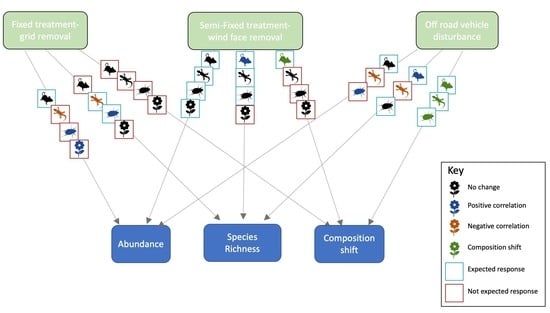
Can Vegetation Removal Successfully Restore Coastal Dune Biodiversity?
Abstract
:1. Introduction
- i.
- How do species compositions of different taxa respond to different removal treatments?
- ii.
- Can indicator species represent assemblage level responses?
- iii.
- Can the different forms of removal contribute to successful coastal dune management?
Hypotheses Tested
2. Materials and Methods
2.1. Study Site
2.2. Removal Experiments
- i.
- Fixed Dune Treatment: In October 2005, perennial vegetation was removed from the entire fixed dune in a grid formation using a bulldozer, to accurately reach the desired amount of remaining vegetation (Figure 1b). The removal reduced the vegetation from 31–50% to approximately 15–20% for each dune [69]. Three dunes were treated and three control dunes were monitored in parallel.
- ii.
- Semi-Fixed Treatment: In November 2012, an experiment was conducted on four semi-fixed dunes, which had a range of 16–30% average PPC prior to treatment. This time, the experiment involved removal of all vegetation from the wind-facing slope of four semi-fixed treated dunes. The vegetation remaining on the crest and slip-face was left intact (Figure 1d). This experimental design was chosen in order to leave some vegetation needed for animal survival, while more closely emulating the distribution of natural vegetation found on mobile dunes [see 77]. Four untreated semi-fixed dunes were selected as controls (Figure 1c).
- iii.
- Disturbed Dunes Treatment: Off-road vehicle (ORV) disturbance has been occurring illegally in the reserve for many years (Figure 1f), which began prior to the initiation of the experimental plots. We began monitoring these dunes in 2012, in order to study the impact of ORVs on dune biodiversity. We consider these dunes as ‘treated’ with an unquantified press disturbance. The exact natural state of these dunes prior to the disturbance is unknown; on the one hand, one might assume they were similar to mobile dunes based on historical aerial photos from 1965 and 1999 [69]. On the other hand, most dunes in the immediate vicinity are semi-fixed. Disturbed dunes were therefore compared with both mobile and un-treated semi-fixed dunes as the reference dunes. Annual plants are almost entirely absent from disturbed dunes and as such this taxon was not monitored on these dunes.
2.3. Data Collection
2.3.1. Rodents
2.3.2. Reptiles
2.3.3. Beetles
2.3.4. Annual Plants
2.4. Data Analysis
2.4.1. Assemblage Abundance and Richness
2.4.2. Composition
2.4.3. Indicator Species
3. Results
3.1. Abundance & Richness
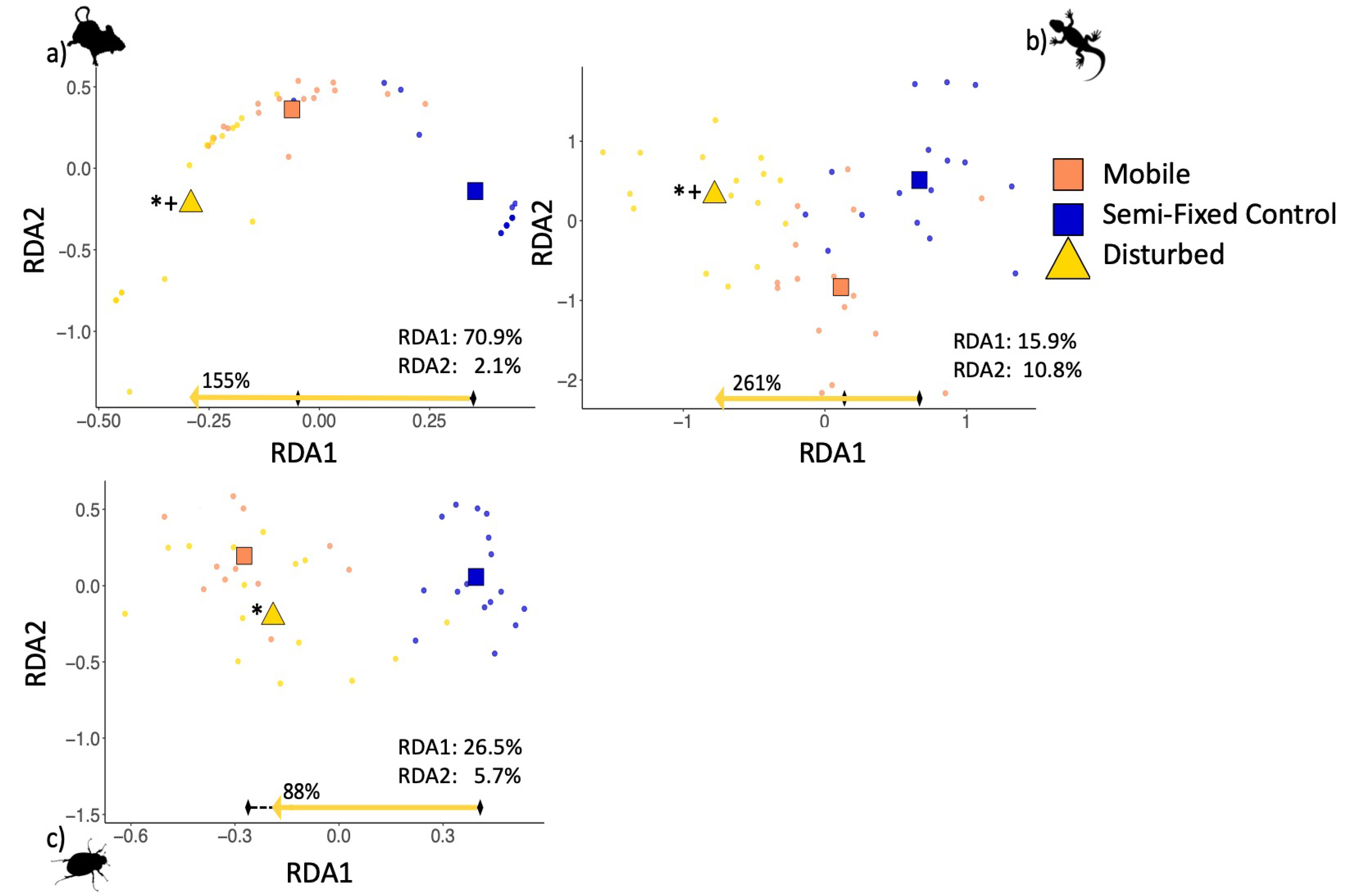
3.2. Composition
3.3. Temporal Trends in Composition
3.4. Indicator Species
4. Discussion
4.1. Effect of Treatment on Semi-Fixed Dunes
4.2. Effect of Fixed-Dune Treatment
4.3. Effect of ORV Disturbance
4.4. Implications for Conservation Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carboni, M.; Dengler, J.; Mantilla-Contreras, J.; Venn, S.; Török, P. Conservation value, management and restoration of Europe’s semi-natural open landscapes. Hacquetia 2015, 14, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Lithgow, D.; Martínez, M.L.; Gallego-Fernández, J.B.; Hesp, P.A.; Flores, P.; Gachuz, S.; Rodríguez-Revelo, N.; Jiménez-Orocio, O.; Mendoza-González, G.; Álvarez-Molina, L.L. Linking restoration ecology with coastal dune restoration. Geomorphology 2013, 199, 214–224. [Google Scholar] [CrossRef]
- Small, C.; Nicholls, R.J.R.J. A global analysis of human settlement in coastal zones. J. Coast. Res. 2003, 19, 584–599. [Google Scholar]
- Arens, S.M.; Geelen, L.H.W.T.; Slings, R.; Wondergem, H. Restoration of Dune Mobility in the Netherlands, Proceedings of the Dunes and Estuaries 2005—International Conference on Nature Restoration Practices in European Coastal Habitats, Koksijde, Belgium, 19–23 September 2005; Herrier, J.L., Mees, J., Salman, A., Seys, J., Nieuwenhuyse, H., Van, I.D., Eds.; VLIZ Special Publication 19, XIV: Koksijde, Belgium, 2005; pp. 129–138. [Google Scholar]
- Grootjans, A.P.; Geelen, H.W.T.; Jansen, A.J.M.; Lammerts, E.J. Restoration of coastal dune slacks in the Netherlands. Hydrobiologia 2002, 478, 181–203. [Google Scholar] [CrossRef]
- Houston, J.A.; Rooney, P.J.; Edmondson, S.E. Coastal Dune Management: Shared Experience of European Conservation Practice; Liverpool University Press: Liverpool, UK, 2001; ISBN 0853238545. [Google Scholar]
- Doody, J.P. Coastal Conservation and Management: An Ecological Perspective; Springer: Berlin/Heidelberg, Germany, 2001; ISBN 1402072481. [Google Scholar]
- Feagin, R.A.; Sherman, D.J.; Grant, W.E. Coastal erosion, global sea-level rise, and the loss of sand dune plant habitats. Front. Ecol. Environ. 2005, 3, 359–364. [Google Scholar] [CrossRef]
- del Vecchio, S.; Prisco, I.; Acosta, A.T.R.; Stanisci, A. Changes in plant species composition of coastal dune habitats over a 20-year period. AoB Plants 2015, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Burak, S.; Doǧan, E.; Gazioǧlu, C. Impact of urbanization and tourism on coastal environment. Ocean Coast. Manag. 2004, 47, 515–527. [Google Scholar] [CrossRef]
- Malavasi, M.; Santoro, R.; Cutini, M.; Acosta, A.T.R.; Carranza, M.L. What has happened to coastal dunes in the last half century? A multitemporal coastal landscape analysis in Central Italy. Landsc. Urban Plan. 2013, 119, 54–63. [Google Scholar] [CrossRef]
- Arens, S.M.; Slings, Q.L.; Geelen, L.H.W.T.; Van der Hagen, H.G.J.M. Implications of environmental change for dune mobility in the Netherlands. In Proceedings of the International Conference on Management and Restoration of Coastal Dunes, Santander, Spain, 3–5 October 2007; pp. 3–5. [Google Scholar]
- Tsoar, H.; Levin, N.; Porat, N.; Maia, L.P.; Herrmann, H.J.; Tatumi, S.H.; Claudino-Sales, V. The effect of climate change on the mobility and stability of coastal sand dunes in Ceará State (NE Brazil). Quat. Res. 2009, 71, 217–226. [Google Scholar] [CrossRef]
- Pardini, E.A.; Parsons, L.S.; Ştefan, V.; Knight, T.M. GLMM BACI environmental impact analysis shows coastal dune restoration reduces seed predation on an endangered plant. Restor. Ecol. 2018, 26, 1190–1194. [Google Scholar] [CrossRef]
- Pye, K.; Blott, S.J.; Howe, M.A. Coastal dune stabilization in Wales and requirements for rejuvenation. J. Coast. Conserv. 2014, 18, 27–54. [Google Scholar] [CrossRef]
- Álvarez-Molina, L.L.; Martínez, M.L.; Pérez-Maqueo, O.; Gallego-Fernández, J.B.; Flores, P. Richness, diversity, and rate of primary succession over 20 year in tropical coastal dunes. Plant Ecol. 2012, 213, 1597–1608. [Google Scholar] [CrossRef]
- Fenu, G.; Cogoni, D.; Ferrara, C.; Pinna, M.S.; Bacchetta, G. Relationships between coastal sand dune properties and plant community distribution: The case of Is Arenas (Sardinia). Plant Biosyst. 2012, 146, 586–602. [Google Scholar] [CrossRef]
- Gornish, E.S.; Miller, T.E. Using long-term census data to inform restoration methods for coastal dune vegetation. Estuaries Coasts 2013, 36, 1014–1023. [Google Scholar] [CrossRef]
- Bonte, D.; Hoffmann, M. Are coastal dune management actions for biodiversity restoration and conservation underpinned by internationally published scientific research? In Proceedings of the Dunes and Estuaries 2005—International Conference on Nature Restoration Practices in European Coastal Habitats, Koksijde, Belgium, 19–23 September 2005; pp. 165–178. [Google Scholar]
- Webb, C.E.; Oliver, I.; Pik, A.J. Does coastal foredune stabilization with Ammophila arenaria restore plant and arthropod communities in southeastern Australia? Restor. Ecol. 2000, 8, 283–288. [Google Scholar] [CrossRef]
- Provoost, S.; Ampe, C.; Bonte, D.; Cosyns, E.; Hoffmann, M. Ecology, management and monitoring of dune grassland in Flanders, Belgium. In Littoral 2002, The Changing Coast; EUROCOAST/EUCC: Porto, Portugal, 2002; pp. 11–20. [Google Scholar]
- Jacques, I.J.; Anderson, M.C.; Bristol, A.E.; Faulkner, J.; Polanski, J. Evaluation of Stability and Restoration of a Michigan Coastal Dune; Calvin College: Grand Rapids, MI, USA, 2017. [Google Scholar]
- Hayes, M.; Kirkpatrick, J.B. Influence of Ammophila arenaria on half a century of vegetation change in eastern Tasmanian sand dune systems. Aust. J. Bot. 2012, 60, 450–460. [Google Scholar] [CrossRef]
- Darke, I.B.; Eamer, J.B.R.; Beaugrand, H.E.R.; Walker, I.J. Monitoring considerations for a dynamic dune restoration project: Pacific Rim National Park Reserve, British Columbia, Canada. Earth Surf. Process. Landforms 2013, 38, 983–993. [Google Scholar] [CrossRef]
- Hilton, M.J. The loss of New Zealand’s active dunes and the spread of marram grass (Ammophila arenaria). N. Z. Geogr. 2006, 62, 105–120. [Google Scholar] [CrossRef]
- Bird, T.L.F.; Dorman, M.; Ramot, A.; Bouskila, A.; Bar Kutiel, P.; Groner, E. Shrub encroachment effects on habitat heterogeneity and beetle diversity in a Mediterranean coastal dune system. Land Degrad. Dev. 2017, 28, 2553–2562. [Google Scholar] [CrossRef]
- Kutiel, P.; Cohen, O.; Shoshany, M.; Shub, M. Vegetation establishment on the southern Israeli coastal sand dunes between the years 1965 and 1999. Landsc. Urban Plan. 2004, 67, 141–156. [Google Scholar] [CrossRef]
- Shacham, B.; Bouskila, A. Vegetation removal as a management tool in Nizzanim dunes (Israel): Preliminary assessment of effects on reptile and mammal populations. In Proceedings of the International Conference on Management and Restoration of Coastal Dunes (ICCD), Santander, Spain, 3–5 October 2007; pp. 144–145. [Google Scholar]
- Acosta, A.; Carranza, M.L.; Izzi, C.F. Are there habitats that contribute best to plant species diversity in coastal dunes? Biodivers. Conserv. 2009, 18, 1087–1098. [Google Scholar] [CrossRef]
- Howe, M.A.; Knight, G.T.; Clee, C. The importance of coastal sand dunes for terrestrial invertebrates in Wales and the UK, with particular reference to aculeate Hymenoptera (bees, wasps & ants). J. Coast. Conserv. 2010, 14, 91–102. [Google Scholar]
- Arens, S.M.; Geelen, L.H.W.T. Dune landscape rejuvenation by intended destabilisation in the Amsterdam water supply dunes. J. Coast. Res. 2006, 22, 1094–1107. [Google Scholar] [CrossRef]
- Doudna, J.W.; Connor, E.F. Response of terrestrial arthropod assemblages to coastal dune restoration. Ecol. Restor. 2012, 30, 20–26. [Google Scholar] [CrossRef]
- Provoost, S.; Jones, M.L.M.; Edmondson, S.E. Changes in landscape and vegetation of coastal dunes in northwest Europe: A review. J. Coast. Conserv. 2011, 15, 207–226. [Google Scholar] [CrossRef]
- Vandvik, V.; Heegaard, E.; Maren, I.E.; Aarrestad, P.A. Managing heterogeneity: The importance of grazing and environmental variation on post-fire succession in heathlands. J. Appl. Ecol. 2005, 42, 139–149. [Google Scholar] [CrossRef]
- Ödman, A.M.; Schnoor, T.K.; Ripa, J.; Olsson, P.A. Soil disturbance as a restoration measure in dry sandy grasslands. Biodivers. Conserv. 2012, 21, 1921–1935. [Google Scholar] [CrossRef]
- Martínez, M.L.; Hesp, P.A.; Gallego-Fernández, J.B. Coastal dune restoration: Trends and perspectives. In Restoration of Coastal Dunes; Martínez, M.L., Hesp, P.A., Gallego-Fernández, J.B., Eds.; Springer Series on Environmental Management: Berlin/Heidelberg, Germany, 2013; pp. 323–339. ISBN 364233444X. [Google Scholar]
- Oost, A.P.; Hoekstra, P.; Wiersma, A.; Flemming, B.; Lammerts, E.J.; Pejrup, M.; Hofstede, J.; van der Valk, B.; Kiden, P.; Bartholdy, J.; et al. Barrier island management: Lessons from the past and directions for the future. Ocean Coast. Manag. 2012, 68, 18–38. [Google Scholar] [CrossRef]
- Jackson, N.L.; Nordstrom, K.F.; Feagin, R.A.; Smith, W.K. Coastal geomorphology and restoration. Geomorphology 2013, 199, 1–7. [Google Scholar] [CrossRef]
- Walker, I.J.; Eamer, J.B.R.; Darke, I.B. Assessing significant geomorphic changes and effectiveness of dynamic restoration in a coastal dune ecosystem. Geomorphology 2013, 199, 192–204. [Google Scholar] [CrossRef]
- Arens, S.M.; Mulder, J.P.M.; Slings, Q.L.; Geelen, L.H.W.T.; Damsma, P. Dynamic dune management, integrating objectives of nature development and coastal safety: Examples from the Netherlands. Geomorphology 2013, 199, 205–213. [Google Scholar] [CrossRef]
- Rooney, P. Changing perspectives in coastal dune management. J. Coast. Conserv. 2010, 14, 71–73. [Google Scholar] [CrossRef] [Green Version]
- van der Meulen, F.; Jungerius, P.D.; Visser, J.H. Perspectives in Coastal Dune Management; SPB Academic Publishing: Devon, UK, 1989. [Google Scholar]
- García-Mora, M.R.; Gallego-Fernández, J.B.; García-Novo, F. Plant functional types in coastal foredunes in relation to disturbance. J. Veg. Sci. 1999, 10, 27–34. [Google Scholar] [CrossRef]
- Brunbjerg, A.K.; Svenning, J.C.; Ejrnæs, R. Experimental evidence for disturbance as key to the conservation of dune grassland. Biol. Conserv. 2014, 174, 101–110. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Kolasa, J.; Armesto, J.J.; Collins, S.L. The ecological concept of disturbance and its expression at various hierarchical levels. Oikos 1989, 54, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Lake, P.S. Disturbance, patchiness, and diversity in streams. J. N. Am. Benthol. Soc. 2000, 19, 573–592. [Google Scholar] [CrossRef] [Green Version]
- Ives, A.R.; Carpenter, S.R. Stability and diversity of ecosystems. Science 2007, 317, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Lake, P.S. Resistance, resilience and restoration. Ecol. Manag. Restor. 2013, 14, 20–24. [Google Scholar] [CrossRef]
- Brunbjerg, A.K.; Jørgensen, G.P.; Nielsen, K.M.; Pedersen, M.L.; Svenning, J.-C.; Ejrnæs, R. Disturbance in dry coastal dunes in Denmark promotes diversity of plants and arthropods. Biol. Conserv. 2015, 182, 243–253. [Google Scholar] [CrossRef]
- van Boxel, J.H.; Jungerius, P.D.; Kieffer, N.; Hampele, N.; van Boxel, J.H.; Jungerius, P.D.; Kieffer, N.; Hampele, N. Ecological effects of reactivation of artificially stabilized blowouts in coastal dunes. J. Coast. Conserv. 1997, 3, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Kutiel, P.; Danin, A.; Orshan, G. Vegetation of the sandy soils near Caesarea, Israel. I. Plant communities, environment and succession. Isreal J. Bot. 1979, 28, 20–35. [Google Scholar]
- Olff, H.; Huisman, J.; Van Tooren, B.F. Species dynamics and nutrient accumulation during early primary succession in coastal sand dunes. J. Ecol. 1993, 81, 693–706. [Google Scholar] [CrossRef] [Green Version]
- Hugenholtz, C.H.; Wolfe, S.A. Biogeomorphic model of dunefield activation and stabilization on the northern Great Plains. Geomorphology 2005, 70, 53–70. [Google Scholar] [CrossRef]
- Pickart, A.J. Dune restoration over two decades at the Lanphere and Ma-le’l Dunes in northern California. In Restoration of Coastal Dunes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 159–171. ISBN 364233444X. [Google Scholar]
- Pye, K.; Tsoar, H. Aeolian Sand and Sand Dunes; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 3540859101. [Google Scholar]
- Tsoar, H.; Shachak, M.; Blumberg, D.G. Ecological aspects of vegetation removal from the coastal sand dunes of Israel. Dunes Estuaries 2005, 19, 487–493. [Google Scholar]
- Buffa, G.; Fantinato, E.; Pizzo, L. Effects of disturbance on sandy coastal ecosystems of N-Adriatic coasts (Italy). In Biodiversity Enrichment in a Diverse World; Lameed, G.A., Ed.; Intech Open Science: London, UK, 2012; pp. 339–372. ISBN 978-953-51-0718-7. [Google Scholar]
- Choi, Y.D. Theories for ecological restoration in changing environment: Toward “futuristic” restoration. Ecol. Res. 2004, 18, 75–81. [Google Scholar] [CrossRef]
- SER. SER International Primer on Ecological Restoration; SER: The Hague, The Netherlands, 2004; Volume 2. [Google Scholar]
- Longcore, T. Terrestrial arthropods as indicators of ecological restoration success in coastal sage scrub (California, U.S.A.). Restor. Ecol. 2003, 11, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-González, G.; Martínez, M.L.; Rojas-Soto, O.R.; Vázquez, G.; Gallego-Fernández, J.B. Ecological niche modeling of coastal dune plants and future potential distribution in response to climate change and sea level rise. Glob. Chang. Biol. 2013, 19, 2524–2535. [Google Scholar] [CrossRef]
- Hobbs, R.J. Setting effective and realistic restoration goals: Key directions for research. Restor. Ecol. 2007, 15, 354–357. [Google Scholar] [CrossRef]
- Ruiz-Jaén, M.C.; Aide, T.M. Vegetation structure, species diversity, and ecosystem processes as measures of restoration success. For. Ecol. Manag. 2005, 218, 159–173. [Google Scholar] [CrossRef]
- Aubin, I.; Venier, L.; Pearce, J.; Moretti, M. Can a trait-based multi-taxa approach improve our assessment of forest management impact on biodiversity? Biodivers. Conserv. 2013, 22, 2957–2975. [Google Scholar] [CrossRef]
- Viterbi, R.; Cerrato, C.; Bassano, B.; Bionda, R.; Hardenberg, A.; Provenzale, A.; Bogliani, G. Patterns of biodiversity in the northwestern Italian Alps: A multi-taxa approach. Community Ecol. 2013, 14, 18–30. [Google Scholar] [CrossRef]
- Fattorini, S.; Dennis, R.L.H.; Cook, L.M. Conserving organisms over large regions requires multi-taxa indicators: One taxon’s diversity-vacant area is another taxon’s diversity zone. Biol. Conserv. 2011, 144, 1690–1701. [Google Scholar] [CrossRef]
- Keenleyside, K.A.; Dudley, N.; Cairns, S.; Hall, C.M.; Stolton, S. Ecological Restoration for Protected Areas: Principles, Guidelines and Best Practices; IUCN: Grand, Switzerland, 2012; 120p. [Google Scholar]
- Kutiel, P.; Peled, Y.; Geffen, E. The effect of removing shrub cover on annual plants and small mammals in a coastal sand dune ecosystem. Biol. Conserv. 2000, 94, 235–242. [Google Scholar] [CrossRef]
- Bar, P. Restoration of coastal sand dunes for conservation of biodiversity: The Israeli experience. In Restoration of Coastal Dunes; Martínez, M.L., Ed.; Springer Series on Environmental Management: Berlin/Heidelberg, Germany, 2013; pp. 173–185. [Google Scholar]
- Tsoar, H.; Blumberg, D.G. Formation of parabolic dunes from barchan and transverse dunes along Israel’s Mediterranean coast. Earth Surf. Process. Landforms 2002, 27, 1147–1161. [Google Scholar] [CrossRef]
- Levin, N. Monitoring, Explaining and Predicting the Stabilization Process of Coastal Dunes in Israel Using Remote Sensing and Geographic Information Systems (GIS) Means: The Case of Ashdod and Nizzanim; Tel Aviv University: Tel Aviv, Isreal, 2006. [Google Scholar]
- Levin, N.; Ben-Dor, E. Monitoring sand dune stabilization along the coastal dunes of Ashdod-Nizanim, Israel, 1945–1999. J. Arid Environ. 2004, 58, 335–355. [Google Scholar] [CrossRef]
- Lake, P.S. On the maturing of restoration: Linking ecological research and restoration. Ecol. Manag. Restor. 2001, 2, 110–115. [Google Scholar] [CrossRef]
- Groner, E.; Novoplansky, A. Reconsidering diversity-productivity relationships: Directness of productivity estimates matters. Ecol. Lett. 2003, 6, 695–699. [Google Scholar] [CrossRef]
- Shacham, B. Dune Management and Reptiles- Implications for Habitat Reconstruction and Conservation Strategies. Ph.D. Thesis, Ben-Gurion University of the Negev, Be’er Sheva, Israel, 2010. [Google Scholar]
- Kutiel, P. Conservation and management of the Mediterranean coastal sand dunes in Israel. J. Coast. Conserv. 2001, 7, 183–192. [Google Scholar] [CrossRef]
- Rubinstein, Y.; Groner, E.; Yizhaq, H.; Svoray, T.; Bar (Kutiel), P. An eco-spatial index for evaluating stabilization state of sand dunes. Aeolian Res. 2013, 9, 75–87. [Google Scholar] [CrossRef]
- Kutiel, P.; Sharon, H. Landscape changes in the last 50 years in the area of HaSharon Park, Israel. Ecol. Environ. 1996, 3, 167–176. [Google Scholar]
- Levin, N.; Kidron, G.J.; Ben-Dor, E. A field quantification of coastal dune perennial plants as indicators of surface stability, erosion or deposition. Sedimentology 2008, 55, 751–772. [Google Scholar] [CrossRef]
- Ramot, A. Effect of Plant Cover on Arthropod Community in Nizzanim Coastal Dunes. Masters’ Thesis, Ben Gurion University of the Negev, Be’er Sheva, Israel, 2007. [Google Scholar]
- Perry, M. Perennial Plants Impact on Annual Plant Diversity in Sand Dunes at Different Spatial Scales. Masters’ Thesis, Ben Gurion University of the Negev, Be’er Sheva, Israel, 2008. [Google Scholar]
- Brittain, S.; Böhning, D. Estimators in capture-recapture studies with two sources. AStA Adv. Stat. Anal. 2009, 93, 23–47. [Google Scholar] [CrossRef]
- Krell, F.T. Parataxonomy vs. taxonomy in biodiversity studies—Pitfalls and applicability of ‘morphospecies’ sorting. Biodivers. Conserv. 2004, 13, 795–812. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 10 October 2018).
- Laird, N.M.; Ware, J.H. Random-effects models for longitudinal data. Biometrics 1982, 38, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. R Core Team Nlme: Linear and Nonlinear Mixed Effects Models_. R Package Version 3. Available online: http://cran.r-project.org/package=nlme (accessed on 19 March 2019).
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Gail, M., Krickeberg, K., Samet, J., Tsiatis, A., Wong, W., Eds.; Statistics for Biology and Health; Springer: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.4-1. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 21 June 2019).
- Lepš, J.; Smilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; ISBN 9780511615146. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- De Cáceres, M.; Jansen, F. Package ‘Indicspecies’ (Version 1.7.6). URL. Available online: https//cran.r-project.org/web/packages/indicspecies/indicspecies.pdf (accessed on 21 June 2019).
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Fearnehough, W.; Fullen, M.A.; Mitchell, D.J.; Trueman, I.C.; Zhang, J. Aeolian deposition and its effect on soil and vegetation changes on stabilised desert dunes in northern China. Geomorphology 1998, 23, 171–182. [Google Scholar] [CrossRef]
- Ovadia, O. Harvest rates and foraging strategies in Negev desert gerbils. Behav. Ecol. 2001, 12, 219–226. [Google Scholar] [CrossRef]
- Abramsky, Z.; Pinshow, B. Changes in foraging effort in two gerbil species correlate with habitat type and intra- and interspecific activity. Oikos 1989, 56, 43. [Google Scholar] [CrossRef]
- Ovadia, O. The effect of intra- and interspecific aggression on patch residence time in Negev Desert gerbils: A competing risk analysis. Behav. Ecol. 2003, 14, 583–591. [Google Scholar] [CrossRef] [Green Version]
- Fenu, G.; Carboni, M.; Acosta, A.T.R.; Bacchetta, G. Environmental factors influencing coastal vegetation pattern: New insights from the Mediterranean Basin. Folia Geobot. 2013, 48, 493–508. [Google Scholar] [CrossRef]
- Remke, E.; Brouwer, E.; Kooijman, A.; Blindow, I.; Roelofs, J.G.M. Low atmospheric Nitrogen loads lead to grass encroachment in coastal dunes, but only on acid soils. Ecosystems 2009, 12, 1173–1188. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.L.M.; Wallace, H.L.; Norris, D.; Brittain, S.A.; Haria, S.; Jones, R.E.; Rhind, P.M.; Reynolds, B.R.; Emmett, B.A. Changes in vegetation and soil characteristics in coastal sand dunes along a gradient of atmospheric nitrogen deposition. Plant Biol. 2004, 6, 598–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparrius, L.B.; Sevink, J.; Kooijman, A.M. Effects of nitrogen deposition on soil and vegetation in primary succession stages in inland drift sands. Plant Soil 2012, 353, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Li, X.R.; Kong, D.S.; Tan, H.J.; Wang, X.P. Changes in soil and vegetation following stabilisation of dunes in the southeastern fringe of the Tengger Desert, China. Plant Soil 2007, 300, 221–231. [Google Scholar] [CrossRef]
- Jones, M.L.M.L.M.; Norman, K.; Rhind, P.M. Topsoil inversion as a restoration measure in sand dunes, early results from a UK field-trial. J. Coast. Conserv. 2010, 14, 139–151. [Google Scholar] [CrossRef]
- Glen, E.; Price, E.A.C.; Caporn, S.J.M.; Carroll, J.A.; Jones, L.M.; Scott, R. Evaluation of topsoil inversion in U.K. habitat creation and restoration schemes. Restor. Ecol. 2017, 25, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Olsson, P.A.; Ödman, A.M. Natural Establishment of Specialist Plant Species after Topsoil Removal and Soil Perturbation in Degraded Calcareous Sandy Grassland. Restor. Ecol. 2014, 22, 49–56. [Google Scholar] [CrossRef]
- Dolman, P.M.; Sutherland, W.J. The Ecological Changes of Breckland Grass Heaths and the Consequences of Management. J. Appl. Ecol. 1992, 29, 402. [Google Scholar] [CrossRef]
- Pedley, S.M.; Franco, A.M.A.; Pankhurst, T.; Dolman, P.M. Physical disturbance enhances ecological networks for heathland biota: A multiple taxa experiment. Biol. Conserv. 2013, 160, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Calvão, T.; Pessoa, M.F.; Lidon, F.C. Impact of human activities on coastal vegetation—A review. Emir. J. Food Agric. 2013, 25, 926–944. [Google Scholar]
- Kindermann, G.; Gormally, M.J. Vehicle damage caused by recreational use of coastal dune systems in a Special Area of Conservation (SAC) on the west coast of Ireland. J. Coast. Conserv. 2010, 14, 173–188. [Google Scholar] [CrossRef]
- Comor, V.; Orgeas, J.; Ponel, P.; Rolando, C.; Delettre, Y.R.; Comor, V.; Orgeas, J.; Ponel, P.; Rolando, C.; Delettre, Y.R. Impact of anthropogenic disturbances on beetle communities of French Mediterranean coastal dunes. Biodivers. Conserv. 2008, 17, 1837–1852. [Google Scholar] [CrossRef]
- Kissling, M.; Hegetschweiler, K.T.; Rusterholz, H.-P.; Baur, B. Short-term and long-term effects of human trampling on above-ground vegetation, soil density, soil organic matter and soil microbial processes in suburban beech forests. Appl. Soil Ecol. 2009, 42, 303–314. [Google Scholar] [CrossRef]
- Hesp, P.; Schmutz, P.; Martinez, M.L.M.; Driskell, L.; Orgera, R.; Renken, K.; Revelo, N.A.R.; Orocio, O.A.J. The effect on coastal vegetation of trampling on a parabolic dune. Aeolian Res. 2010, 2, 105–111. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Dugan, J.; Schoeman, D.S.; Lastra, M.; Jones, A.; Scapini, F.; McLachlan, A.; Defeo, O. Sandy beaches at the brink. Divers. Distrib. 2007, 13, 556–560. [Google Scholar] [CrossRef] [Green Version]
- Kutiel, P.; Eden, Z.; Zhevelev, H. The impact of motorcycle traffic on soil and vegetation of stabilized coastal dunes, Israel. J. Coast. Conserv. 2001, 7, 81–90. [Google Scholar] [CrossRef]
- Kutiel, P.; Eden, E.; Zhevelev, Y. Effect of experimental trampling and off-road motorcycle traffic on soil and vegetation of stabilized coastal dunes, Israel. Environ. Conserv. 2000, 27, 14–23. [Google Scholar] [CrossRef]
- Santoro, R.; Jucker, T.; Prisco, I.; Carboni, M.; Battisti, C.; Acosta, A.T.R. Effects of trampling limitation on coastal dune plant communities. Environ. Manag. 2012, 49, 534–542. [Google Scholar] [CrossRef]
- Liddle, M.J. A selective review of the ecological effects of human trampling on natural ecosystems. Biol. Conserv. 1975, 7, 17–36. [Google Scholar] [CrossRef]
- Davenport, J.; Davenport, J.L. The impact of tourism and personal leisure transport on coastal environments: A review. Estuar. Coast. Shelf Sci. 2006, 67, 280–292. [Google Scholar] [CrossRef]
- Defeo, O.; McLachlan, A.; Schoeman, D.S.; Schlacher, T.A.; Dugan, J.E.; Jones, A.; Lastra, M.; Scapini, F. Threats to sandy beach ecosystems: A review. Estuar. Coast. Shelf Sci. 2009, 81, 1–12. [Google Scholar] [CrossRef]
- Luckenbach, R.A.; Bury, R.B. Effects of off-road vehicles on the biota of the Algodones Dunes, Imperial County, California. J. Appl. Ecol. 1983, 20, 265–283. [Google Scholar] [CrossRef]
- Priskin, J. Physical impacts of four-wheel drive related tourism and recreation in a semi-arid, natural coastal environment. Ocean Coast. Manag. 2003, 46, 127–155. [Google Scholar] [CrossRef]
- Van Dam, A.R.; Dam, M.H. Van Impact of off-road vehicle use on dune endemic Coleoptera. Ann. Entomol. Soc. Am. 2008, 101, 411–417. [Google Scholar] [CrossRef]
- Vega, L.E.; Bellagamba, P.J.; Fitzgerald, L.A. Long-term effects of anthropogenic habitat disturbance on a lizard assemblage inhabiting coastal dunes in Argentina. Can. J. Zool. 2000, 78, 1653–1660. [Google Scholar] [CrossRef]
- Rocha, C.F.; Bergallo, H. Population decrease: The case of Liolaemus lutzae, an endemic lizard of Southeastern Brazil. Ciencia e Cultura, Sao Paulo, Brazil. J. Braz. Assoc. Adv. Sci. 1992, 44, 52–54. [Google Scholar]
- Abramsky, Z.; Rosenzweig, M.L.; Pinshow, B.; Brown, J.S.; Kotler, B.; Mitchell, W.A. Habitat selection: An experimental field test with two gerbil species. Ecology 1990, 71, 2358–2369. [Google Scholar] [CrossRef]
- Ruessink, B.G.; Arens, S.M.; Kuipers, M.; Donker, J.J.A. Coastal dune dynamics in response to excavated foredune notches. Aeolian Res. 2018, 31, 3–17. [Google Scholar] [CrossRef]
- Plassmann, K.; Jones, M.L.M.; Edwards-Jones, G. Effects of long-term grazing management on sand dune vegetation of high conservation interest. Appl. Veg. Sci. 2010, 13, 100–112. [Google Scholar] [CrossRef]
- Valdés-Correcher, E.; Rodriguez, E.; Kemp, Y.J.M.; Wassen, M.J.; Cromsigt, J.P.G.M. Comparing the impact of a grazing regime with European bison versus one with free-ranging cattle on coastal dune vegetation in the Netherlands. Mammal Res. 2018, 63, 455–466. [Google Scholar] [CrossRef]
- Rainio, J.; Niemela, J. Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers. Conserv. 2003, 12, 487–506. [Google Scholar] [CrossRef]
- Wallis De Vries, M.F.; Raemakers, I. Does extensive grazing benefit butterflies in coastal dunes? Restor. Ecol. 2001, 9, 179–188. [Google Scholar] [CrossRef]
- Sirami, C.; Nespoulous, A.; Cheylan, J.P.; Marty, P.; Hvenegaard, G.T.; Geniez, P.; Schatz, B.; Martin, J.L. Long-term anthropogenic and ecological dynamics of a Mediterranean landscape: Impacts on multiple taxa. Landsc. Urban Plan. 2010, 96, 214–223. [Google Scholar] [CrossRef]
- Read, J.L. Experimental trial of Australian arid zone reptiles as early warning indicators of overgrazing by cattle. Austral Ecol. 2002, 27, 55–66. [Google Scholar] [CrossRef]
- Attum, O.; Eason, P.; Cobbs, G.; Baha El Din, S.M. Response of a desert lizard community to habitat degradation: Do ideas about habitat specialists/generalists hold? Biol. Conserv. 2006, 133, 52–62. [Google Scholar] [CrossRef]
- Tahmasebi Kohyani, P.; Kohyani, P.T.; Bossuyt, B.; Bonte, D.; Hoffmann, M. Grazing impact on plant spatial distribution and community composition. Plant Ecol. Evol. 2011, 144, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Sandom, C.; Donlan, C.J.; Svenning, J.-C.; Hansen, D. Rewilding. In Key Topics in Conservation Biology 2; John Wiley & Sons: Oxford, UK, 2013; pp. 430–451. [Google Scholar]
- IUCN SSC Antelope Specialist Group Gazella Gazella. The IUCN Red List of Threatened Species 2017; IUCN SSC Antelope Specialist Group Gazella Gazella: Grand, Switzerland, 2017. [Google Scholar]
- Katz, O.; Kam, M.; Carmi, A.; Degen, A.A.; Henkin, Z.; Bar (Kutiel), P. Activity and short-term impacts of dromedary camels (Camelus dromedarius) foraging on perennial coastal sand dune vegetation. J. Arid Environ. 2016, 133, 47–53. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Norton, D.A. Towards a conceptual framework for restoration ecology. Restor. Ecol. 1996, 4, 93–110. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011; ISBN 9788578110796. [Google Scholar]
- Magnuson, W. Uncertainty and the design of in-situ biodiversity-monitoring programs. Nat. Conserv. 2014, 8, 77–94. [Google Scholar] [CrossRef]
- Michener, W.K. Quantitatively evaluating restoration experiments: Research design, statistical analysis, and data management considerations. Restor. Ecol. 1997, 5, 324–337. [Google Scholar] [CrossRef]











| Taxa | Species | Affiliated Dune Type | IV % (Affinity) | wPPC% |
|---|---|---|---|---|
| Rodents | Gerbillus pyramidum * | Mobile | 91.29 | 11.43 |
| Gerbillus andersoni allenbyi ** | Semi-Fixed | 61.67 | 23.81 | |
| Reptiles | Acanthodactylus scutellatus * | Mobile | 76.05 | 13.38 |
| Stenodactylus sthenodactylus | Semi-Fixed | 61.10 | 18.77 | |
| Chalcides ocellatus | Fixed | 70.68 | 25.28 | |
| Acanthodactylus schreiberi ** | Fixed | 84.92 | 29.18 | |
| Beetles | Scarites striatus * | Mobile | 95.90 | 10.91 |
| Mecynotarsus bison | Mobile | 98.13 | 10.89 | |
| Cardiophorus reitteri | Mobile | 60.97 | 12.72 | |
| Eurycaulus henoni | Mobile | 69.49 | 15.07 | |
| Tentyrina orbiculata | Mobile | 63.70 | 19.26 | |
| Erodius dejeani | Fixed | 62.61 | 24.11 | |
| Cheirodes spp. | Fixed | 60.77 | 25.66 | |
| Mesostena angustata | Fixed | 59.41 | 27.04 | |
| Graphopterus sharonae ** | Fixed | 79.17 | 33.00 | |
| Annuals | Cutandia memphitica * | Mobile | 64.42 | 18.64 |
| Ifloga spicata | Semi-Fixed | 69.78 | 18.89 | |
| Lotus halophilus | Semi-Fixed | 64.34 | 19.86 | |
| Crepis aculeate | Semi-Fixed | 68.24 | 20.62 | |
| Polycarpon succulentum | Semi-Fixed | 66.85 | 20.70 | |
| Rumex pictus | Semi-Fixed | 65.60 | 25.06 | |
| Erodium laciniatum | Fixed | 65.50 | 29.89 | |
| Daucus glaber | Fixed | 72.58 | 31.56 | |
| Hormuzakia aggregata | Fixed | 70.76 | 31.62 | |
| Geranium robertianum | Fixed | 73.60 | 32.13 | |
| Lupinus palaestinus | Fixed | 62.36 | 32.46 | |
| Asphodelus tenuifolius | Fixed | 70.71 | 32.89 | |
| Maresia pulchella | Fixed | 64.19 | 33.02 | |
| Anagallis arvensis | Fixed | 77.99 | 33.17 | |
| Brassica tournefortii | Fixed | 83.86 | 33.99 | |
| Rumex bucephalophorus | Fixed | 92.59 | 34.11 | |
| Bromus rigidus ** | Fixed | 63.51 | 34.20 |
| Indicators | Taxa | Species | ST | CT | DT |
|---|---|---|---|---|---|
| (i) Mobile IS | (a) Rodent | G. pyramidum | 126% | 15% | 0% |
| (b) Reptile | A. scutellatus | 67% | 15% | 4% | |
| (c) Beetle | S. striatus | 42% | 27% | 0% | |
| (d) Annual | C. memphitica | - | 114% | −181% | |
| (ii) Fixed IS | (a) Rodent | G. a. andersoni | 158% | 12% | 30% |
| (b) Reptile | A. schreiberi | 180% | 36% | −2% | |
| (c) Beetle | G. sharonae | 127% | 30% | 33% | |
| (d) Annual | B. rigidus | - | 42% | 21% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bird, T.L.F.; Bouskila, A.; Groner, E.; Bar Kutiel, P. Can Vegetation Removal Successfully Restore Coastal Dune Biodiversity? Appl. Sci. 2020, 10, 2310. https://doi.org/10.3390/app10072310
Bird TLF, Bouskila A, Groner E, Bar Kutiel P. Can Vegetation Removal Successfully Restore Coastal Dune Biodiversity? Applied Sciences. 2020; 10(7):2310. https://doi.org/10.3390/app10072310
Chicago/Turabian StyleBird, Tania Leah Fairfax, Amos Bouskila, Elli Groner, and Pua Bar Kutiel. 2020. "Can Vegetation Removal Successfully Restore Coastal Dune Biodiversity?" Applied Sciences 10, no. 7: 2310. https://doi.org/10.3390/app10072310
APA StyleBird, T. L. F., Bouskila, A., Groner, E., & Bar Kutiel, P. (2020). Can Vegetation Removal Successfully Restore Coastal Dune Biodiversity? Applied Sciences, 10(7), 2310. https://doi.org/10.3390/app10072310