Application of a Multiscale Approach in the Substitution and Reduction of NaCl in Costeño-Type Artisan Cheese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Costeño-Type Cheese-Making
2.2. Physicochemical Analysis
2.3. Texture Profile Analysis
2.4. Rheological Analysis
2.5. Scanning Electron Microscopy
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis of Milk
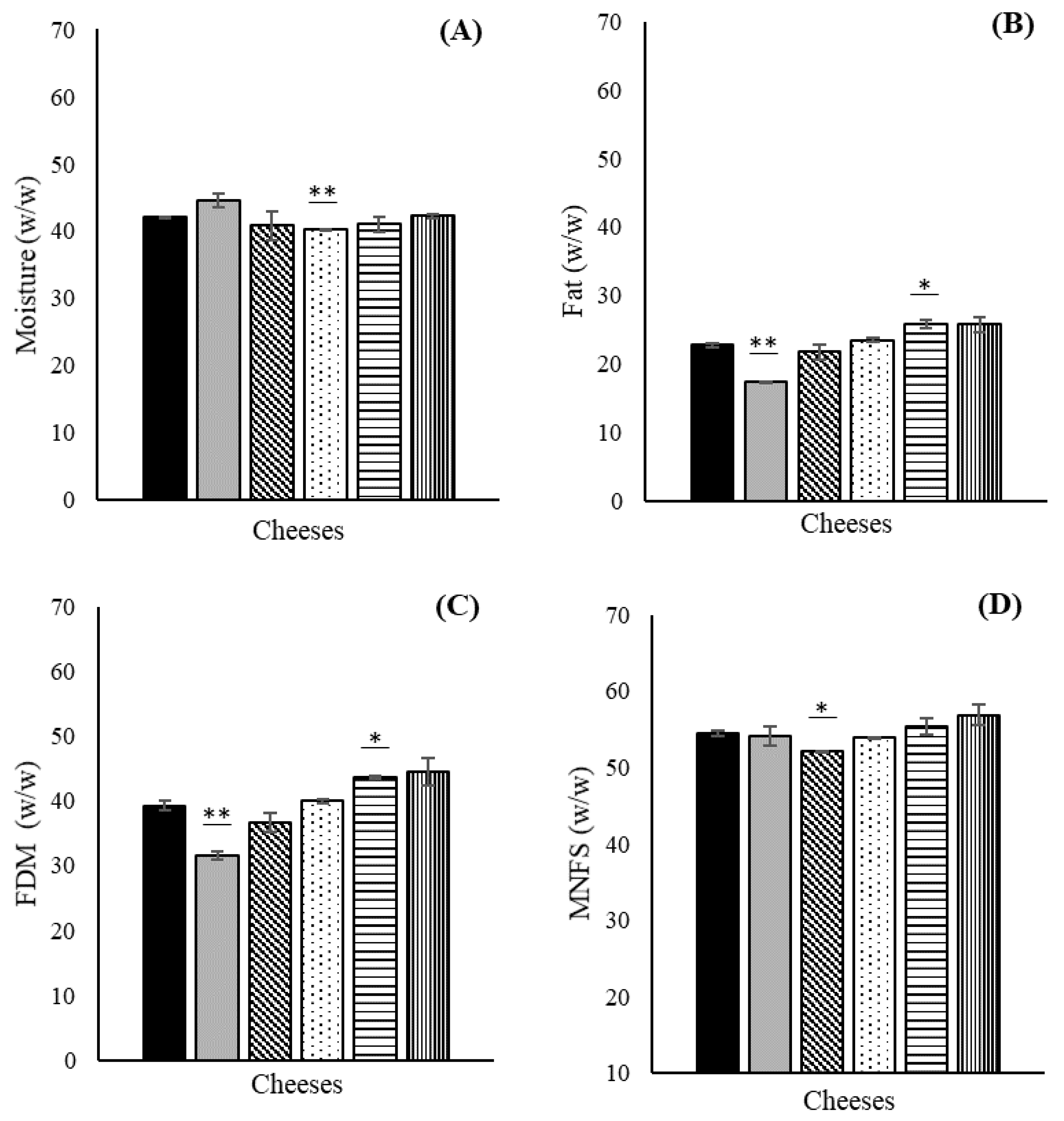
3.2. Physicochemical Analysis in Costeño-Type Cheese
3.3. Texture Profile Analysis
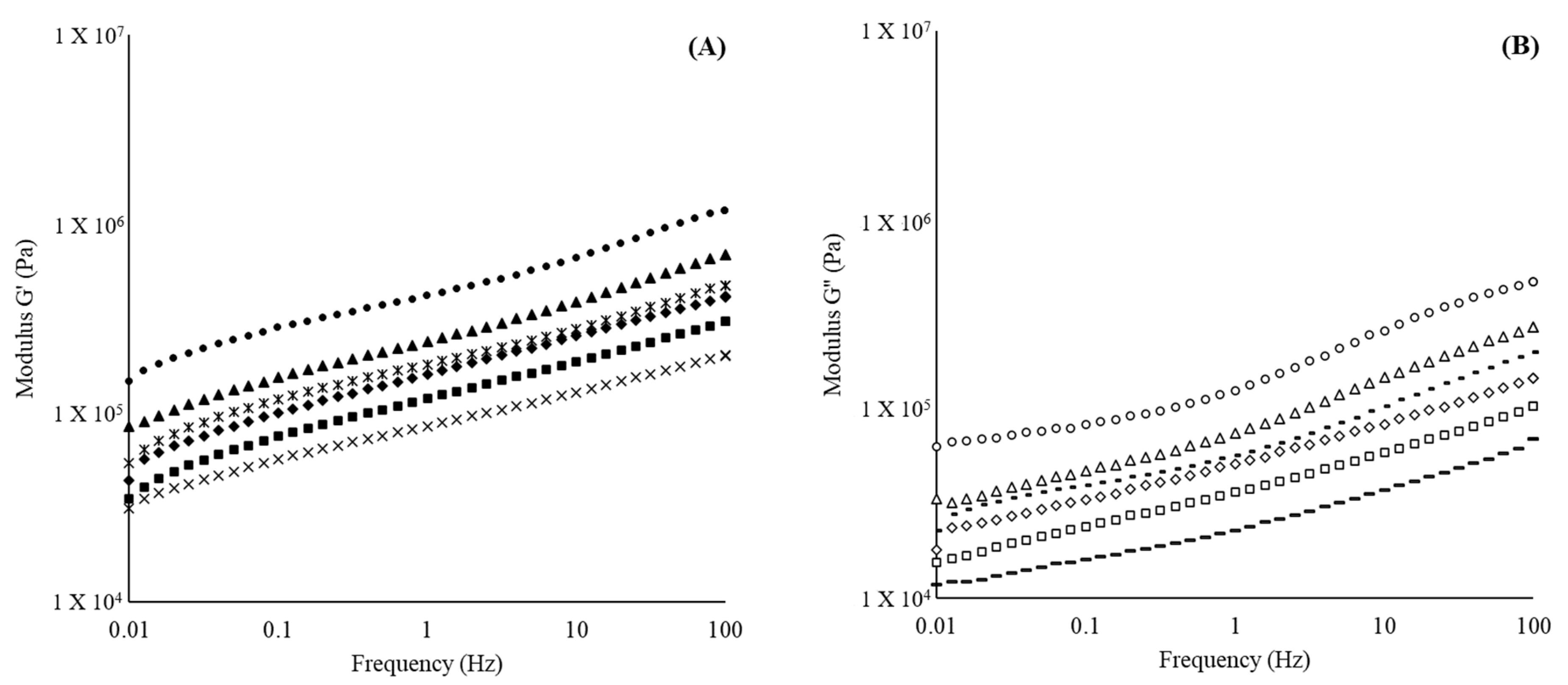
3.4. Rheological Analysis
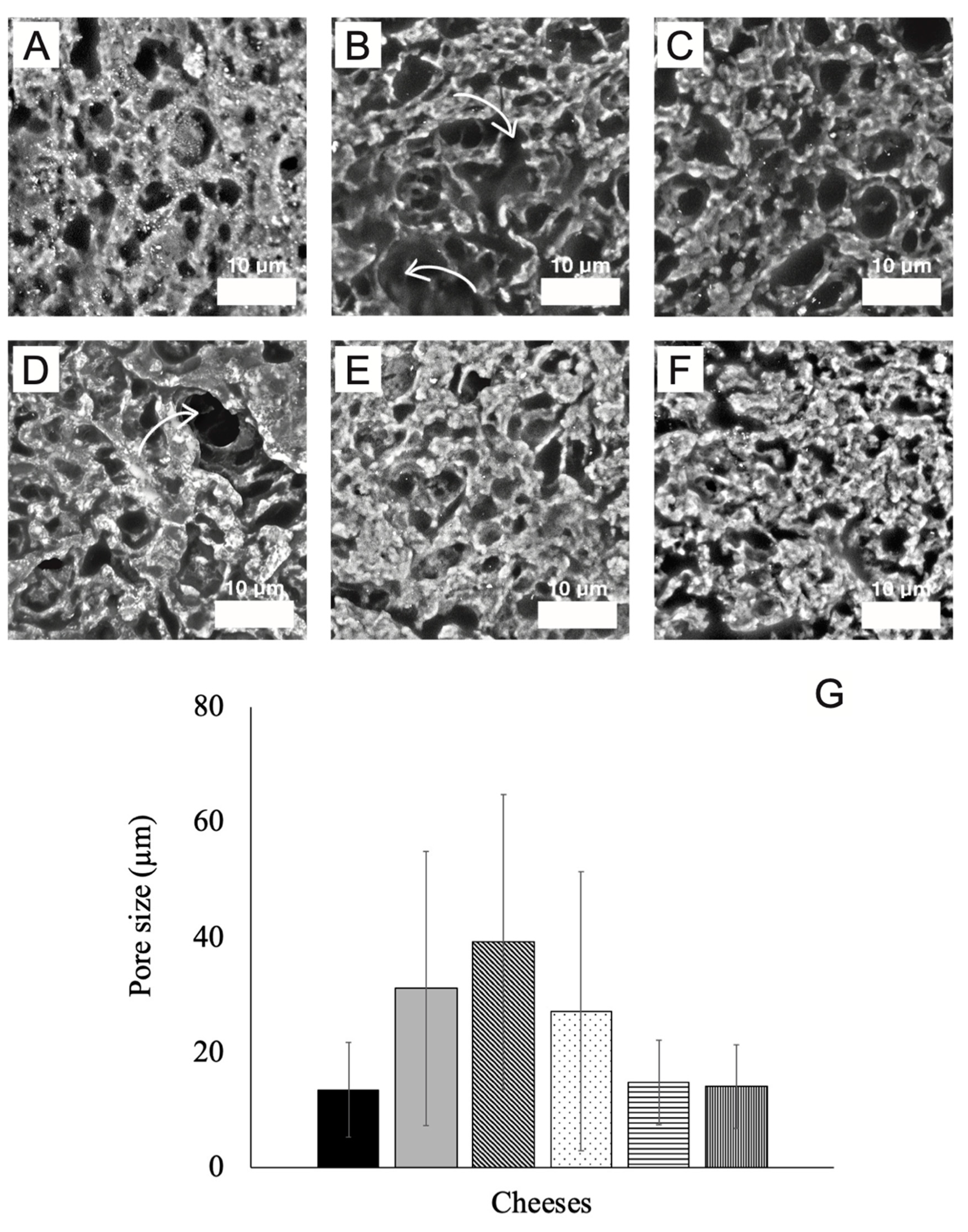
3.5. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doyle, M.E.; Glass, K.A. Sodium Reduction and Its Effect on Food Safety, Food Quality, and Human Health. Compr. Rev. Food Sci. Food Saf. 2010, 9, 44–56. [Google Scholar] [CrossRef]
- He, F.; Brown, M.; Tan, M.; MacGregor, G.A. Reducing population salt intake-An update on latest evidence and global action. J. Clin. Hypertens. 2019, 21, 1596–1601. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sodium Intake for Adults and Children. Available online: https://www.who.int/nutrition/publications/guidelines/sodium_intake_printversion.pdf (accessed on 20 August 2020).
- Arboatti, A.S.; Olivares, M.; Sabbag, N.G.; Costa, S.C.; Zorrilla, S.E.; Sihufe, G.A. The influence of sodium chloride reduction on physicochemical, biochemical, rheological and sensory characteristics of Mozzarella cheese. Dairy Sci. Technol. 2014, 94, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, B.; Brown, K.; Irish, D.A.; Brothersen, C.; McMahon, N.J. Manufacture and sensory analysis of reduced and low-sodium Cheddar and Mozzarella cheeses. J. Dairy Sci. 2014, 97, 1970–1982. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.G.; Faria, J.A.; Pollonio, M.A.; Bolini, H.M.; Celeghini, R.M.; Granato, D.; Shah, N.P. Cheeses with reduced sodium content: Effects on functionality, public health benefits and sensory properties. Trends Food Sci. Technol. 2011, 22, 276–291. [Google Scholar] [CrossRef]
- Carmi, I.K.; Benjamin, O. Reduction in sodium content of fresh, semihard Tzfat cheese using salt replacer mixtures: Taste, texture and shelf-life evaluation. Int. J. Dairy Technol. 2016, 70, 354–364. [Google Scholar] [CrossRef]
- Gomes, A.P.; Cruz, A.G.; Cadena, R.S.; Celeghini, R.M.S.; Faria, J.A.F.; Bolini, H.M.A.; Pollonio, M.A.R.; Granato, D. Manufacture of low-sodium Minas fresh cheese: Effect of the partial replacement of sodium chloride with potassium chloride. J. Dairy Sci. 2011, 94, 2701–2706. [Google Scholar] [CrossRef] [Green Version]
- Grummer, J.; Bobowski, N.; Karalus, M.; Vickers, Z.; Schoenfuss, T. Use of potassium chloride and flavor enhancers in low sodium Cheddar cheese. J. Dairy Sci. 2013, 96, 1401–1418. [Google Scholar] [CrossRef]
- Ayyash, M.; Sherkat, F.; Shah, N. The effect of NaCl substitution with KCl on Akawi cheese: Chemical composition, proteolysis, angiotensin-converting enzyme-inhibitory activity, probiotic survival, texture profile, and sensory properties. J. Dairy Sci. 2012, 95, 4747–4759. [Google Scholar] [CrossRef] [Green Version]
- Horita, C.; Morgano, M.; Celeghini, R.; Pollonio, M. Physico-chemical and sensory properties of reduced-fat mortadella prepared with blends of calcium, magnesium and potassium chloride as partial substitutes for sodium chloride. Meat Sci. 2011, 89, 426–433. [Google Scholar] [CrossRef]
- Grummer, J.; Karalus, M.; Zhang, K.; Vickers, Z.; Schoenfuss, T. Manufacture of reduced-sodium Cheddar-style cheese with mineral salt replacers. J. Dairy Sci. 2012, 95, 2830–2839. [Google Scholar] [CrossRef] [PubMed]
- Guinee, T.P.; Fox, P.F. Salt in Cheese: Physical, Chemical and Biological Aspects BT-Starter Cultures: General Aspects, 4th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2004; Volume 1. [Google Scholar]
- Lucey, J.; Johnson, M.; Horne, D. Invited Review: Perspectives on the Basis of the Rheology and Texture Properties of Cheese. J. Dairy Sci. 2003, 86, 2725–2743. [Google Scholar] [CrossRef] [Green Version]
- Acevedo, D.; Jaimes, J.D.; Espitia, C.R. Efecto de la Adición de Lactosuero al Queso Costeño Amasado. Inf. Tecnológica 2015, 26, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Serpa, J.G.; Pérez, T.I.; Hernández, E.J. Effect of pasteurization and starter cultures on physicochemical and microbiological properties of costeño cheese. Rev. Fac. Nac. Agron. Medellín 2016, 69, 8007–8014. [Google Scholar] [CrossRef]
- Tirado, D.F.; Acevedo, D.; Torres-Gallo, R. Effect of deformation history on the stress relaxation behaviour of Colombian Caribbean coastal cheese from goat milk. Food Sci. Technol. Int. 2018, 24, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Floury, J.; Camier, B.; Rousseau, F.; Lopez, C.; Tissier, J.-P.; Famelart, M.-H. Reducing salt level in food: Part 1. Factors affecting the manufacture of model cheese systems and their structure–texture relationships. LWT 2009, 42, 1611–1620. [Google Scholar] [CrossRef]
- Cussler, E.L.; Moggridge, G.D.; Cussler, E.L.; Moggridge, G.D. An Introduction to Chemical Product Design. In Chemical Product Design; Cambridge University Press: Cambridge, UK, 2012; pp. 1–16. [Google Scholar]
- Mora, H.B.; Piñeros, M.; Moreno, D.E.; Restrepo, S.R.; Jaramillo, J.C.; Solano, Ó.A. Álvarez; Fernandez-Niño, M.; Barrios, A.G. Multiscale design of a dairy beverage model composed of Candida utilis single cell protein supplemented with oleic acid. J. Dairy Sci. 2019, 102, 9749–9762. [Google Scholar] [CrossRef]
- Pradilla, D.; Vargas, W.L.; Alvarez, O.A. The application of a multi-scale approach to the manufacture of concentrated and highly concentrated emulsions. Chem. Eng. Res. Des. 2015, 95, 162–172. [Google Scholar] [CrossRef]
- Lamichhane, P.; Kelly, A.L.; Sheehan, J.J. Symposium review: Structure-function relationships in cheese. J. Dairy Sci. 2018, 101, 2692–2709. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Fox, P.F. Advanced Dairy Chemistry; Springer: New York, NY, USA, 2009; ISBN 9780387848648. [Google Scholar]
- Ho, Q.T.; Carmeliet, J.; Datta, A.K.; Defraeye, T.; Delele, M.A.; Herremans, E.; Opara, L.; Ramon, H.; Tijskens, E.; Van Der Sman, R.; et al. Multiscale modeling in food engineering. J. Food Eng. 2013, 114, 279–291. [Google Scholar] [CrossRef]
- Ballesta Rodriguez, I. Evaluación de la Calidad del Queso Costeño Elaborado con Diferentes Tipos de Cuajo (Animal y Microbiano) y la Adición o no de Cultivos Lácticos (Lactococcus Lactis Subps. Lactis y Lactococcus Lactis Subps. Cremoris). Master’s Thesis, Universidad Nacional De Colombia Sede Medellin Facultad, Medellín, Columbia, 2014. [Google Scholar]
- AOAC Official Methods of Analysis of AOAC International, 16th ed.; AOAC: Rockville, MD, USA, 1995; Volume 2.
- AOAC Official Methods of Analysis of AOAC International; AOAC: Rockville, MD, USA, 2005; ISBN 0935584544.
- Tunick, M.H.; Van Hekken, D.L. Rheology and Texture of Commercial Queso Fresco Cheeses Made from Raw and Pasteurized milk. J. Food Qual. 2010, 33, 204–215. [Google Scholar] [CrossRef]
- Bähler, B.; Back, R.; Hinrichs, J. Evaluation of oscillatory and shear strain behaviour for thermo-rheological plasticisation of non-ripened cheese curd: Effect of water, protein, and fat. Int. Dairy J. 2015, 46, 63–70. [Google Scholar] [CrossRef]
- Katsiari, M.; Voutsinas, L.; Alichanidis, E.; Roussis, I. Manufacture of Kefalograviera cheese with less sodium by partial replacement of NaCl with KCl. Food Chem. 1998, 61, 63–70. [Google Scholar] [CrossRef]
- Foegeding, E.; Drake, M. Invited Review: Sensory and Mechanical Properties of Cheese Texture. J. Dairy Sci. 2007, 90, 1611–1624. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Ak, M.M. Cheese Rheology and Texture; CRC Press: Boca Raton, FL, USA, 2003; ISBN 1587160218. [Google Scholar]
- Castro, A.C.; Novoa, C.F.; Algecira, N.; Buitrago, G. Reología y Textura de Quesos Bajos en Grasa; Revista de Ciencia y Tecnología: Bogotá, Colombia, 2014; Volume 16, pp. 58–66. [Google Scholar]
- Grummer, J.; Schoenfuss, T. Determining salt concentrations for equivalent water activity in reduced-sodium cheese by use of a model system. J. Dairy Sci. 2011, 94, 4360–4365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamleh, R.; Olabi, A.; Toufeili, I.; Daroub, H.; Younis, T.; Ajib, R. The effect of partial substitution of NaCl with KCl on the physicochemical, microbiological and sensory properties of Akkawi cheese. J. Sci. Food Agric. 2015, 95, 1940–1948. [Google Scholar] [CrossRef]
- Codex Alimentarius. Codex Standard for Milk and Milk Products. Group standard for cheese including fresh cheese CODEX STAN 221-2001. General standard for cheese CODEX STAN 283-1978. 2011. Available online: http://www.fao.org/home/en/ (accessed on 23 November 2020).
- Pastorino, A.; Hansen, C.; McMahon, D.J. Effect of Salt on Structure-Function Relationships of Cheese. J. Dairy Sci. 2003, 86, 60–69. [Google Scholar] [CrossRef] [Green Version]
- McSweeney, P.L.H. Cheese Problems Solved; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9781845690601. [Google Scholar]
- Brown, J.; Foegeding, E.A.; Daubert, C.; Drake, M.; Gumpertz, M. Relationships Among Rheological and Sensorial Properties of Young Cheeses. J. Dairy Sci. 2003, 86, 3054–3067. [Google Scholar] [CrossRef]
- Rogers, N.; McMahon, D.; Daubert, C.; Berry, T.; Foegeding, E.A. Rheological properties and microstructure of Cheddar cheese made with different fat contents. J. Dairy Sci. 2010, 93, 4565–4576. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, D.J.; Guinee, T.P. Rheology and Texture of Cheese BT-Starter cultures: General aspects. In Cheese: Chemistry, Physics and Microbiology, 3rd ed.; Fox, F.P., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 1, pp. 511–540. [Google Scholar]

| Experimental Cheese | Cook Temperature (°C) | Added Salt (%) | |
|---|---|---|---|
| NaCl (w/w) | KCl (w/w) | ||
| Qc (Control) | 45 | 10.00 | 0.00 |
| Q1 | 45 | 7.50 | 0.00 |
| Q2 | 55 | 5.00 | 0.00 |
| Q3 | 55 | 2.50 | 0.00 |
| Q4 | 45 | 5.00 | 5.00 |
| Q5 | 45 | 2.50 | 7.50 |
| Composition | Raw Bovine Milk |
|---|---|
| Density (g/cm3) | 1.027 ± 0.010 |
| Titratable acidity (ºD) | 14.20 ± 1.17 |
| pH | 6.70 ± 0.12 |
| Fat (%, w/w) | 3.20 ± 0.32 |
| Total Solids (%, w/w) | 10.70 ± 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Bustamante, M.L.; Reyes, L.H.; Solano, O.A.A. Application of a Multiscale Approach in the Substitution and Reduction of NaCl in Costeño-Type Artisan Cheese. Appl. Sci. 2020, 10, 9008. https://doi.org/10.3390/app10249008
Diaz-Bustamante ML, Reyes LH, Solano OAA. Application of a Multiscale Approach in the Substitution and Reduction of NaCl in Costeño-Type Artisan Cheese. Applied Sciences. 2020; 10(24):9008. https://doi.org/10.3390/app10249008
Chicago/Turabian StyleDiaz-Bustamante, Martha L., Luis H. Reyes, and Oscar Alberto Alvarez Solano. 2020. "Application of a Multiscale Approach in the Substitution and Reduction of NaCl in Costeño-Type Artisan Cheese" Applied Sciences 10, no. 24: 9008. https://doi.org/10.3390/app10249008





