.; Crespo, J.L. Chloroplast damage induced by the inhibition of fatty acid synthesis triggers autophagy in Chlamydomonas. Plant Physiol. 2018, 178, 1112–1129. [Google Scholar] [CrossRef] [Green Version]


| Pathway | Category | Up-gene | Down-gene | p Value | FDR |
|---|---|---|---|---|---|
| Control vs. Low | |||||
| Photosynthesis—antenna proteins | Energy metabolism | lhca2, lhca4, lhcb1 | - | 1.43 × 10−7 | 4.30 × 10−7 |
| Porphyrin and chlorophyll metabolism | Metabolism of cofactors and vitamins | hemE, hepe | - | 0.0044 | 0.0065 |
| photosynthesis | Energy metabolism | psbY | - | 0.096 | 0.096 |
| Control vs. Medium | |||||
| Photosynthesis - antenna proteins | Energy metabolism | lhca4, lhcb1, lhcb2 | - | 2.60 × 10−16 | 2.34 × 10−15 |
| Porphyrin and chlorophyll metabolism | Metabolism of cofactors and vitamins | chlD, chlH, hemA, hemD, hemE, hemL, por | - | 2.51× 10−8 | 1.13× 10−7 |
| Control vs. High | |||||
| Pentose and glucuronate interconversions | Carbohydrate metabolism | rpe, xk1 | ugdh, ugp2, ugt | 0.0022 | 0.15 |
| Starch and sucrose metabolism | Carbohydrate metabolism | glgA, isa, spp, tps, waxy | E2.4.4.14, E3.2.1.2, E3.2.1.4, ugp2 | 0.0034 | 0.15 |
| Amino sugar and nucleotide sugar metabolism | Carbohydrate metabolism | E3.2.1.14, uap1 | E1.6.2.2, gmpp, rhm, ugdh, ugp2 | 0.056 | 0.74 |
| Taurine and hypotaurine metabolism | Metabolism of other amino acids | - | ado, cdo1 | 0.014 | 0.41 |
| Arginine and proline metabolism | Amino acid metabolism | aguA, E3.5.1.4 | p4ha, pip | 0.065 | 0.74 |
| Purine metabolism | Nucleotide metabolism | apa1_2, ndk, pk | adk, ade2, adprm, E2.7.1.20, fhit, purH, relA, rrm1, rrm2 | 0.038 | 0.74 |
| Pyrimidine metabolism | Nucleotide metabolism | ndk, udk, upb1 | cmpk1, pyrB, rrm1, rrm2 | 0.098 | 0.90 |
| Carbon fixation in photosynthetic organisms | Energy metabolism | aldo, gapa, tbl1 | Gapdh, ppdK | 0.063 | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Bai, Y.; Chen, Z.; Mo, J.; Tian, Y.; Guo, J. Lincomycin-Induced Transcriptional Alterations in the Green Alga Raphidocelis subcapitata. Appl. Sci. 2020, 10, 8565. https://doi.org/10.3390/app10238565
Zhang Q, Bai Y, Chen Z, Mo J, Tian Y, Guo J. Lincomycin-Induced Transcriptional Alterations in the Green Alga Raphidocelis subcapitata. Applied Sciences. 2020; 10(23):8565. https://doi.org/10.3390/app10238565
Chicago/Turabian StyleZhang, Qiang, Yi Bai, Zhi Chen, Jiezhang Mo, Yulu Tian, and Jiahua Guo. 2020. "Lincomycin-Induced Transcriptional Alterations in the Green Alga Raphidocelis subcapitata" Applied Sciences 10, no. 23: 8565. https://doi.org/10.3390/app10238565




