Can Encapsulation of the Biocide DCOIT Affect the Anti-Fouling Efficacy and Toxicity on Tropical Bivalves?
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Compounds
2.2. Animals
2.3. Fertilization Assay
2.4. Embryo-Larval Development Assay
2.5. Short-Term Exposure Assay with Juveniles
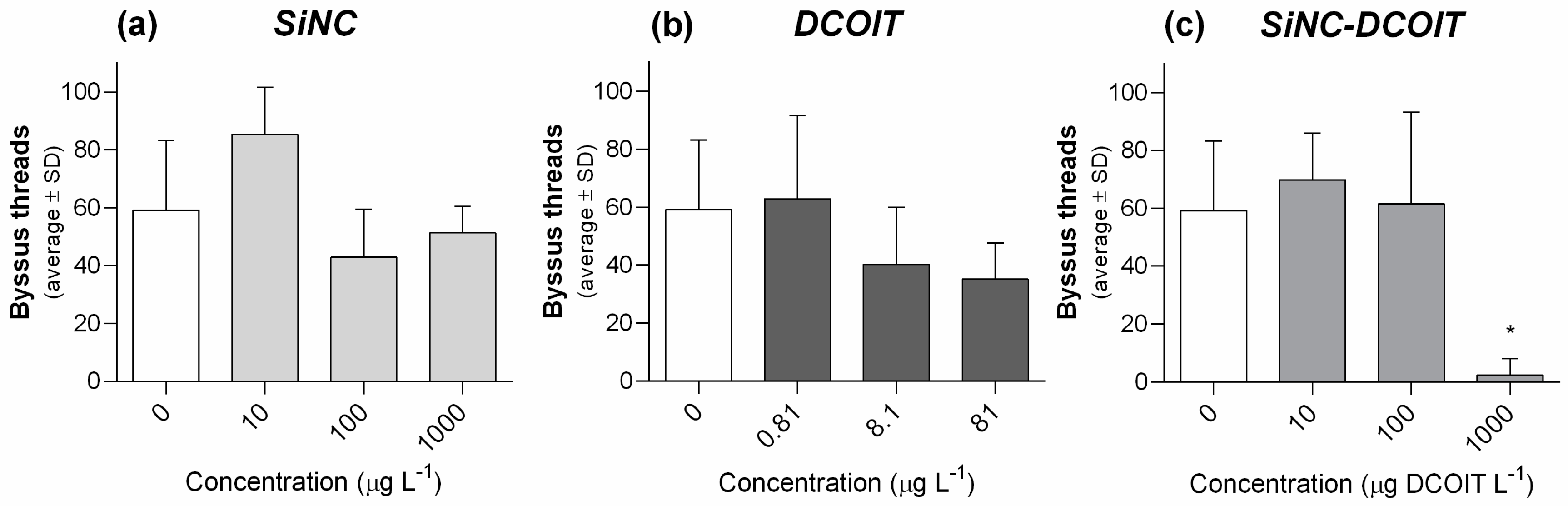
2.5.1. Byssus Threads Formation
2.5.2. Survival-in-Air Test
2.6. Statistical Analysis
3. Results and Discussion
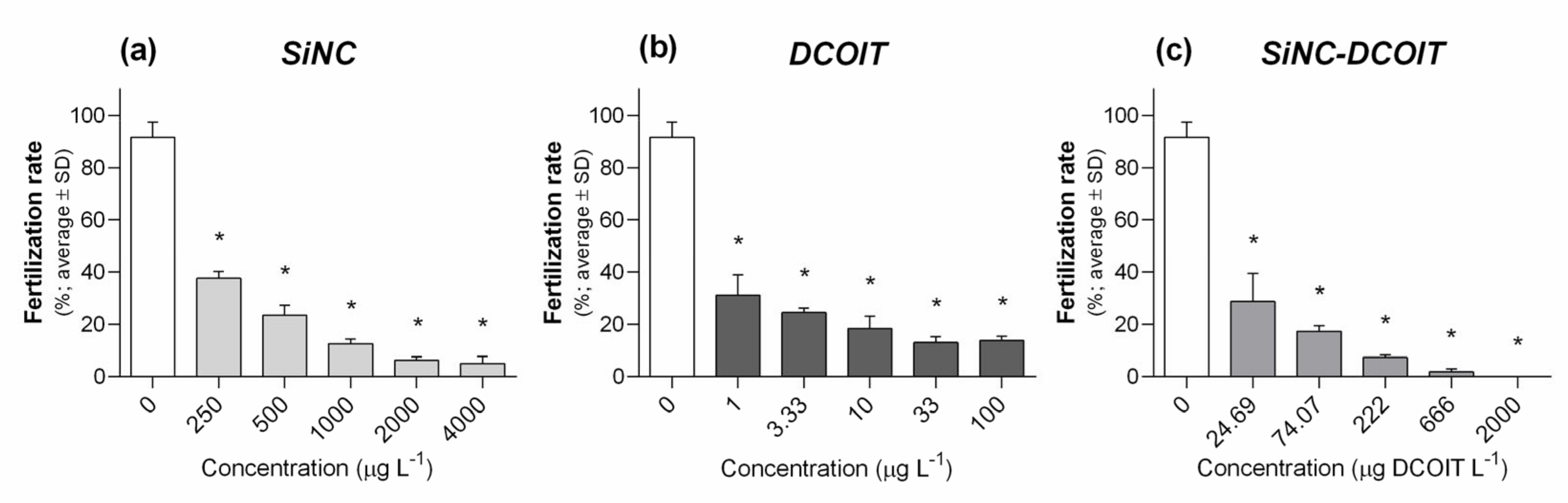
3.1. Fertilization Assay
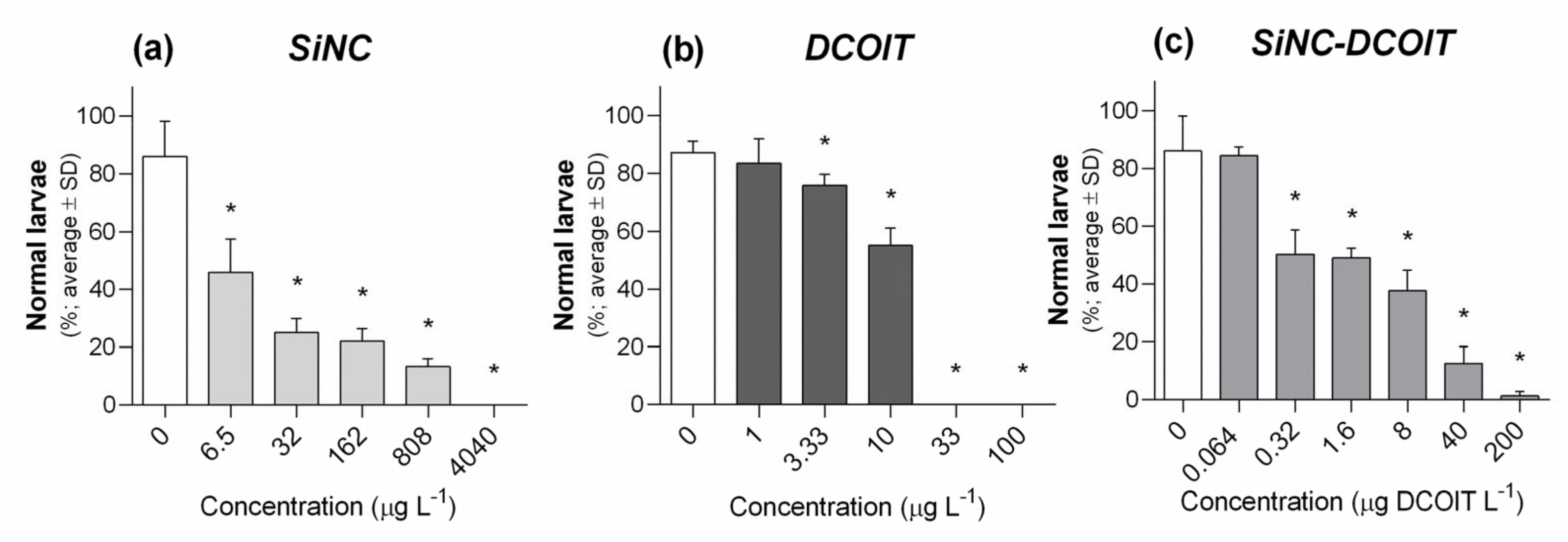
3.2. Embryo-Larval Development Assay
3.3. Short-Term Exposure Assay with Juvenile Mussel Stages
3.4. Environmental Relevance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Lam, J.C.W. SeaNine 211 as antifouling biocide: A coastal pollutant of emerging concern. J. Environ. Sci. 2017, 61, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.M.; Birchenough, A.C.; Brancato, M.S. The TBT Ban: Out of the Frying Pan into the Fire? Mar. Pollut. Bull. 2000, 40, 204–211. [Google Scholar] [CrossRef]
- Fernandes, J.A.; Santos, L.; Vance, T.; Fileman, T.; Smith, D.; Bishop, J.D.D.; Viard, F.; Queirós, A.M.; Merino, G.; Buisman, E.; et al. Costs and benefits to European shipping of ballast-water and hull-fouling treatment: Impacts of native and non-indigenous species. Mar. Policy 2016, 64, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Voulvoulis, N. Antifouling Paint Booster Biocides: Occurrence and Partitioning in Water and Sediments. In Handbook of Environmental Chemistry: Water Pollution; Konstantinou, I.K., Ed.; Springer: Berlin, Germany, 2006; Volume 50, pp. 155–170. [Google Scholar]
- Arai, T.; Harino, H. Contamination by Organotin Compounds in Asia. In Ecotoxicology of Antifouling Biocides; Arai, T., Harino, H., Ohji, M., Langston, W.J., Eds.; Springer: Tokyo, Japan, 2009; pp. 61–74. [Google Scholar]
- Perina, F.C.; Abessa, D.M.S.; Pinho, G.L.L.; Fillmann, G. Comparative toxicity of antifouling compounds on the development of sea urchin. Ecotoxicology 2011, 20, 1870–1880. [Google Scholar] [CrossRef]
- Castro, Í.B.; Westphal, E.; Fillmann, G. Third generation antifouling paints: New biocides in the aquatic environment. Quim. Nova 2011, 34, 1021–1031. [Google Scholar] [CrossRef]
- [USEPA] United States Environmental Protection Agency. Presidential Green Chemistry Challenge: 1996 Designing Greener Chemicals Award Rohm and Haas Company (now a subsidiary of The Dow Chemical Company). Designing an Environmentally Safe Marine Antifoulant. Available online: https://www.epa.gov/greenchemistry/presidential-green-chemistry-challenge-1996-designing-greener-chemicals-award (accessed on 27 October 2020).
- Thomas, K.V. The environmental fate and behaviour of antifouling paint booster biocides: A review. Biofouling 2001, 17, 73–86. [Google Scholar] [CrossRef]
- Shade, W.D.; Hurt, S.S.; Jacobson, A.H.; Reinert, K.H. Ecological Risk Assessment of a Novel Marine Antifoulant. In Environmental Toxicology and Risk Assessment: Second Volume; STP1216-EB; Gorsuch, J.W., Dwyer, F.J., Ingersoll, C.G., LaPoint, T.W., Eds.; ASTM International: Philadelphia, PA, USA, 1993; Volume 2, pp. 408–1993. [Google Scholar]
- Chen, L.; Xu, Y.; Wang, W.; Qian, P.Y. Degradation kinetics of a potent antifouling agent, butenolide, under various environmental conditions. Chemosphere 2015, 119, 1075–1083. [Google Scholar] [CrossRef]
- Figueiredo, J.; Oliveira, T.; Ferreira, V.; Sushkova, A.; Silva, S.; Carneiro, D.; Cardoso, D.N.; Gonçalves, S.F.; Maia, F.; Rocha, C.; et al. Toxicity of innovative anti-fouling nano-based solutions to marine species. Environ. Sci. Nano 2019, 6, 1418–1429. [Google Scholar] [CrossRef]
- Sakkas, V.A.; Konstantinou, I.K.; Lambropoulou, D.A.; Albanis, T.A. Survey for the occurrence of antifouling paint booster biocides in the aquatic environment of Greece. Environ. Sci. Pollut. Res. 2002, 9, 327–332. [Google Scholar] [CrossRef]
- Tsunemasa, N.; Yamazaki, H. Concentration of antifouling biocides and metals in sediment core samples in the northern part of Hiroshima bay. Int. J. Mol. Sci. 2014, 15, 9991–10004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, K.; Barceló, D. Determination of antifouling pesticides and their degradation products in marine sediments by means of ultrasonic extraction and HPLC-APCI-MS. Anal. Bioanal. Chem. 2001, 370, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Bae, J.S.; Kang, S.G.; Son, J.S.; Jeon, J.H.; Lee, H.J.; Jeon, J.Y.; Sidharthan, M.; Ryu, S.H.; Shin, H.W. Acute toxicity of organic antifouling biocides to phytoplankton Nitzschia pungens and zooplankton Artemia larvae. Mar. Pollut. Bull. 2017, 124, 811–818. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. 4,5-dichloro-2-octyl-2H-isothiazol-3-one. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.058.930 (accessed on 27 October 2020).
- Geiger, T.; Delavy, P.; Hany, R.; Schleuniger, J.; Zinn, M. Encapsulated Zosteric Acid Embedded in Poly [3-hydroxyalkanoate] Coatings—Protection against Biofouling. Polym. Bull. 2004, 52, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Hart, R.L.; Virgallito, D.R.; Work, D.E. Microencapsulation of Biocides and Antifoulingagents. U.S. Patent 7,938,897, 10 May 2011. [Google Scholar]
- Szabó, T.; Molnár-Nagy, L.; Bognár, J.; Nyikos, L.; Telegdi, J. Self-healing microcapsules and slow release microspheres in paints. Prog. Org. Coat. 2011, 72, 52–57. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, X.; Schenderlein, M.; Borisova, D.; Cao, R.; Möhwald, H.; Shchukin, D. Self-healing and antifouling multifunctional coatings based on pH and sulfide ion sensitive nanocontainers. Adv. Funct. Mater. 2013, 23, 3307–3314. [Google Scholar] [CrossRef]
- Avelelas, F.; Martins, R.; Oliveira, T.; Maia, F.; Malheiro, E.; Soares, A.M.V.M.; Loureiro, S.; Tedim, J. Efficacy and Ecotoxicity of Novel Anti-Fouling Nanomaterials in Target and Non-Target Marine Species. Mar. Biotechnol. 2017, 19, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Maia, F.; Silva, A.P.; Fernandes, S.; Cunha, A.; Almeida, A.; Tedim, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Incorporation of biocides in nanocapsules for protective coatings used in maritime applications. Chem. Eng. J. 2015, 270, 150–157. [Google Scholar] [CrossRef]
- Maia, F.; Tedim, J.; Lisenkov, A.D.; Salak, A.N.; Zheludkevich, M.L.; Ferreira, M.G.S. Silica nanocontainers for active corrosion protection. Nanoscale 2012, 4, 1287–1298. [Google Scholar] [CrossRef]
- Lourenço, C.R.; Nicastro, K.R.; McQuaid, C.D.; Chefaoui, R.M.; Assis, J.; Taleb, M.Z.; Zardi, G.I. Evidence for rangewide panmixia despite multiple barriers to dispersal in a marine mussel. Sci. Rep. 2017, 7, 10279. [Google Scholar] [CrossRef] [Green Version]
- Pierri, B.S.; Fossari, T.D.; Magalhães, A.M.R. The brown mussel Perna perna in Brazil: Native or exotic? Arq. Bras. Med. Veterinária Zootec. 2016, 68, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Zaroni, L.P.; Abessa, D.M.S.; Lotufo, G.R.; Sousa, E.C.P.M.; Pinto, Y.A. Toxicity Testing with Embryos of Marine Mussels: Protocol Standardization for Perna perna (Linnaeus, 1758). Bull. Environ. Contam. Toxicol. 2005, 74, 793–800. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms, EPA-821-R-02-014, 3rd ed.; United States Environmental Protection Agency: Washington, DC, USA, 2002. [Google Scholar]
- Associação Brasileira de Normas Técnicas. Aquatic Ecotoxicology—Short Term Method Test with Bivalve Embryos (Mollusca—Bivalvae); NBR 16456; Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Bellas, J. Toxicity of the booster biocide Sea-Nine to the early developmental stages of the sea urchin Paracentrotus lividus. Aquat. Toxicol. 2007, 83, 52–61. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Martins, R.; Figueiredo, J.; Loureiro, S.; Tedim, J. Environmental behaviour and ecotoxicity of cationic surfactants towards marine organisms. J. Hazard. Mater. 2020, 392, 122299. [Google Scholar] [CrossRef] [PubMed]
- Gutner-Hoch, E.; Martins, R.; Maia, F.; Oliveira, T.; Shpigel, M.; Weis, M.; Tedim, J.; Benayahu, Y. Toxicity of engineered micro- and nanomaterials with antifouling properties to the brine shrimp Artemia salina and embryonic stages of the sea urchin Paracentrotus lividus. Environ. Pollut. 2019, 251, 530–537. [Google Scholar] [CrossRef]
- Gutner-Hoch, E.; Martins, R.; Oliveira, T.; Maia, F.; Soares, A.M.V.M.; Loureiro, S.; Piller, C.; Preiss, I.; Weis, M.; Larroze, S.B.; et al. Antimacrofouling efficacy of innovative inorganic nanomaterials loaded with booster biocides. J. Mar. Sci. Eng. 2018, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Kaczerewska, O.; Sousa, I.; Martins, R.; Figueiredo, J.; Loureiro, S.; Tedim, J. Gemini surfactant as a template agent for the synthesis of more eco-friendly silica nanocapsules. Appl. Sci. 2020, 10, 8085. [Google Scholar] [CrossRef]
- Bellas, J. Comparative toxicity and of alternative antifouling biocides on embryos larvae of marine invertebrates. Sci. Total Environ. 2006, 367, 573–585. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Pesticide Ecotoxicity Database (Formerly: Environmental Effects Database—EEDB); ECOREF #344; U.S. Environmental Protection Agency, Environmental Fate and Effects Division: Washington, DC, USA, 2000. Available online: https://cfpub.epa.gov/ecotox/search.cfm (accessed on 11 August 2020).
- Harino, H.; Kitano, M.; Mori, Y.; Mochida, K.; Kakuno, A.; Arima, S. Degradation of antifouling booster biocides in water. J. Mar. Biol. Assoc. UK 2005, 85, 33–38. [Google Scholar] [CrossRef]
- Readman, J.W. Development, occurrence and regulation of antifouling paint biocides: Historical review and future trends. In Handbook of Environmental Chemistry: Water Pollution; Konstantinou, I.K., Ed.; Springer: Berlin, Germany, 2006; Volume 50, pp. 1–15. [Google Scholar]
- Martínez, K.; Ferrer, I.; Hernando, M.D.; Fernández-Alba, A.R.; Marcé, R.M.; Borrull, F.; Barceló, D. Occurrence of antifouling biocides in the spanish mediterranean marine environment. Environ. Technol. 2001, 22, 543–552. [Google Scholar] [CrossRef]
- Figueiredo, J.; Loureiro, S.; Martins, R. Hazard of novel anti-fouling nanomaterials and biocides DCOIT and silver to marine organisms. Environ. Sci. Nano 2020, 7, 1670–1680. [Google Scholar] [CrossRef]
- Fonseca, V.B.; Guerreiro, A.S.; Vargas, M.A.; Sandrini, J.Z. Effects of DCOIT (4,5-dichloro-2-octyl-4-isothiazolin-3-one) to the haemocytes of mussels Perna perna. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 232, 108737. [Google Scholar] [CrossRef] [PubMed]
- Resgalla, C.; Brasil, E.S.; Laitano, K.S.; Filho, R.W.R. Physioecology of the mussel Perna perna (Mytilidae) in Southern Brazil. Aquaculture 2007, 270, 464–474. [Google Scholar] [CrossRef]

| Parameter | NOEC | LOEC | EC50 | 95% CI | |
|---|---|---|---|---|---|
| SiNC | Fertilization | <250.0 | 250.0 | 161.3 | 139.3–186.8 |
| Embryotoxicity | <6.5 | 6.5 | 39.8 | 10.5–150.8 | |
| Byssus threads | 1000 | >1000 | 1323 | 218.2–8019 | |
| Air survival capacity | 1000 | >1000 | nd | – | |
| DCOIT | Fertilization | <1.0 | 1.0 | 0.063 | 0.016–0.255 |
| Embryotoxicity | 1.0 | 3.3 | 12.4 | 9.9–15.4 | |
| Byssus threads | 81.0 | >81.0 | 96.1 | 6.3–470 | |
| Air survival capacity | <0.810 | 0.810 | nd | – | |
| SiNC-DCOIT | Fertilization | <24.7 | 24.7 | 8.6 | 4.9–15.0 |
| Embryotoxicity | 0.064 | 0.320 | 6.8 | 2.7–16.9 | |
| Byssus threads | 100.0 | 1000 | 305.5 | 124.2–751.5 | |
| Air survival capacity | <10.0 | 10.0 | nd | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.V.N.d.; Martins, R.; Fontes, M.K.; Campos, B.G.d.; Silva, M.B.M.d.P.e.; Maia, F.; Abessa, D.M.d.S.; Perina, F.C. Can Encapsulation of the Biocide DCOIT Affect the Anti-Fouling Efficacy and Toxicity on Tropical Bivalves? Appl. Sci. 2020, 10, 8579. https://doi.org/10.3390/app10238579
Santos JVNd, Martins R, Fontes MK, Campos BGd, Silva MBMdPe, Maia F, Abessa DMdS, Perina FC. Can Encapsulation of the Biocide DCOIT Affect the Anti-Fouling Efficacy and Toxicity on Tropical Bivalves? Applied Sciences. 2020; 10(23):8579. https://doi.org/10.3390/app10238579
Chicago/Turabian StyleSantos, Juliana Vitoria Nicolau dos, Roberto Martins, Mayana Karoline Fontes, Bruno Galvão de Campos, Mariana Bruni Marques do Prado e Silva, Frederico Maia, Denis Moledo de Souza Abessa, and Fernando Cesar Perina. 2020. "Can Encapsulation of the Biocide DCOIT Affect the Anti-Fouling Efficacy and Toxicity on Tropical Bivalves?" Applied Sciences 10, no. 23: 8579. https://doi.org/10.3390/app10238579






