Hyperlipidemic Rabbit Models for Anti-Atherosclerotic Drug Development
Abstract
:1. Introduction
2. Hyperlipidemic Rabbit Models
2.1. Diet-Induced Hyperlipidemic Rabbits
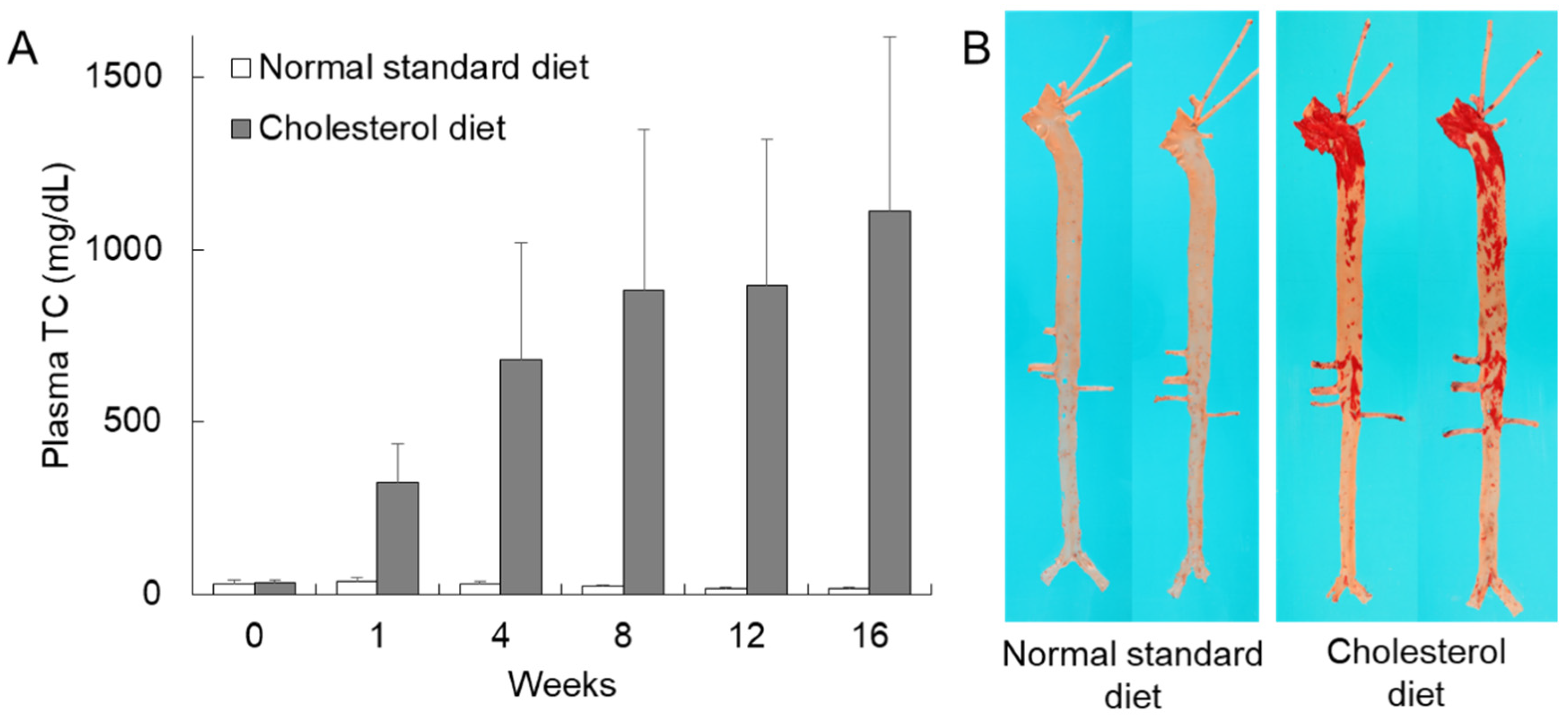
2.1.1. Cholesterol-Fed Rabbits
2.1.2. Casein-Fed Rabbits
2.2. Spontaneous Hyperlipidemic Rabbits
2.2.1. Watanabe Heritable Hyperlipidemic (WHHL) Rabbits
2.2.2. St. Thomas’ Mixed Hyperlipidemic (SMHL) Rabbits
2.2.3. Postprandial Hypertriglyceridemic (PHT) Rabbits
2.3. Gene-Manipulated Rabbits
2.3.1. Transgenic (Tg) Rabbits
2.3.2. Knockout (KO) Rabbits
3. Hyperlipidemic Rabbit Models for the Development of Drugs
3.1. Statins
| Rabbit | Diet | Drug | Plasma TC | Atherosclerosis | Reference |
|---|---|---|---|---|---|
| Wild-type rabbits | |||||
| JW (Female) | Normal standard | Compactin (5 mg/kg) | 16% ↓ | ‒ | Watanabe [54] |
| Compactin (25 mg/kg) | 41% ↓ | ||||
| for 2 weeks | (2 weeks) | ||||
| NZW (Male) | 27% casein semi-purified | Lovastatin (2 mg/kg) | 37% ↓ * | ‒ | Kroon [60] |
| Lovastatin (6 mg/kg) | 48% ↓ * | ||||
| for 39 days | (39 days) | ||||
| NZW (Male) | 2% cholesterol 6% corn oil | Lovastatin (2.5 mg/kg) | 54% ↓ * | 43% ↓ * | Kritchevsky [61] |
| for 8 weeks | (8 weeks) | (Aortic arch, scale graded gross lesion) | |||
| JW (Male) | 1% cholesterol | Lovastatin (5 mg/kg) | 50% ↓ * | 74% ↓ * | Kobayashi [62] |
| Simvastatin (5 mg/kg) | 80% ↓ * | 81% ↓ * | |||
| for 12 weeks | (12 weeks) | (Aorta, gross lesion) | |||
| NZW (Male) | 27% casein semi-purified | Lovastatin (3 mg/kg) | 20% ↓ * | ‒ | Auerbach [63] |
| Atorvastatin (3 mg/kg) | 57% ↓ * | ||||
| for 6 weeks | (6 weeks) | ||||
| NZW (Male) | 0.5% cholesterol 3% peanut oil 3% coconut oil | Lovastain (2.5 mg/kg) | 40% ↓ * | 39% ↓ | Bocan [58] |
| Simvastatin (2.5 mg/kg) | 43% ↓ * | 41% ↓ | |||
| Pravastatin (2.5 mg/kg) | 37% ↓ * | 13% ↓ | |||
| Atorvastatin (2.5 mg/kg) | 32% ↓ * | 69% ↓ * | |||
| for 8 weeks | (AUC) | (Iliac–femoral artery, microscopic lesion) | |||
| JW (Male) | 0.5% cholesterol | Fluvastain (2 mg/kg) | 14% ↓ | 62% ↓ * | Rikitake [64] |
| for 12 weeks | (12 weeks) | (Aorta, microscopic lesion) | |||
| NZW (Female) | 0.3% cholesterol | Pitavastatin (0.1 mg/kg) | 27% ↓ * | 64% ↓ * | Hayashi [65] |
| for 12 weeks | (12 weeks) | (Aortic arch, microscopic lesion) | |||
| WHHL rabbits | |||||
| (Female) | Normal standard | Lovastatin (0.03% diet) | 40% ↓ | ‒ | Ma [66] |
| for 10 days | (10 days) | ||||
| (Male and female) | Normal standard | Pravastatin (50 mg/kg) | 33% ↓ * | ‒ | Tsujita [67] |
| for 8 weeks | (8 weeks) | ||||
| (Male and female) | Normal standard | Pravastatin (50 mg/kg) | 28% ↓ * | 54% ↓ * | Watanabe [59] |
| for 24 weeks | (24 weeks) | (Coronary artery, microscopic lesion) | |||
| (Male and female) | Normal standard | Cerivastatin (0.6 mg/kg, subcutaneously) | 39% ↓ * | 37% ↓ * | Shiomi [68] |
| for 32 weeks | (32 weeks) | (Thoracic aorta, microscopic lesion) | |||
| (Male) | Normal standard | Pitavastatin (0.5 mg/kg) | 29% ↓ * | 39% ↓ * | Suzuki [69] |
| for 16 weeks | (16 weeks) | (Aorta, gross lesion) | |||
3.2. Fibrates
3.3. Ezetimibe
3.4. Probucol
3.5. CETP Inhibitors
3.6. HDL, Apo A-I, Apo A-IMilano, and Apo A-I Mimetic Peptides
| Agent | Rabbit, Manipulation, and Treatment | Outcome | Reference |
|---|---|---|---|
| HDL | |||
| HDL + VHDL (d = 1.063–1.25 g/mL) |
|
| Badimon [105] |
| HDL + VHDL (d = 1.063–1.25 g/mL) |
|
| Badimon [106] |
| HDL (isolated from HC and NC rabbits) |
|
| Ben-Aicha [107] |
| Apo A-I and apo A-IMilano | |||
| Apo A-I |
|
| Miyazaki [108] |
| Apo A-IMilano + PC |
|
| Soma [109] |
| Apo A-IMilano + DPPC |
|
| Chiesa [110] |
| Apo A-IMilano + POPC (ETC-216) |
|
| Ibanez [111] |
| Apo A-I mimetic peptide | |||
| Ac-hE18A-NH2 |
|
| Gupta [116] |
| D-4F L-4F |
|
| Van Lenten [117] |
| EPS24218 + DPPC + SM (ETC-462) |
|
| Iwata [118] |
4. Limitation of Rabbit Models
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Apo | apolipoprotein |
| CETP | cholesteryl ester transfer protein |
| CHD | coronary heart disease |
| CM | chylomicron |
| FH | familial hypercholesterolemia |
| HDL | high-density lipoprotein |
| HDL-C | high-density lipoprotein cholesterol |
| HL | hepatic lipase |
| JW | Japanese white |
| KO | knockout |
| LDL | low-density lipoprotein |
| LDL-C | low-density lipoprotein cholesterol |
| LDLR | low-density lipoprotein receptor |
| MRI | magnetic resonance imaging |
| NZW | New Zealand white |
| PC | phosphatidylcholine |
| TC | total cholesterol |
| TG | triglycerides |
| Tg | transgenic |
| VHDL | very-high-density lipoprotein |
| VLDL | very-low-density lipoprotein |
| VLDL-C | very-low-density lipoprotein cholesterol |
| WHHL | Watanabe heritable hyperlipidemic |
| WHHLMI | myocardial infarction-prone Watanabe heritable hyperlipidemic |
References
- Ross, R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Beaumont, J.L.; Carlson, L.A.; Cooper, G.R.; Fejfar, Z.; Fredrickson, D.S.; Strasser, T. Classification of hyperlipidaemias and hyperlipoproteinaemias. Bull. World Health Organ. 1970, 43, 891–915. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Chen, Y.; Yan, H.; Niimi, M.; Wang, Y.; Liang, J. Principles and applications of rabbit models for atherosclerosis research. J. Atheroscler. Thromb. 2018, 25, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Duff, G.L. Experimental cholesterol arteriosclerosis and its relationship to human arteriosclerosis. Arch. Pathol. 1935, 20, 81–123. [Google Scholar]
- Zhang, S.H.; Reddick, R.L.; Piedrahita, J.A.; Maeda, N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992, 258, 468–471. [Google Scholar] [CrossRef]
- Ishibashi, S.; Brown, M.S.; Goldstein, J.L.; Gerard, R.D.; Hammer, R.E.; Herz, J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Investig. 1993, 92, 883–893. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Kitajima, S.; Watanabe, T.; Xu, J.; Zhang, J.; Liu, E.; Chen, Y.E. Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol. Ther. 2015, 146, 104–119. [Google Scholar] [CrossRef] [Green Version]
- Ha, Y.; Barter, P. Differences in plasma cholesteryl ester transfer activity in sixteen vertebrate species. Comp. Biochem. Physiol. Part B Biochem. 1982, 71, 265–269. [Google Scholar] [CrossRef]
- Greeve, J.; Altkemper, I.; Dieterich, J.H.; Greten, H.; Windler, E. Apolipoprotein B mRNA editing in 12 different mammalian species: Hepatic expression is reflected in low concentrations of apoB-containing plasma lipoproteins. J. Lipid Res. 1993, 34, 1367–1383. [Google Scholar]
- Steinberg, D. Thematic review series: Living history of lipids: In celebration of the 100th anniversary of the lipid hypothesis of atherosclerosis. J. Lipid Res. 2013, 54, 2946–2949. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Watanabe, T. Cholesterol-fed and transgenic rabbit models for the study of atherosclerosis. J. Atheroscler. Thromb. 2000, 7, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Kolodgie, F.D.; Katocs, A.S.; Largis, E.E.; Wrenn, S.M.; Cornhill, J.F.; Herderick, E.E.; Lee, S.J.; Virmani, R. Hypercholesterolemia in the Rabbit Induced by Feeding Graded Amounts of Low-Level Cholesterol. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1454–1464. [Google Scholar] [CrossRef]
- Mahley, R.W.; Innerarity, T.L.; Brown, M.S.; Ho, Y.K.; Goldstein, J.L. Cholesteryl ester synthesis in macrophages: Stimulation by beta-very low density lipoproteins from cholesterol-fed animals of several species. J. Lipid Res. 1980, 21, 970–980. [Google Scholar]
- Keyamura, Y.; Nagano, C.; Kohashi, M.; Niimi, M.; Nozako, M.; Koyama, T.; Itabe, H.; Yoshikawa, T. Dietary cholesterol atherogenic changes in juvenile rabbits. Biol. Pharm. Bull. 2015, 38, 785–788. [Google Scholar] [CrossRef] [Green Version]
- Huff, M.W.; Hamilton, R.M.G.; Carroll, K.K. Plasma cholesterol levels in rabbits fed low fat, cholesterol-free, semipurified diets: Effects of dietary proteins, protein hydrolysates and amino acid mixtures. Atherosclerosis 1977, 28, 187–195. [Google Scholar] [CrossRef]
- Daley, S.J.; Herderick, E.E.; Cornhill, J.F.; Rogers, K.A. Cholesterol-fed and casein-fed rabbit models of atherosclerosis—Part 1: Differing lesion area and volume despite equal plasma cholesterol levels. Arterioscler. Thromb. 1994, 14, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Beynen, A.C.; Van der Meer, R.; West, C.E. Mechanism of casein-induced hypercholesterolemia: Primary and secondary features. Atherosclerosis 1986, 60, 291–293. [Google Scholar] [CrossRef]
- Huff, M.W.; Carroll, K.K. Effects of dietary protein on turnover, oxidation, and absorption of cholesterol, and on steroid excretion in rabbits. J. Lipid Res. 1980, 21, 546–548. [Google Scholar]
- Chao, Y.; Yamin, T.T.; Alberts, A.W. Effects of cholestyramine on low density lipoprotein binding sites on liver membranes from rabbits with endogenous hypercholesterolemia induced by a wheat starch-casein diet. J. Biol. Chem. 1982, 257, 3623–3627. [Google Scholar]
- Watanabe, Y.; Ito, T.; Kondo, T. Breeding of a Rabbit Strain of Hyperlipidemia and Characteristic of These Strain. Exp. Anim. 1977, 26, 35–41. [Google Scholar] [CrossRef]
- Watanabe, Y. Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit). Incidence and development of atherosclerosis and xanthoma. Atherosclerosis 1980, 36, 261–268. [Google Scholar] [CrossRef]
- Soutar, A.K.; Naoumova, R.P. Mechanisms of disease: Genetic causes of familial hypercholesterolemia. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Bishop, R.W.; Brown, M.S.; Goldstein, J.L.; Russell, D.W. Deletion in Cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science 1986, 232, 1230–1237. [Google Scholar] [CrossRef] [Green Version]
- Kita, T.; Brown, M.S.; Watanabe, Y.; Goldstein, J.L. Deficiency of low density lipoprotein receptors in liver and adrenal gland of the WHHL rabbit, an animal model of familial hypercholesterolemia. Proc. Natl. Acad. Sci. USA 1981, 78, 2268–2272. [Google Scholar] [CrossRef] [Green Version]
- Havel, R.J.; Kita, T.; Kotite, L.; Kane, J.P.; Hamilton, R.L.; Goldstein, J.L.; Brown, M.S. Concentration and composition of lipoproteins in blood plasma of the WHHL rabbit. An animal model of human familial hypercholesterolemia. Arteriosclerosis 1982, 2, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, J.L.; Kita, T.; Brown, M.S. Defective Lipoprotein Receptors and Atherosclerosis: Lessons from an Animal Counterpart of Familial Hypercholesterolemia. N. Engl. J. Med. 1983, 309, 288–296. [Google Scholar] [CrossRef]
- Shiomi, M.; Ito, T.; Fujioka, T.; Tsujita, Y. Age-associated decrease in plasma cholesterol and changes in cholesterol metabolism in homozygous Watanabe heritable hyperlipidemic rabbits. Metabolism 2000, 49, 552–556. [Google Scholar] [CrossRef]
- Atkinson, J.B.; Hoover, R.L.; Berry, K.K.; Swift, L.L. Cholesterol-fed heterozygous Watanabe heritable hyperlipidemic rabbits: A new model for atherosclerosis. Atherosclerosis 1989, 78, 123–136. [Google Scholar] [CrossRef]
- Zhang, B.; Saku, K.; Hirata, K.; Liu, R.; Tateishi, K.; Yamamoto, K.; Arakawa, K. Insulin resistance observed in WHHL rabbits. Atherosclerosis 1991, 91, 277–278. [Google Scholar] [CrossRef]
- Shiomi, M.; Kobayashi, T.; Kuniyoshi, N.; Yamada, S.; Ito, T. Myocardial Infarction-Prone Watanabe Heritable Hyperlipidemic Rabbits with Mesenteric Fat Accumulation Are a Novel Animal Model for Metabolic Syndrome. Pathobiology 2012, 79, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, M.; Ito, T.; Yamada, S.; Kawashima, S.; Fan, J. Development of an Animal Model for Spontaneous Myocardial Infarction (WHHLMI Rabbit). Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1239–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiomi, M.; Fan, J. Unstable coronary plaques and cardiac events in myocardial infarction-prone Watanabe heritable hyperlipidemic rabbits: Questions and quandaries. Curr. Opin. Lipidol. 2008, 19, 631–636. [Google Scholar] [CrossRef] [PubMed]
- La Ville, A.; Turner, P.R.; Pittilo, R.M.; Martini, S.; Marenah, C.B.; Rowles, P.M.; Morris, G.; Thomson, G.A.; Woolf, N.; Lewis, B. Hereditary hyperlipidemia in the rabbit due to overproduction of lipoproteins. I. Biochemical studies. Arteriosclerosis 1987, 7, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Seddon, A.M.; Woolf, N.; La Ville, A.; Pittilo, R.M.; Rowles, P.M.; Turner, P.R.; Lewis, B. Hereditary hyperlipidemia and atherosclerosis in the rabbit due to overproduction of lipoproteins. II. Preliminary report of arterial pathology. Arteriosclerosis 1987, 7, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Ardern, H.A.; Benson, G.M.; Suckling, K.E.; Caslake, M.J.; Shepherd, J.; Packard, C.J. Apolipoprotein B overproduction by the perfused liver of the St. Thomas’ mixed hyperlipidemic (SMHL) rabbit. J. Lipid Res. 1999, 40, 2234–2243. [Google Scholar]
- de Roos, B.; Caslake, M.J.; Milliner, K.; Benson, G.M.; Suckling, K.E.; Packard, C.J. Characterisation of the lipoprotein structure in the St. Thomas’ Mixed Hyperlipidaemic (SMHL) rabbit. Atherosclerosis 2005, 181, 63–68. [Google Scholar] [CrossRef]
- Mitsuguchi, Y.; Ito, T.; Ohwada, K. Pathologic findings in rabbit models of hereditary hypertriglyceridemia and hereditary postprandial hypertriglyceridemia. Comp. Med. 2008, 58, 465–480. [Google Scholar]
- Kawai, T.; Ito, T.; Ohwada, K.; Mera, Y.; Matsushita, M.; Tomoike, H. Hereditary postprandial hypertriglyceridemic rabbit exhibits insulin resistance and central obesity: A novel model of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2752–2757. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; McCormick, S.P.A.; Krauss, R.M.; Taylor, S.; Quan, R.; Taylor, J.M.; Young, S.G. Overexpression of Human Apolipoprotein B-100 in Transgenic Rabbits Results in Increased Levels of LDL and Decreased Levels of HDL. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1889–1899. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Zhu, H.; Fan, J.; Yu, L.; Liu, G.; Liu, E. Hypertriglyceridemia and delayed clearance of fat load in transgenic rabbits expressing human apolipoprotein CIII. Transgenic Res. 2011, 20, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ji, Z.S.; Huang, Y.; de Silva, H.; Sanan, D.; Mahley, R.W.; Innerarity, T.L.; Taylor, J.M. Increased expression of apolipoprotein E in transgenic rabbits results in reduced levels of very low density lipoproteins and an accumulation of low density lipoproteins in plasma. J. Clin. Investig. 1998, 101, 2151–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Ji, Z.-S.; Brecht, W.J.; Rall, S.C.; Taylor, J.M.; Mahley, R.W. Overexpression of Apolipoprotein E3 in Transgenic Rabbits Causes Combined Hyperlipidemia by Stimulating Hepatic VLDL Production and Impairing VLDL Lipolysis. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2952–2959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Schwendner, S.W.; Rall, S.C.; Sanan, D.A.; Mahley, R.W. Apolipoprotein E2 Transgenic Rabbits. J. Biol. Chem. 1997, 272, 22685–22694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Xu, J.; Zhu, T.; Fan, J.; Lai, L.; Zhang, J.; Chen, Y.E. Effective gene targeting in rabbits using RNA-guided Cas9 nucleases. J. Mol. Cell Biol. 2014, 6, 97–99. [Google Scholar] [CrossRef] [Green Version]
- Ji, D.; Zhao, G.; Songstad, A.; Cui, X.; Weinstein, E.J. Efficient creation of an APOE knockout rabbit. Transgenic Res. 2015, 24, 227–235. [Google Scholar] [CrossRef]
- Lu, R.; Yuan, T.; Wang, Y.; Zhang, T.; Yuan, Y.; Wu, D.; Zhou, M.; He, Z.; Lu, Y.; Chen, Y.; et al. Spontaneous severe hypercholesterolemia and atherosclerosis lesions in rabbits with deficiency of low-density lipoprotein receptor (LDLR) on exon 7. EBioMedicine 2018, 36, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Niimi, M.; Yang, D.; Kitajima, S.; Ning, B.; Wang, C.; Li, S.; Liu, E.; Zhang, J.; Chen, Y.E.; Fan, J. ApoE knockout rabbits: A novel model for the study of human hyperlipidemia. Atherosclerosis 2016, 245, 187–193. [Google Scholar] [CrossRef]
- Yuan, T.; Zhong, Y.; Wang, Y.; Zhang, T.; Lu, R.; Zhou, M.; Lu, Y.; Yan, K.; Chen, Y.; Hu, Z.; et al. Generation of hyperlipidemic rabbit models using multiple sgRNAs targeted CRISPR/Cas9 gene editing system. Lipids Health Dis. 2019, 18, 69. [Google Scholar] [CrossRef] [Green Version]
- Shiomi, M.; Koike, T.; Ito, T. Contribution of the WHHL rabbit, an animal model of familial hypercholesterolemia, to elucidation of the anti-atherosclerotic effects of statins. Atherosclerosis 2013, 231, 39–47. [Google Scholar] [CrossRef]
- Endo, A.; Kuroda, M.; Tanzawa, K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme a reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976, 72, 323–326. [Google Scholar] [CrossRef] [Green Version]
- Endo, A.; Tsujita, Y.; Kuroda, M.; Tanzawa, K. Effects of ML-236B on cholesterol metabolism in mice and rats: Lack of hypocholesterolemic activity in normal animals. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1979, 575, 266–276. [Google Scholar] [CrossRef]
- Endo, A. Regulation of cholesterol synthesis, as focused on the regulation of HMG-CoA reductase. Seikagaku 1980, 52, 1033–1049. [Google Scholar] [PubMed]
- Watanabe, Y.; Ito, T.; Saeki, M.; Kuroda, M.; Tanzawa, K.; Mochizuki, M.; Tsujita, Y.; Arai, M. Hypolipidemic effects of CS-500 (ML-236B) in WHHL-rabbit, a heritable animal model for hyperlipidemia. Atherosclerosis 1981, 38, 27–31. [Google Scholar] [CrossRef]
- Tsujita, Y.; Kuroda, M.; Tanzawa, K.; Kitano, N.; Endo, A. Hypolipidemic effects in dogs of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme a reductase. Atherosclerosis 1979, 32, 307–313. [Google Scholar] [CrossRef]
- Kuroda, M.; Tsujita, Y.; Tanzawa, K.; Endo, A. Hypolipidemic effects in monkeys of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Lipids 1979, 14, 585–589. [Google Scholar] [CrossRef]
- Knopp, R.H. Drug Treatment of Lipid Disorders. N. Engl. J. Med. 1999, 341, 498–511. [Google Scholar] [CrossRef]
- Bocan, T.M.A.; Mazur, M.J.; Bak Mueller, S.; Brown, E.Q.; Sliskovic, D.R.; O’Brien, P.M.; Creswell, M.W.; Lee, H.; Uhlendorf, P.D.; Roth, B.D.; et al. Antiatherosclerotic activity of inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase in cholesterol-fed rabbits: A biochemical and morphological evaluation. Atherosclerosis 1994, 111, 127–142. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ito, T.; Masashi, S.; Tsujita, Y.; Kuroda, M.; Arai, M.; Fukamim, M.; Tamura, A. Preventive effect of pravastatin sodium, a potent inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, on coronary atherosclerosis and xanthoma in WHHL rabbits. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1988, 960, 294–302. [Google Scholar] [CrossRef]
- Kroon, P.A.; Hand, K.M.; Huff, J.W.; Alberts, A.W. The effects of mevinolin on serum cholesterol levels of rabbits with endogenous hypercholesterolemia. Atherosclerosis 1982, 44, 41–48. [Google Scholar] [CrossRef]
- Kritchevsky, D.; Tepper, S.A.; Klurfeld, D.M. Influence of mevinolin on experimental atherosclerosis in rabbits. Pharmacol. Res. Commun. 1981, 13, 921–926. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ishida, F.; Takahashi, T.; Taguchi, K.; Watanabe, K.; Ohmura, I.; Kamei, T. Preventive effect of MK-733 (simvastatin), an inhibitor of HMG-CoA reductase, on hypercholesterolemia and atherosclerosis induced by cholesterol feeding in rabbits. Jpn. J. Pharmacol. 1989, 49, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, B.J.; Krause, B.R.; Bisgaier, C.L.; Newton, R.S. Comparative effects of HMG-CoA reductase inhibitors on apo B production in the casein-fed rabbit: Atorvastatin versus lovastatin. Atherosclerosis 1995, 115, 173–180. [Google Scholar] [CrossRef]
- Rikitake, Y.; Kawashima, S.; Takeshita, S.; Yamashita, T.; Azumi, H.; Yasuhara, M.; Nishi, H.; Inoue, N.; Yokoyama, M. Anti-oxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis 2001, 154, 87–96. [Google Scholar] [CrossRef]
- Hayashi, T.; Rani P, J.A.; Fukatsu, A.; Matsui-Hirai, H.; Osawa, M.; Miyazaki, A.; Tsunekawa, T.; Kano-Hayashi, H.; Iguchi, A.; Sumi, D.; et al. A new HMG-CoA reductase inhibitor, pitavastatin remarkably retards the progression of high cholesterol induced atherosclerosis in rabbits. Atherosclerosis 2004, 176, 255–263. [Google Scholar] [CrossRef]
- Ma, P.T.; Gil, G.; Südhof, T.C.; Bilheimer, D.W.; Goldstein, J.L.; Brown, M.S. Mevinolin, an inhibitor of cholesterol synthesis, induces mRNA for low density lipoprotein receptor in livers of hamsters and rabbits. Proc. Natl. Acad. Sci. USA 1986, 83, 8370–8374. [Google Scholar] [CrossRef] [Green Version]
- Tsujita, Y.; Kuroda, M.; Shimada, Y.; Tanzawa, K.; Arai, M.; Kaneko, I.; Tanaka, M.; Masuda, H.; Tarumi, C.; Watanabe, Y.; et al. CS-514, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase: Tissue-selective inhibition of sterol synthesis and hypolipidemic effect on various animal species. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1986, 877, 50–60. [Google Scholar] [CrossRef]
- Shiomi, M.; Ito, T. Effect of cerivastatin sodium, a new inhibitor of HMG-CoA reductase, on plasma lipid levels, progression of atherosclerosis, and the lesional composition in the plaques of WHHL rabbits. Br. J. Pharmacol. 1999, 126, 961–968. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Kobayashi, H.; Sato, F.; Yonemitsu, Y.; Nakashima, Y.; Sueishi, K. Plaque-stabilizing effect of pitavastatin in Watanabe heritable hyperlipidemic (WHHL) rabbits. J. Atheroscler. Thromb. 2003, 10, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.-C. Mechanism of Action of Fibrates on Lipid and Lipoprotein Metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef] [Green Version]
- Krause, B.R.; Princen, H.M.G. Lack of predictability of classical animal models for hypolipidemic activity: A good time for mice? Atherosclerosis 1998, 140, 15–24. [Google Scholar] [CrossRef]
- Staels, B.; Auwerx, J. Regulation of apo A-I gene expression by fibrates. Atherosclerosis 1998, 137, S19–S23. [Google Scholar] [CrossRef]
- Saitoh, K.; MoriI, T.; Kasai, H.; Nagayama, T.; Tsuchiya, A.; Ohbayashi, S. Anti-atheromatous effects of fenofibrate, a hypolipidemic drug. I. Anti-atheromatous effects are independent of its hypolipidemic effect in cholesterol-fed rabbits. Folia Pharmacol. Jpn. 1995, 106, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corti, R.; Osende, J.; Hutter, R.; Viles-Gonzalez, J.F.; Zafar, U.; Valdivieso, C.; Mizsei, G.; Fallon, J.T.; Fuster, V.; Badimon, J.J. Fenofibrate induces plaque regression in hypercholesterolemic atherosclerotic rabbits: In vivo demonstration by high-resolution MRI. Atherosclerosis 2007, 190, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Jeanpierre, E.; Le Tourneau, T.; Zawadzki, C.; Van Belle, E.; Mouquet, F.; Susen, S.; Ezekowitz, M.D.; Staels, B.; Jude, B.; Corseaux, D. Beneficial effects of fenofibrate on plaque thrombogenicity and plaque stability in atherosclerotic rabbits. Cardiovasc. Pathol. 2009, 18, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, A.; Kajani, Z.; Miedema, M.D.; Virani, S.S. The role of ezetimibe in the treatment of cardiovascular disease. Curr. Atheroscler. Rep. 2016, 18, 8. [Google Scholar] [CrossRef]
- Gómez-Garre, D.; Muñoz-Pacheco, P.; González-Rubio, M.L.; Aragoncillo, P.; Granados, R.; Fernández-Cruz, A. Ezetimibe reduces plaque inflammation in a rabbit model of atherosclerosis and inhibits monocyte migration in addition to its lipid-lowering effect. Br. J. Pharmacol. 2009, 156, 1218–1227. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.; Janoudi, A.; Vedre, A.; Aziz, K.; Tamhane, U.; Rubinstein, J.; Abela, O.G.; Berger, K.; Abela, G.S. Plaque rupture and thrombosis are reduced by lowering cholesterol levels and crystallization with ezetimibe and are correlated with fluorodeoxyglucose positron emission tomography. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2007–2014. [Google Scholar] [CrossRef] [Green Version]
- Honda, K.; Matoba, T.; Antoku, Y.; Koga, J.I.; Ichi, I.; Nakano, K.; Tsutsui, H.; Egashira, K. Lipid-Lowering Therapy with Ezetimibe Decreases Spontaneous Atherothrombotic Occlusions in a Rabbit Model of Plaque Erosion. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 757–771. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, T.; Fujii, H.; Yoshizato, K.; Kawada, N. A human-type nonalcoholic steatohepatitis model with advanced fibrosis in rabbits. Am. J. Pathol. 2010, 177, 153–165. [Google Scholar] [CrossRef]
- Barnhart, J.W.; Rytter, D.J.; Molello, J.A. An overview of the biochemical pharmacology of probucol. Lipids 1977, 12, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Matsuzawa, Y. Where are we with probucol: A new life for an old drug? Atherosclerosis 2009, 207, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Naruszewicz, M.; Carew, T.E.; Pittman, R.C.; Witztum, J.L.; Steinberg, D. A novel mechanism by which probucol lowers low density lipoprotein levels demonstrated in the LDL receptor-deficient rabbit. J. Lipid Res. 1984, 25, 1206–1213. [Google Scholar] [PubMed]
- Kita, T.; Nagano, Y.; Yokode, M.; Ishii, K.; Kume, N.; Ooshima, A.; Yoshida, H.; Kawai, C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc. Natl. Acad. Sci. USA 1987, 84, 5928–5931. [Google Scholar] [CrossRef] [Green Version]
- Carew, T.E.; Schwenke, D.C.; Steinberg, D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect. Proc. Natl. Acad. Sci. USA 1987, 84, 7725–7729. [Google Scholar] [CrossRef] [Green Version]
- Daugherty, A.; Zweifel, B.S.; Schonfeld, G. Probucol attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Br. J. Pharmacol. 1989, 98, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Oshima, R.; Ikeda, T.; Watanabe, K.; Itakura, H.; Sugiyama, N. Probucol treatment attenuates the aortic atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Atherosclerosis 1998, 137, 13–22. [Google Scholar] [CrossRef]
- Daugherty, A.; Zweifel, B.S.; Schonfeld, G. The effects of probucol on the progression of atherosclerosis in mature Watanabe heritable hyperlipidaemic rabbits. Br. J. Pharmacol. 1991, 103, 1013–1018. [Google Scholar] [CrossRef] [Green Version]
- Niimi, M.; Keyamura, Y.; Nozako, M.; Koyama, T.; Kohashi, M.; Yasufuku, R.; Yoshikawa, T.; Fan, J. Probucol inhibits the initiation of atherosclerosis in cholesterol-fed rabbits. Lipids Health Dis. 2013, 12, 166. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Liang, J.; Niimi, M.; Waqar, A.B.; Kang, D.; Koike, T.; Wang, Y.; Shiomi, M.; Fan, J. Probucol suppresses macrophage infiltration and MMP expression in atherosclerotic plaques of WHHL rabbits. J. Atheroscler. Thromb. 2014, 21, 648–658. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, D. The LDL modification hypothesis of atherogenesis: An update. J. Lipid Res. 2009, 50, S376–S381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, T.; Castelli, W.P.; Hjortland, M.C.; Kannel, W.B.; Dawber, T.R. High density lipoprotein as a protective factor against coronary heart disease: The Framingham study. Am. J. Med. 1977, 62, 707–714. [Google Scholar] [CrossRef]
- Chapman, M.J.; Le Goff, W.; Guerin, M.; Kontush, A. Cholesteryl ester transfer protein: At the heart of the action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur. Heart J. 2010, 31, 149–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, J.; Okamoto, H.; Otabe, M.; Bujo, H.; Saito, Y. Effect of HDL, from Japanese white rabbit administered a new cholesteryl ester transfer protein inhibitor JTT-705, on cholesteryl ester accumulation induced by acetylated low density lipoprotein in J774 macrophage. Atherosclerosis 2002, 162, 131–135. [Google Scholar] [CrossRef]
- Brodeur, M.R.; Rhainds, D.; Charpentier, D.; Mihalache-Avram, T.; Mecteau, M.; Brand, G.; Chaput, E.; Perez, A.; Niesor, E.J.; Rhéaume, E.; et al. Dalcetrapib and anacetrapib differently impact HDL structure and function in rabbits and monkeys. J. Lipid Res. 2017, 58, 1282–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, H.; Yonemori, F.; Wakitani, K.; Minowa, T.; Maeda, K.; Shinkai, H. A cholesteryl ester transfer protein inhibitor attenuate atherosclerosis in rabbits. Nature 2000, 406, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Inazu, A.; Nohara, A.; Higashikata, T.; Mabuchi, H. Cholesteryl ester transfer protein inhibitor (JTT-705) and the development of atherosclerosis in rabbits with severe hypercholesterolaemia. Clin. Sci. 2002, 103, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Morehouse, L.A.; Sugarman, E.D.; Bourassa, P.A.; Sand, T.M.; Zimetti, F.; Gao, F.; Rothblat, G.H.; Milici, A.J. Inhibition of CETP activity by torcetrapib reduces susceptibility to diet-induced atherosclerosis in New Zealand White rabbits. J. Lipid Res. 2007, 48, 1263–1272. [Google Scholar] [CrossRef] [Green Version]
- Miyosawa, K.; Watanabe, Y.; Murakami, K.; Murakami, T.; Shibata, H.; Iwashita, M.; Yamazaki, H.; Yamazaki, K.; Ohgiya, T.; Shibuya, K.; et al. New CETP inhibitor K-312 reduces PCSK9 expression: A potential effect on LDL cholesterol metabolism. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E177–E190. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Niimi, M.; Yang, D.; Liang, J.; Xu, J.; Kimura, T.; Mathew, A.V.; Guo, Y.; Fan, Y.; Zhu, T.; et al. Deficiency of Cholesteryl Ester Transfer Protein Protects Against Atherosclerosis in Rabbits. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1068–1075. [Google Scholar] [CrossRef] [Green Version]
- Tall, A.R.; Rader, D.J. Trials and Tribulations of CETP Inhibitors. Circ. Res. 2018, 122, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer, H.B.; Ansell, B.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Translation of high-density lipoprotein function into clinical practice: Current prospects and future challenges. Circulation 2013, 128, 1256–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riwanto, M.; Landmesser, U. High density lipoproteins and endothelial functions: Mechanistic insights and alterations in cardiovascular disease. J. Lipid Res. 2013, 54, 3227–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenson, R.S.; Brewer, H.B.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.C.; Phillips, M.C.; Rader, D.J.; et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 2012, 125, 1905–1919. [Google Scholar] [CrossRef] [Green Version]
- Badimon, J.; Badimon, L.; Galvez, A.; Dische, R.; Fuster, V. High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol-fed rabbits. Lab. Investig. 1989, 60, 455–461. [Google Scholar]
- Badimon, J.J.; Badimon, L.; Fuster, V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J. Clin. Investig. 1990, 85, 1234–1241. [Google Scholar] [CrossRef] [Green Version]
- Ben-Aicha, S.; Casaní, L.; Muñoz-García, N.; Joan-Babot, O.; Peña, E.; Aržanauskaitė, M.; Gutierrez, M.; Mendieta, G.; Padró, T.; Badimon, L.; et al. HDL (High-Density Lipoprotein) Remodeling and Magnetic Resonance Imaging-Assessed Atherosclerotic Plaque Burden: Study in a Preclinical Experimental Model. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2481–2493. [Google Scholar] [CrossRef]
- Miyazaki, A.; Sakuma, S.; Morikawa, W.; Takiue, T.; Miake, F.; Terano, T.; Sakai, M.; Hakamata, H.; Sakamoto, Y.I.; Naito, M.; et al. Intravenous injection of rabbit apolipoprotein A-I inhibits the progression of atherosclerosis in cholesterol-fed rabbits. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1882–1888. [Google Scholar] [CrossRef]
- Soma, M.R.; Donetti, E.; Parolini, C.; Sirtori, C.R.; Fumagalli, R.; Franceschini, G. Recombinant apolipoprotein A-IMilano dimer inhibits carotid intimai thickening induced by perivascular manipulation in rabbits. Circ. Res. 1995, 76, 405–411. [Google Scholar] [CrossRef]
- Chiesa, G.; Monteggia, E.; Marchesi, M.; Lorenzon, P.; Laucello, M.; Lorusso, V.; Di Mario, C.; Karvouni, E.; Newton, R.S.; Bisgaier, C.L.; et al. Recombinant apolipoprotein A-IMilano infusion into rabbit carotid artery rapidly removes lipid from fatty streaks. Circ. Res. 2002, 90, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Ibanez, B.; Vilahur, G.; Cimmino, G.; Speidl, W.S.; Pinero, A.; Choi, B.G.; Zafar, M.U.; Santos-Gallego, C.G.; Krause, B.; Badimon, L.; et al. Rapid Change in Plaque Size, Composition, and Molecular Footprint After Recombinant Apolipoprotein A-IMilano (ETC-216) Administration. Magnetic Resonance Imaging Study in an Experimental Model of Atherosclerosis. J. Am. Coll. Cardiol. 2008, 51, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Duverger, N.; Kruth, H.; Emmanuel, F.; Caillaud, J.-M.; Viglietta, C.; Castro, G.; Tailleux, A.; Fievet, C.; Fruchart, J.C.; Houdebine, L.M.; et al. Inhibition of Atherosclerosis Development in Cholesterol-Fed Human Apolipoprotein A-I–Transgenic Rabbits. Circulation 1996, 94, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niimi, M.; Nishijima, K.; Waqar, A.B.; Yu, Y.; Koike, T.; Kitajima, S.; Liu, E.; Inoue, T.; Kohashi, M.; et al. Human apolipoprotein A-II protects against diet-induced atherosclerosis in transgenic rabbits. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 224–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anantharamaiah, G.; Jones, J.; Brouillette, C.; Schmidt, C.; Chung, B.; Hughes, T.; Bhown, A.; Segrest, J. Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J. Biol. Chem. 1985, 260, 10248–10255. [Google Scholar]
- White, C.R.; Datta, G.; Zhang, Z.; Gupta, H.; Garber, D.W.; Mishra, V.K.; Palgunachari, M.N.; Handattu, S.P.; Chaddha, M.; Anantharamaiah, G.M. HDL therapy for cardiovascular diseases: The road to HDL mimetics. Curr. Atheroscler. Rep. 2008, 10, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; White, C.R.; Handattu, S.; Garber, D.W.; Datta, G.; Chaddha, M.; Dai, L.; Gianturco, S.H.; Bradley, W.A.; Anantharamaiah, G.M. Apolipoprotein E mimetic peptide dramatically lowers plasma cholesterol and restores endothelial function in Watanabe heritable hyperlipidemic rabbits. Circulation 2005, 111, 3112–3118. [Google Scholar] [CrossRef] [Green Version]
- Van Lenten, B.J.; Wagner, A.C.; Navab, M.; Anantharamaiah, G.M.; Hama, S.; Reddy, S.T.; Fogelman, A.M. Lipoprotein inflammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J. Lipid Res. 2007, 48, 2344–2353. [Google Scholar] [CrossRef] [Green Version]
- Iwata, A.; Miura, S.I.; Zhang, B.; Imaizumi, S.; Uehara, Y.; Shiomi, M.; Saku, K. Antiatherogenic effects of newly developed apolipoprotein A-I mimetic peptide/phospholipid complexes against aortic plaque burden in Watanabe-heritable hyperlipidemic rabbits. Atherosclerosis 2011, 218, 300–307. [Google Scholar] [CrossRef]
- Overturf, M.L.; Smith, S.A.; Hewett-Emmett, D.; Loose-Mitchell, D.S.; Soma, M.R.; Gotto, A.M., Jr.; Morrisett, J.D. Development and partial metabolic characterization of a dietary cholesterol-resistant colony of rabbits. J. Lipid Res. 1989, 30, 263–273. [Google Scholar]
- Beynen, A.C.; Meijer, G.W.; Lemmens, A.G.; Glatz, J.F.C.; Versluis, A.; Katan, M.B.; Van Zutphen, L.F.M. Sterol balance and cholesterol absorption in inbred strains of rabbits hypo- or hyperresponsive to dietary cholesterol. Atherosclerosis 1989, 77, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhao, S.; Huang, B.; Wang, Y.; Li, Y.; Waqar, A.B.; Liu, R.; Bai, L.; Fan, J.; Liu, E. Probucol and cilostazol exert a combinatorial anti-atherogenic effect in cholesterol-fed rabbits. Thromb. Res. 2013, 132, 565–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clay, M.A.; Hopkins, G.J.; Ehnholm, C.P.; Barter, P.J. The rabbit as an animal model of hepatic lipase deficiency. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1989, 1002, 173–181. [Google Scholar] [CrossRef]
- Warren, R.J.; Ebert, D.L.; Mitchell, A.; Barter, P.J. Rabbit hepatic lipase cDNA sequence: Low activity is associated with low messenger RNA levels. J. Lipid Res. 1991, 32, 1333–1339. [Google Scholar] [PubMed]
- Kimura, N.; Kikumori, A.; Kawase, D.; Okano, M.; Fukamachi, K.; Ishida, T.; Nakajima, K.; Shiomi, M. Species differences in lipoprotein lipase and hepatic lipase activities: Comparative studies of animal models of lifestyle-related diseases. Exp. Anim. 2019, 68, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Target Disease Rabbit and Manipulation | Drug | Plasma TC | Outcome | Reference |
|---|---|---|---|---|
Atherosclerosis
| Ezetimibe (0.6 mg/kg) | 8% ↓ | 13% ↓ * | Gómez-Garre [77] |
| Simvastatin (5 mg/kg) | 26% ↓ | 27% ↓ * | ||
| Combination (Eze + Sim) | 37% ↓ | 28% ↓ * | ||
| for 6 weeks | (6 weeks) | (Femoral artery, intima/media ratio) | ||
Atherothrombosis
| Ezetimibe (1 mg/kg) | 86% ↓ * | Plaque area: 38% ↓ * | Patel [78] |
| for 3 months | (6 months) | Thrombus area: 93% ↓ * | ||
Atherothrombosis
| Ezetimibe (0.6 mg/kg) | 18% ↓ * | 51% ↓ * | Honda [79] |
| Rosvastatin (1 mg/kg) | 23% ↓ * | 12% ↓ | ||
| for 2–8 weeks | (4 weeks) | (Thrombotic occlusion) | ||
NASH
| Ezetimibe (0.6 mg/kg) | ≈ 40% ↓ * | Lipid droplet: ≈15% ↓ * | Ogawa [80] |
| for 8 weeks | (8 weeks) | Hepatic TC: ≈26% ↓ * | ||
| Hepatic TG: ≈63% ↓ * |
| Rabbit | Drug | Plasma Lipids | Atherosclerosis | Findings | Reference |
|---|---|---|---|---|---|
| WHHL rabbits (Normal standard diet) | |||||
| (Male and female) | 1% probucol in diet (4 weeks) | TC: 24% ↓ VLDL-C: 4% ↓ IDL-C: 2% ↓ LDL-C: 36% ↓ HDL-C: 53% ↓ (4 weeks vs. pre-treatment) | - | up-regulation of LDL fractional catabolic rate | Naruszewicz [83] |
| (Male and female) | 1% probucol in diet (6 months) | TC: 17% ↓ (6 months) | 87% ↓ * (Thoracic aorta, gross lesion) | protective effect of LDL oxidization | Kita [84] |
| (Male and female) | 1% probucol in diet (≈33 weeks) | TC: 12% ↓ (during treatment) | 65% ↓ * (Aorta, gross lesion) | protective effect of lesional LDL degradation | Carew [85] |
| (Male) | 1% probucol in diet (5 months) | TC: 35% ↓ * VLDL-C: 51% ↓ * IDL-C: 19% ↓ LDL-C: 33% ↓ * HDL-C: 45% ↓ * (5 months) | ≈39% ↓ * (Aorta, gross lesion) | regression of atherosclerosis (≈34% ↓ * vs. baseline lesion) | Oshima [87] |
| (Male) | 0.3% probucol in diet (16 weeks) | TC: 12% ↓ * HDL-C: 12% ↓ (16 weeks) | 41% ↓ * (Coronary artery, stenosis) | protective effect of coronary atherosclerosis | Li [90] |
| Wild-type rabbits (Cholesterol diet) | |||||
| NZW 2% Chol diet | 1% probucol in diet (60 days) | TC: 16% ↓ (60 days) | Thoracic: 79% ↓ * Abdominal: 85% ↓ * (Aorta, gross lesion) | anti-atherosclerotic effect without plasma lipid lowering | Daugherty [86] |
| NZW (male) 0.5% Chol diet | 0.3% probucol in diet (4 weeks) | TC: 4% ↓ HDL-C: 49% ↓ * (5 weeks) | 65% ↓ * (Aorta, gross lesion) | protective effect of initial atherosclerosis | Niimi [89] |
| Rabbit | Drug | Plasma Lipids | Atherosclerosis | Reference | ||
|---|---|---|---|---|---|---|
| HDL-C | LDL-C | TC | ||||
| Normal standard diet | ||||||
| JW (male) | Dalcetrapib (300 mg/kg) | 2.1-fold ↑ * | 4% ↑ | 73% ↑ * | - | Kobayashi [94] |
| for 7 days | (7 days) | (7 days) | (7 days) | |||
| NZW (male) | Dalcetrapib (300 mg/kg) | 3.3-fold ↑ * | 7% ↑ | 86% ↑ * | - | Brodeur [95] |
| Anacetrapib (30 mg/kg) | 2.7-fold ↑ * | 21% ↑ | 72% ↑ * | |||
| for 2 weeks | (2 weeks) | (2 weeks) | (2 weeks) | |||
| High-cholesterol diet | ||||||
| JW (male) 0.2% Chol diet | Dalcetrapib | 2.0-fold ↑ * | - | 24% ↓ | 70% ↓ * | Okamoto [96] |
| (≈225 mg/kg) | (6 months) | (6 months) | (Aortic arch, gross lesion) | |||
| for 6 months | ||||||
| JW (male) 0.25% Chol diet | Dalcetrapib (100 mg/kg) | 1.3-fold ↑ | - | 6% ↓ | 12% ↑ | Huang [97] |
| Dalcetrapib (300 mg/kg) | 1.9-fold ↑ * | 21% ↓ | 3% ↑ | |||
| for 12 weeks | (12 weeks) | (12 weeks) | (Aorta, gross lesion) | |||
| NZW (male) 0.2% Chol, 10% coconut oil diet | Torcetrapib | 3.6-fold ↑ | - | 28% ↑ | 59% ↓ * | Morehouse [98] |
| (60–90 mg/kg) | (16 weeks) | (16 weeks) | (Aorta, gross lesion) | |||
| for 16 weeks | ||||||
| NZW (male) 0.25% Chol diet | K-312 (10 mg/kg) | ≈4.7-fold ↑ * | ≈33% ↑ | - | ≈54% ↓ * | Miyosawa [99] |
| K-312 (30 mg/kg) | ≈5.1-fold ↑ * | ≈18% ↑ | ≈55% ↓ * | |||
| for 18 weeks | (18 weeks) | (18 weeks) | (Aorta, gross lesion) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niimi, M.; Chen, Y.; Yan, H.; Wang, Y.; Koike, T.; Fan, J. Hyperlipidemic Rabbit Models for Anti-Atherosclerotic Drug Development. Appl. Sci. 2020, 10, 8681. https://doi.org/10.3390/app10238681
Niimi M, Chen Y, Yan H, Wang Y, Koike T, Fan J. Hyperlipidemic Rabbit Models for Anti-Atherosclerotic Drug Development. Applied Sciences. 2020; 10(23):8681. https://doi.org/10.3390/app10238681
Chicago/Turabian StyleNiimi, Manabu, Yajie Chen, Haizhao Yan, Yao Wang, Tomonari Koike, and Jianglin Fan. 2020. "Hyperlipidemic Rabbit Models for Anti-Atherosclerotic Drug Development" Applied Sciences 10, no. 23: 8681. https://doi.org/10.3390/app10238681





