Development of Novel Polymer Supported Nanocomposite GO/TiO2 Films, Based on poly(L-lactic acid) for Photocatalytic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Films
2.3. Characterization of PLLA–GO–TiO2 Composite Films
2.3.1. Wide Angle X-Ray Scattering
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Scanning Electron Microscopy (SEM)
2.4. TiO2 Photocatalytic Degradation Experiment
2.5. Analytical Procedures
2.5.1. Kinetic Studies—LC-MS Analysis
2.5.2. Mineralization Studies
2.5.3. Toxicity Analysis
3. Results and Discussion
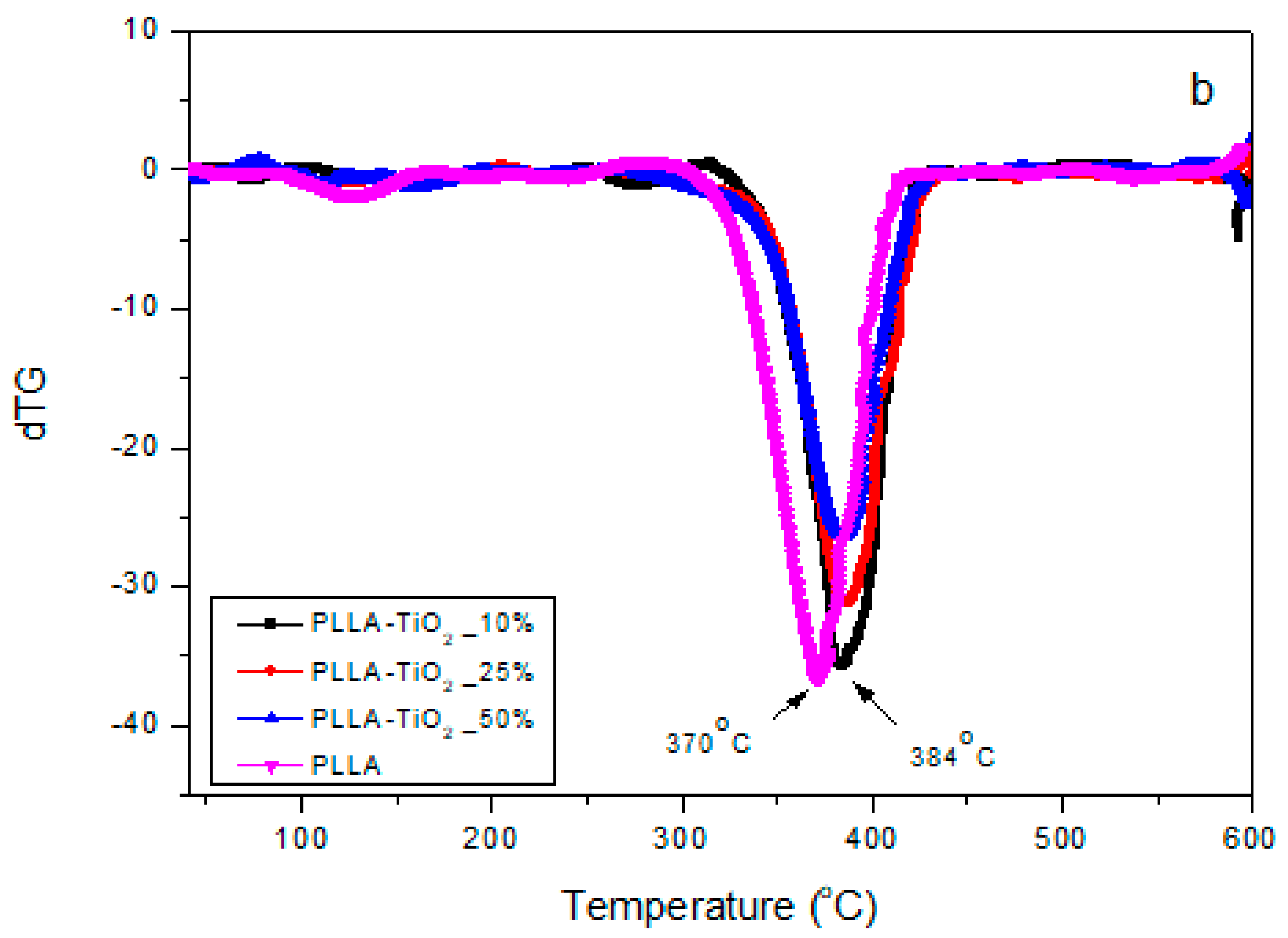
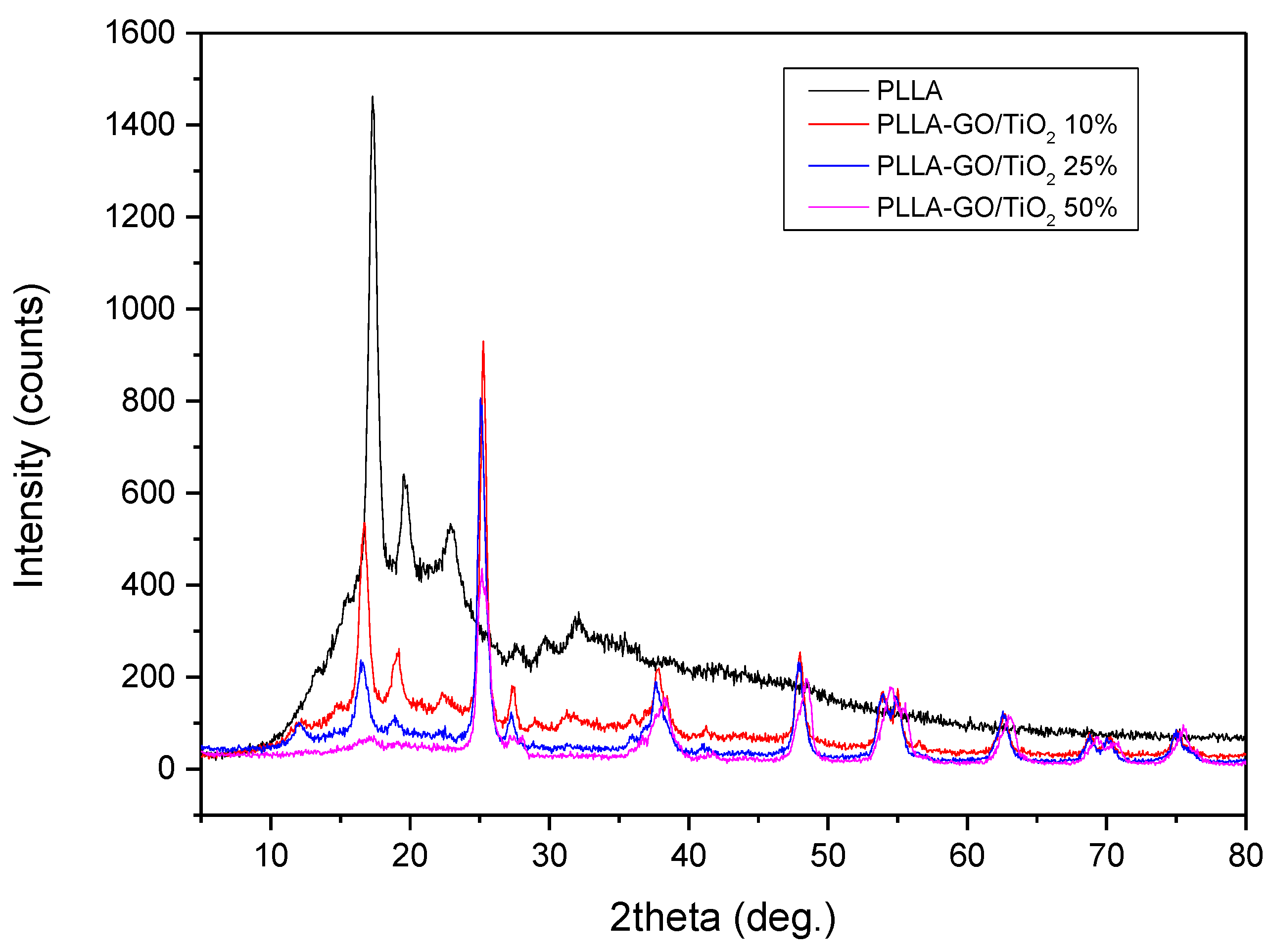
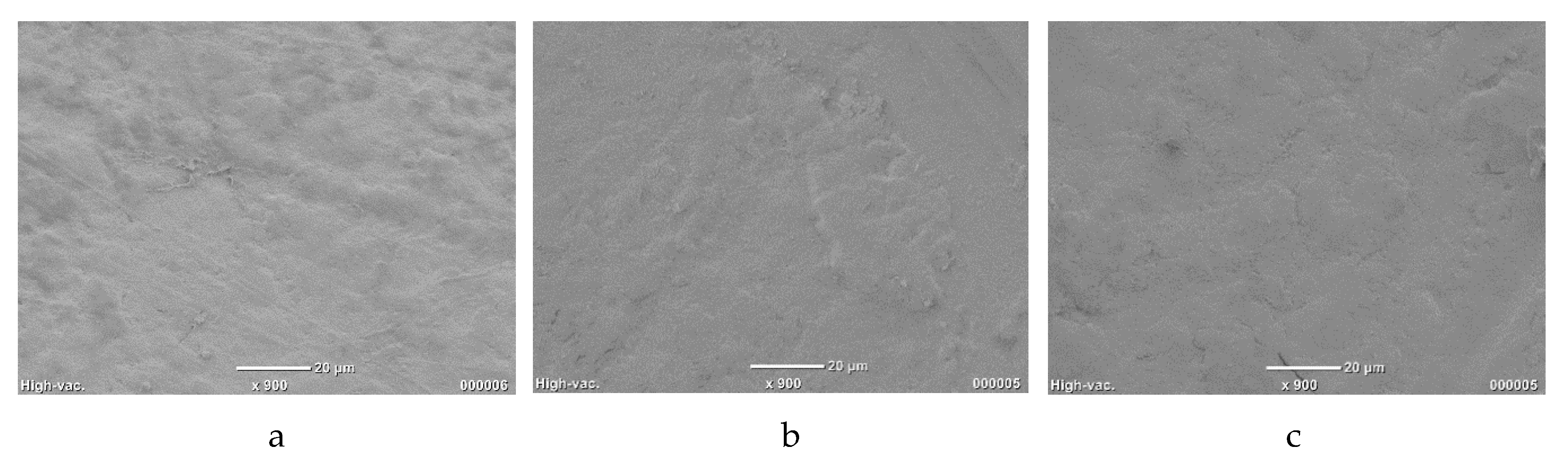
3.1. PLLA–GO–TiO2 Characterization
3.2. PLLA–GO–TiO2-Based Photocatalysis
3.2.1. Photocatalytic Degradation
3.2.2. Effect of TiO2 Content on PLLA Film
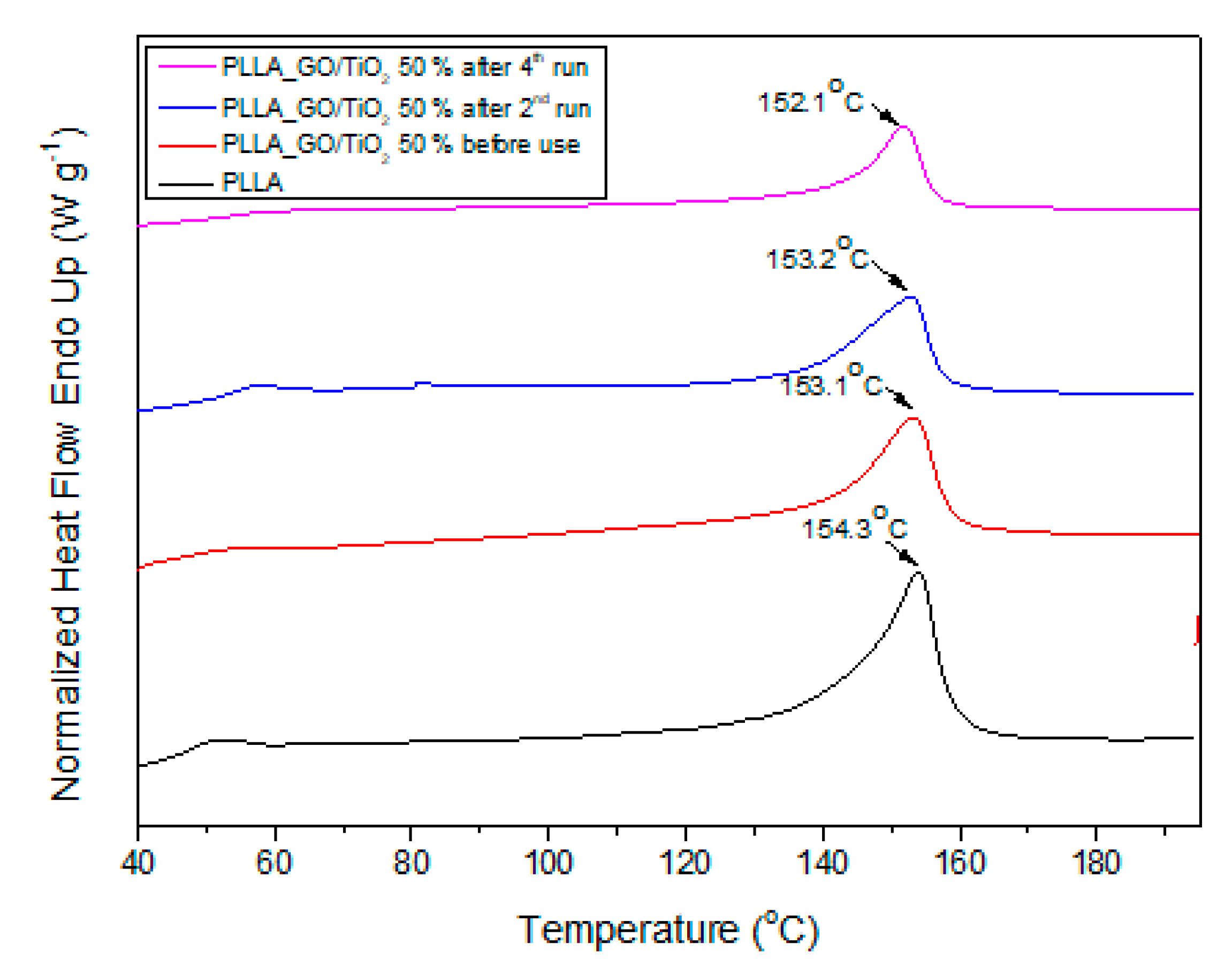
3.2.3. Reusability Study of the PLLA–GO–50%wt TiO2 Composite Film
3.2.4. Mineralization and Toxicity Evaluation
3.2.5. Evaluation of the Photocatalytic Efficiency of the PLLA–GO–50 wt% TiO2 Composite Film on the Photodegradation of the Mixture of Antibiotics in a Wastewater Matrix
4. Conclusions
Author Contributions
Funding

Acknowledgments
Conflicts of Interest
References
- De Vidales, M.M.; Nieto-márquez, A.; Morcuende, D.; Atanes, E.; Blaya, F.; Soriano, E.; Fernández-martínez, F. 3D printed floating photocatalysts for wastewater treatment. Catal. Today 2019, 328, 157–163. [Google Scholar] [CrossRef]
- Neghi, N.; Krishnan, N.R.; Kumar, M. Analysis of metronidazole removal and micro-toxicity in photolytic systems: Effects of persulfate dosage, anions and reactor operation-mode. J. Environ. Chem. Eng. 2018, 6, 754–761. [Google Scholar] [CrossRef]
- Gonzalez, B.D.; Bayarri, O.; Acena, B.; Perez, J. Treatment Technologies for Wastewater Reuse: Fate of Contaminants of Emerging Concern. Adv. Treat. Technol. Urban Wastewater Reuse 2015, 20, 95–100. [Google Scholar]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Deng, J.; Sun, P.; Liu, J.; Ji, Y.; Nakada, N.; Qiao, Z.; Tanaka, H.; Yang, Y. Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: Systematic review and bibliometric analysis. Sci. Total Environ. 2018, 627, 1253–1263. [Google Scholar] [CrossRef]
- Kyzas, Z.G.; Fu, J.; Lazaridis, K.N.; Bikiaris, N.D.; Matis, A.K. New approaches on the removal of pharmaceuticals from wastewaters with adsorbent materials. J. Mol. Liq. 2015, 209, 87–93. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Sancho, K.V.; Serrano, R.; Hernández, F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 2012, 87, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Topkaya, E.; Konyar, M.; Yatmaz, H.C.; Öztürk, K. A comparative study for the degradation of azo dye, pesticide and antibiotic in aqueous solutions. J. Colloid Interface Sci. 2014, 430, 6–11. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Stuer-lauridsen, F. Review of passive accumulation devices for monitoring organic micropollutants in the aquatic environment. Environ. Pollut. 2005, 136, 503–524. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Liu, Y.; Lu, S.; Qin, P.; Guo, X.; Bi, B.; Wang, L.; Xi, B.; Wu, F.; et al. Occurrence and fate of antibiotics and antibiotic resistance genes in typical urban water of Beijing, China. Environ. Pollut. 2019, 246, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhou, J.; Zhou, S.; Wu, P.; Tsang, Y.F. Occurrence and fate of antibiotics in a wastewater treatment plant and their biological effects on receiving waters in Guizhou. Process Saf. Environ. Prot. 2018, 113, 483–490. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.; Zhang, H.; Alvarez, P.J.J. Occurrence and Transport of Tetracycline, Sulfonamide, Quinolone, and Macrolide Antibiotics in the Haihe River Basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, I.T.; Santos, J.L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, A.; Eckert, E.M.; Urso, S.D.; Bertoni, R.; Gillan, D.C.; Wattiez, R.; Corno, G. Co-occurrence of integrase 1, antibiotic and heavy metal resistance genes in municipal wastewater treatment plants. Water Res. 2016, 94, 208–214. [Google Scholar] [CrossRef]
- Wang, W.L.; Wu, Q.Y.; Huang, N.; Bin Xu, Z.; Lee, M.Y.; Hu, H.Y. Potential risks from UV/H2O2 oxidation and UV photocatalysis: A review of toxic, assimilable, and sensory-unpleasant transformation products. Water Res. 2018, 141, 109–125. [Google Scholar] [CrossRef]
- Cardinal, P.; Anderson, J.C.; Carlson, J.C.; Low, J.E.; Challis, J.K.; Beattie, S.A.; Bartel, C.N.; Elliott, A.D.; Montero, O.F.; Lokesh, S.; et al. Macrophytes may not contribute significantly to removal of nutrients, pharmaceuticals, and antibiotic resistance in model surface constructed wetlands. Sci. Total Environ. 2014, 482–483, 294–304. [Google Scholar] [CrossRef]
- Mulla, S.I.; Hu, A.; Sun, Q.; Li, J.; Ashfaq, M.; Yu, C. Biodegradation of sulfamethoxazole in bacteria from three different origins. J. Environ. Manag. 2018, 206, 93–102. [Google Scholar] [CrossRef]
- Ashfaq, M.; Li, Y.; Wang, Y.; Chen, W.; Wang, H.; Chen, X.; Wu, W.; Huang, Z.; Yu, C.; Sun, Q. Occurrence, fate, and mass balance of different classes of pharmaceuticals and personal care products in an anaerobic-anoxic-oxic wastewater treatment plant in Xiamen, China. Water Res. 2017, 123, 655–667. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, O.; Bayarri, B.; Acena, J.; Perez, S.; Barcelo, D. Treatment Technologies for Wastewater Reuse: Fate of Contaminants of Emerging Concern. Handb. Environ. Chem. 2015, 363, 5–37. [Google Scholar]
- Malesic-Eleftheriadou, N.; Evgenidou, E.; Kyzas, G.Z.; Bikiaris, N.D.; Lambropoulou, D.A. Removal of antibiotics in aqueous media by using new synthesized bio-based poly(ethylene terephthalate)-TiO2 photocatalysts. Chemosphere 2019, 234, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Schaider, L.A.; Rodgers, K.M.; Rudel, R.A. Review of Organic Wastewater Compound Concentrations and Removal in Onsite Wastewater Treatment Systems. Environ. Sci. Technol. 2017, 51, 7304–7317. [Google Scholar] [CrossRef] [Green Version]
- Koltsakidou, A.; Antonopoulou, M.; Εvgenidou, E.; Konstantinou, I.; Giannakas, A.E.; Papadaki, M.; Bikiaris, N.D.; Lambropoulou, D.A. Photocatalytical removal of fluorouracil using TiO2-P25 and N/S doped TiO2 catalysts: A kinetic and mechanistic study. Sci. Total Environ. 2017, 578, 257–267. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, P.; Ji, Y.; Tian, J.; Du, Z. Photocatalytic degradation of four non-steroidal anti-inflammatory drugs in water under visible light by P25-TiO2/tetraethyl orthosilicate film and determination via ultra performance liquid chromatography electrospray tandem mass spectrometry. Chem. Eng. J. 2015, 262, 1108–1115. [Google Scholar] [CrossRef]
- Luiz, D.B.; Jose, H.J.; Moreira, R.F.P.M. A Discussion Paper on Challenges and Proposals for Advanced Treatments for Potabilization of Wastewater in the Food Industry. Sci. Heal. Soc. 2012, 3–24. [Google Scholar] [CrossRef] [Green Version]
- Fagan, R.; Mccormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2015, 42, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Liu, F.; Yue, C.; Ling, C.; Li, A. A recyclable nanosheet of Mo/N-doped TiO2 nanorods decorated on carbon nanofibers for organic pollutants degradation under simulated sunlight irradiation. Chemosphere 2019, 215, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Thuy-Duong Nguyen-Phan, S.K.; Pham, V.H.; Shin, E.W.; Pham, H.; Jin, S.H.H.; Chung, S.; Kim, E.J. The role of graphene oxide content on the adsorption-enhanced photocatalysis of titanium dioxide/graphene oxide composites. Chem. Eng. J. 2011, 170, 226–232. [Google Scholar] [CrossRef]
- Shan, A.Y.; Idaty, T.; Ghazi, M.; Rashid, S.A. General Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Alhaji, M.H.; Khan, K.S.A.; Muhammad, A.H.A. Recent developments in immobilizing titanium dioxide on supports for degradation of organic pollutants in wastewater—A review. Int. J. Environ. Sci. Technol. 2017, 14, 2039–2052. [Google Scholar] [CrossRef]
- Xing, Z.; Li, J.; Wang, Q.; Zhou, W.; Tian, G.; Pan, K.; Tian, C.; Zou, J.; Fu, H. A Floating Porous Crystalline TiO2 Ceramic with Enhanced Photocatalytic Performance for Wastewater Decontamination. Eur. J. Inorg. Chem. 2013, 2013, 2411–2417. [Google Scholar] [CrossRef]
- Buzarovska, A.; Gualandi, C.; Parrilli, A.; Scandola, M. Effect of TiO2 nanoparticle loading on Poly(L-lactic acid) porous scaffolds fabricated by TIPS. Compos. Part B Eng. 2015, 81, 189–195. [Google Scholar] [CrossRef]
- Athanasoulia, I.; Mikropoulou, M.; Karapati, S. Study of thermomechanical and antibacterial properties of TiO2/Poly(lactic acid) nanocomposites. Mater. Today Proc. 2018, 5, 27553–27562. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Roberto, A. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Shaikh, K.H.; Rathore, T.; Kaur, H. Poly(Lactic Acid) Grafting of TiO2 Nanoparticles: A Shift in Dye Degradation Performance of TiO2 from UV to Solar Light. Chem. Sel. 2017, 2, 6901–6908. [Google Scholar]
- Zhu, Y.; Buonocore, G.G.; Lavorgna, M. Photocatalytic Activity of PLA/TiO2 Nanocomposites and TiO2-active Multilayered Hybrid Coatings. Ital. J. Food Sci. 2012, 24, 102–107. [Google Scholar]
- Zhu, Y.; Buonocore, G.G.; Lavorgna, M.; Ambrosio, L. Poly(lactic acid)/Titanium Dioxide Nanocomposite Films: Influence of Processing Procedure on Dispersion of Titanium Dioxide and Photocatalytic Activity. Polym. Compos. 2011, 32, 519–528. [Google Scholar] [CrossRef]
- Hou, X.; Yibing, C.; Muhammad, M.; Xiaofei, S.; Qiang, Y.; Fenglin, H.; Qufu, W. Deposition of TiO2 Nanoparticles on Porous Polylactic Acid Fibrous Substrates and Its Photocatalytic Capability. J. Nanomed. Nanotechnol. 2018, 18, 5617–5623. [Google Scholar] [CrossRef] [PubMed]
- Kaseem, Z.U.; Hamad, M.; Rehman, K. Review of Recent Advances in Polylactic. Mater. Sci. Eng. C 2019, 12, 3659. [Google Scholar]
- Carpenter, D.O.; Arcaro, K.; Spink, D.C. Understanding the Human Health Effects of Chemical Mixtures. Environ. Health Perspect. 2002, 110, 25–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stergiou, D.V.; Diamanti, K.; Gournis, D.; Prodromidis, M.I. Comparative study of different types of graphenes as electrocatalysts for ascorbic acid. Electrochem. Commun. 2010, 12, 1307–1309. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Terzopoulou, Z.; Bikiaris, N.D.; Triantafyllidis, K.S.; Diamanti, E.; Gournis, D.; Klonos, P.; Giannoulidis, E.; Pissis, P. Evaluation of the formed interface in biodegradable poly(l-lactic acid)/graphene oxide nanocomposites and the effect of nanofillers on mechanical and thermal properties. Thermochim. Acta 2014, 597, 48–57. [Google Scholar] [CrossRef]
- Nerantzaki, M.; Prokopiou, L.; Bikiaris, N.D.; Patsiaoura, D.; Chrissafis, K.; Klonos, P.; Kyritsis, A.; Pissis, P. In situ prepared poly(DL-lactic acid)/silica nanocomposites: Study of molecular composition, thermal stability, glass transition and molecular dynamics. Thermochim. Acta 2018, 669, 16–29. [Google Scholar] [CrossRef]
- Bet-Moushoul, E.; Mansourpanah, Y.; Farhadi, K.; Tabatabaei, M. TiO2 nanocomposite based polymeric membranes: A review on performance improvement for various applications in chemical engineering processes. Chem. Eng. J. 2016, 283, 29–46. [Google Scholar] [CrossRef]
- Klonos, P.; Kripotou, S.; Kyritsis, A.; Papageorgiou, G.Z.; Bikiaris, N.D.; Gournis, D. Glass transition and segmental dynamics in poly(l-lactic acid)/graphene oxide nanocomposites. Thermochim. Acta 2015, 617, 44–53. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Gournis, D.; Petridis, D.; Szabó, T.; Szeri, A.; Dékány, I. Graphite oxide: Chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids. Langmuir 2003, 19, 6050–6055. [Google Scholar] [CrossRef]
- Klonos, P.; Terzopoulou, Z.; Koutsoumpis, S.; Zidropoulos, S.; Kripotou, S.; Papageorgiou, G.Z.; Bikiaris, D.N.; Kyritsis, A.; Pissis, P. Rigid amorphous fraction and segmental dynamics in nanocomposites based on poly(L–lactic acid) and nano-inclusions of 1–3D geometry studied by thermal and dielectric techniques. Eur. Polym. J. 2016, 82, 16–34. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Klonos, P.A.; Kyritsis, A.; Tziolas, A.; Avgeropoulos, A.; Papageorgiou, G.Z.; Bikiaris, D.N. Interfacial interactions, crystallization and molecular mobility in nanocomposites of Poly(lactic acid) filled with new hybrid inclusions based on graphene oxide and silica nanoparticles. Polymer 2019, 166, 1–12. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Achilias, D.S.; Nanaki, S.; Beslikas, T.; Bikiaris, N.D. PLA nanocomposites: Effect of filler type on non-isothermal crystallization. Thermochim. Acta 2010, 511, 129–139. [Google Scholar] [CrossRef]
- Babić, S.; Periša, M.; Škorić, I. Photolytic degradation of norfloxacin, enrofloxacin and ciprofloxacin in various aqueous media. Chemosphere 2013, 91, 1635–1642. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Hir, Z.A.M.; Moradihamedani, P.; Abdullah, A.H.; Mohamed, M.A. Immobilization of TiO2 into polyethersulfone matrix as hybrid film photocatalyst for effective degradation of methyl orange dye. Mater. Sci. Semicond. Process. 2017, 57, 157–165. [Google Scholar] [CrossRef]
- Tahir, C.; Qazi, I.A.; Anwar, M.; Arshad, M.; Quddos, A. Enhanced photodegradation of titania loaded polyethylene films in a humid environment. Int. Biodeterior. Biodegrad. 2016, 113, 287–296. [Google Scholar]
- Agüera, A.; Perez Estrada, L.A.; Ferrer, I.; Thurman, E.M.; Malato, S.; Fernandez-Alba, A.R. Application of time-of-flight mass spectrometry to the analysis of phototransformation products of diclofenac in water under natural sunlight. J. Mass Spectrom. 2005, 40, 908–915. [Google Scholar] [CrossRef]
- Koltsakidou, A.; Antonopoulou, M.; Εvgenidou, E.; Konstantinou, I. A comparative study on the photo-catalytic degradation of Cytarabine anticancer drug under Fe3+/H2O2, Fe3+/S2O82−, and [Fe(C2O4)3]3−/H2O2 processes. Kinetics, identification, and in silico toxicity assessment of generated transformation products. Environ. Sci. Pollut. Res. 2019, 26, 7772–7784. [Google Scholar] [CrossRef]









| Categories | Antibiotics | Photocatalysis | Photolysis | ||||
|---|---|---|---|---|---|---|---|
| k | t1/2 | R2 | k | t1/2 | R2 | ||
| Fluoroquinolones | moxifloxacin | 0.014 | 49.5 | 0.98 | 0.003 | 231.0 | 0.96 |
| levofloxacin | 0.019 | 36.5 | 0.99 | 0.005 | 138.6 | 0.97 | |
| norfloxacin | 0.031 | 22.4 | 0.99 | 0.020 | 34.7 | 0.98 | |
| Sulfonamides | sulfamethoxazole | 0.022 | 31.5 | 0.97 | 0.010 | 69.3 | 0.99 |
| sulfadiazine | 0.015 | 46.2 | 0.97 | 0.014 | 49.5 | 0.98 | |
| Diaminopyrimidines | trimethoprim | 0.008 | 86.6 | 0.99 | 0.001 | 69.1 | 0.96 |
| Lincosamides | lincomycin | 0.013 | 53.3 | 0.98 | 0.007 | 99.0 | 0.98 |
| Imidazoles | metronidazole | 0.037 | 18.7 | 0.99 | 0.033 | 21.0 | 0.99 |
| Hydrazide derivatives | isoniazid | 0.017 | 40.8 | 0.98 | 0.006 | 11.5 | 0.97 |
| Antibiotics | PLLA–10% wt TiO2 | PLLA–25% wt TiO2 | PLLA–50 wt% TiO2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fluoro-Quinolones | k | t1/2 | R2 | k | t1/2 | R2 | k | t1/2 | R2 |
| moxifloxacin | 0.013 | 53.3 | 0.97 | 0.014 | 49.5 | 0.98 | 0.014 | 49.5 | 0.98 |
| levofloxacin | 0.009 | 77.0 | 0.98 | 0.014 | 49.5 | 0.98 | 0.019 | 36.5 | 0.99 |
| norfloxacin | 0.020 | 34.7 | 0.97 | 0.023 | 30.1 | 0.96 | 0.031 | 22.4 | 0.99 |
| Sulfonamides | |||||||||
| sulfamethoxazole | 0.010 | 69.3 | 0.98 | 0.011 | 63.0 | 0.99 | 0.022 | 31.5 | 0.97 |
| sulfadiazine | 0.013 | 53.3 | 0.98 | 0.014 | 49.5 | 0.98 | 0.015 | 46.2 | 0.97 |
| Diaminopyrimidines | |||||||||
| trimethoprim | 0.003 | 231.0 | 0.99 | 0.005 | 138.6 | 0.98 | 0.008 | 86.6 | 0.99 |
| Lincosamides | |||||||||
| lincomycin | 0.008 | 86.6 | 0.97 | 0.012 | 57.8 | 0.98 | 0.013 | 53.3 | 0.98 |
| Imidazoles | |||||||||
| metronidazole | 0.032 | 21.7 | 0.96 | 0.033 | 21.0 | 0.99 | 0.037 | 18.7 | 0.99 |
| Hydrazide derivatives | |||||||||
| isoniazid | 0.009 | 77.0 | 0.98 | 0.016 | 43.3 | 0.97 | 0.017 | 40.8 | 0.98 |
| Categories | Antibiotics | Cycles | |||
|---|---|---|---|---|---|
| k (min−1) | |||||
| 1st | 2nd | 3rd | 4th | ||
| Fluoroquinolones | moxifloxacin | 0.014 | 0.013 | 0.014 | 0.014 |
| levofloxacin | 0.019 | 0.019 | 0.018 | 0.018 | |
| norfloxacin | 0.031 | 0.032 | 0.030 | 0.030 | |
| Sulfonamides | sulfamethoxazole | 0.022 | 0.022 | 0.021 | 0.022 |
| sulfadiazine | 0.015 | 0.014 | 0.015 | 0.016 | |
| Diaminopyrimidines | trimethoprim | 0.008 | 0.009 | 0.007 | 0.008 |
| Lincosamides | lincomycin | 0.013 | 0.013 | 0.011 | 0.012 |
| Imidazoles | metronidazole | 0.037 | 0.035 | 0.037 | 0.033 |
| Hydrazide derivatives | isoniazid | 0.017 | 0.017 | 0.017 | 0.017 |
| Physicochemical Parameters | Values |
|---|---|
| BOD (mgL−1) | 11 |
| COD (mgL−1) | 59 |
| SS (mgL−1) | 8.4 |
| N–NH3 (mgL−1) | 9.3 |
| N–NO3 (mgL−1) | 1.5 |
| Ptot (mgL−1) | 1.2 |
| Bicarbonate (mgL−1) | 380 |
| Chloride (mg−1) | 124 |
| TOC (mgL−1) | 14.3 |
| pH | 7.9 |
| Categories | Antibiotics | Photocatalysis in Wastewater Effluent | ||
|---|---|---|---|---|
| k | t1/2 | R2 | ||
| Fluoroquinolones | moxifloxacin | 0.0035 | 198.0 | 0.99 |
| levofloxacin | 0.0048 | 145.9 | 0.98 | |
| norfloxacin | 0.0078 | 89.4 | 0.97 | |
| Sulfonamides | sulfamethoxazole | 0.0055 | 126.0 | 0.96 |
| sulfadiazine | 0.0038 | 184.8 | 0.97 | |
| Diaminopyrimidines | trimethoprim | 0.002 | 346.6 | 0.99 |
| Lincosamides | lincomycin | 0.0033 | 213.3 | 0.99 |
| Imidazoles | metronidazole | 0.0093 | 74.9 | 0.99 |
| Hydrazide derivatives | isoniazid | 0.0043 | 163.1 | 0.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malesic Eleftheriadou, N.; Ofrydopoulou, A.; Papageorgiou, M.; Lambropoulou, D. Development of Novel Polymer Supported Nanocomposite GO/TiO2 Films, Based on poly(L-lactic acid) for Photocatalytic Applications. Appl. Sci. 2020, 10, 2368. https://doi.org/10.3390/app10072368
Malesic Eleftheriadou N, Ofrydopoulou A, Papageorgiou M, Lambropoulou D. Development of Novel Polymer Supported Nanocomposite GO/TiO2 Films, Based on poly(L-lactic acid) for Photocatalytic Applications. Applied Sciences. 2020; 10(7):2368. https://doi.org/10.3390/app10072368
Chicago/Turabian StyleMalesic Eleftheriadou, Neda, Anna Ofrydopoulou, Myrsini Papageorgiou, and Dimitra Lambropoulou. 2020. "Development of Novel Polymer Supported Nanocomposite GO/TiO2 Films, Based on poly(L-lactic acid) for Photocatalytic Applications" Applied Sciences 10, no. 7: 2368. https://doi.org/10.3390/app10072368





