Natural Extracts from White Common Bean (Phaseolus vulgaris L.) Inhibit 3T3-L1 Adipocytes Differentiation
Abstract
:1. Introduction
2. Material and Method
2.1. Chemicals
2.2. Materials
2.3. Cell Cultures
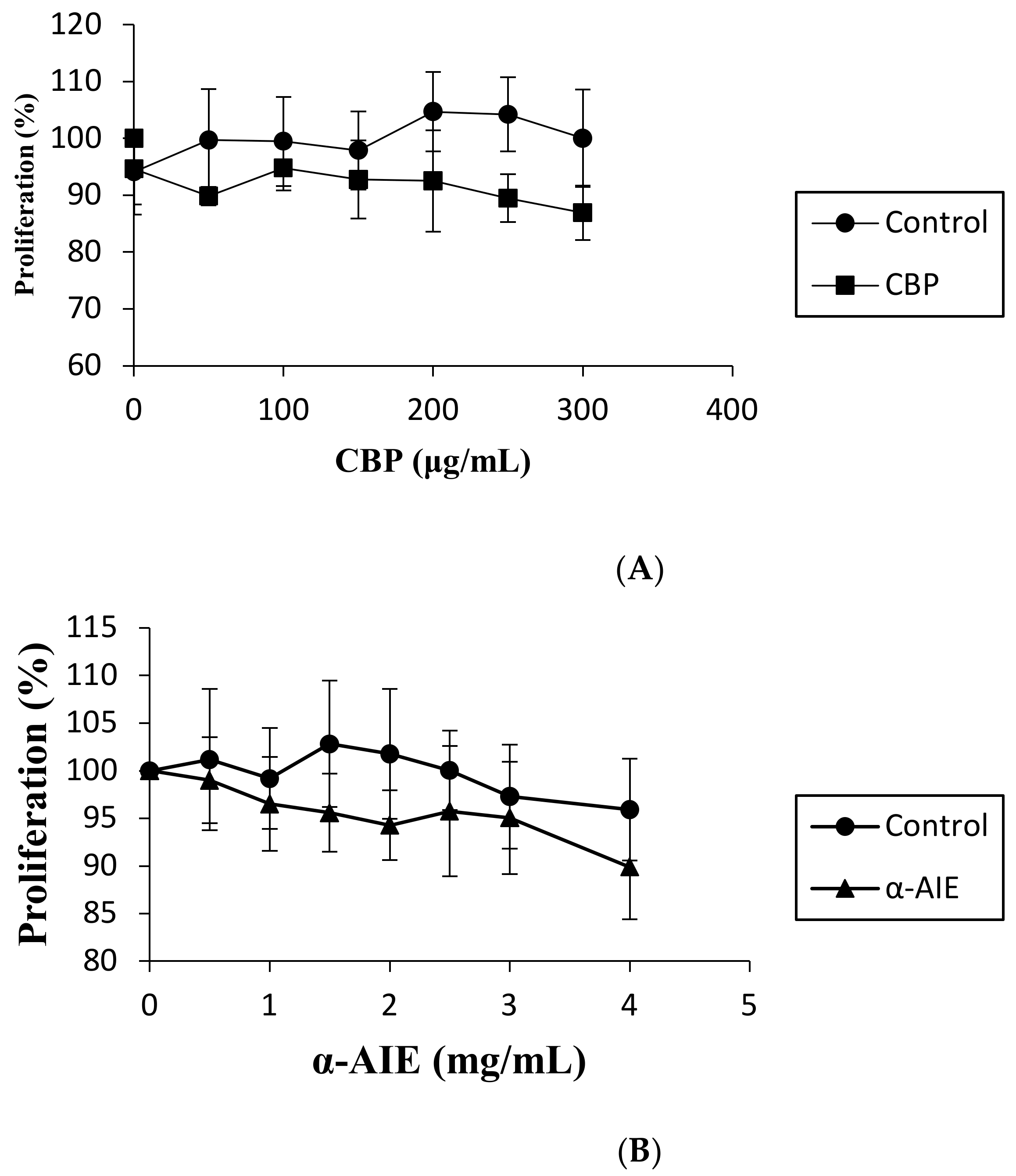
2.4. Cell Cytotoxicity Assay
2.5. 3T3-L1 Adipocyte Differentiation
2.6. Oil Red O Staining
2.7. mRNA Expression Analysis
2.8. Protein Analysis
2.9. Statistics
3. Results and Discussion
3.1. Quality Analysis of Different Common Bean Extracts
3.2. Cell Cytotoxicity Assay
3.3. Intracellular Lipid Accumulation
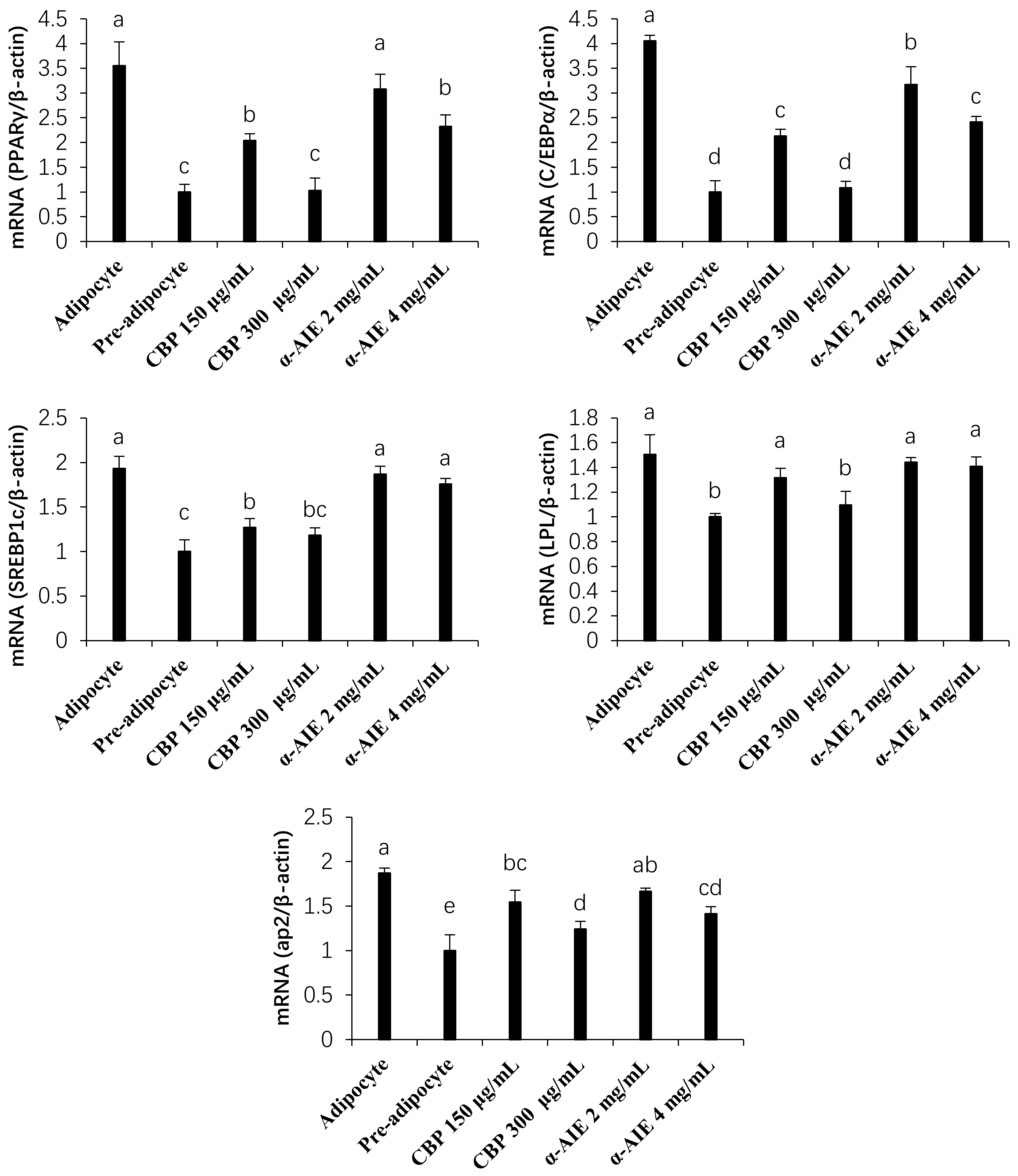
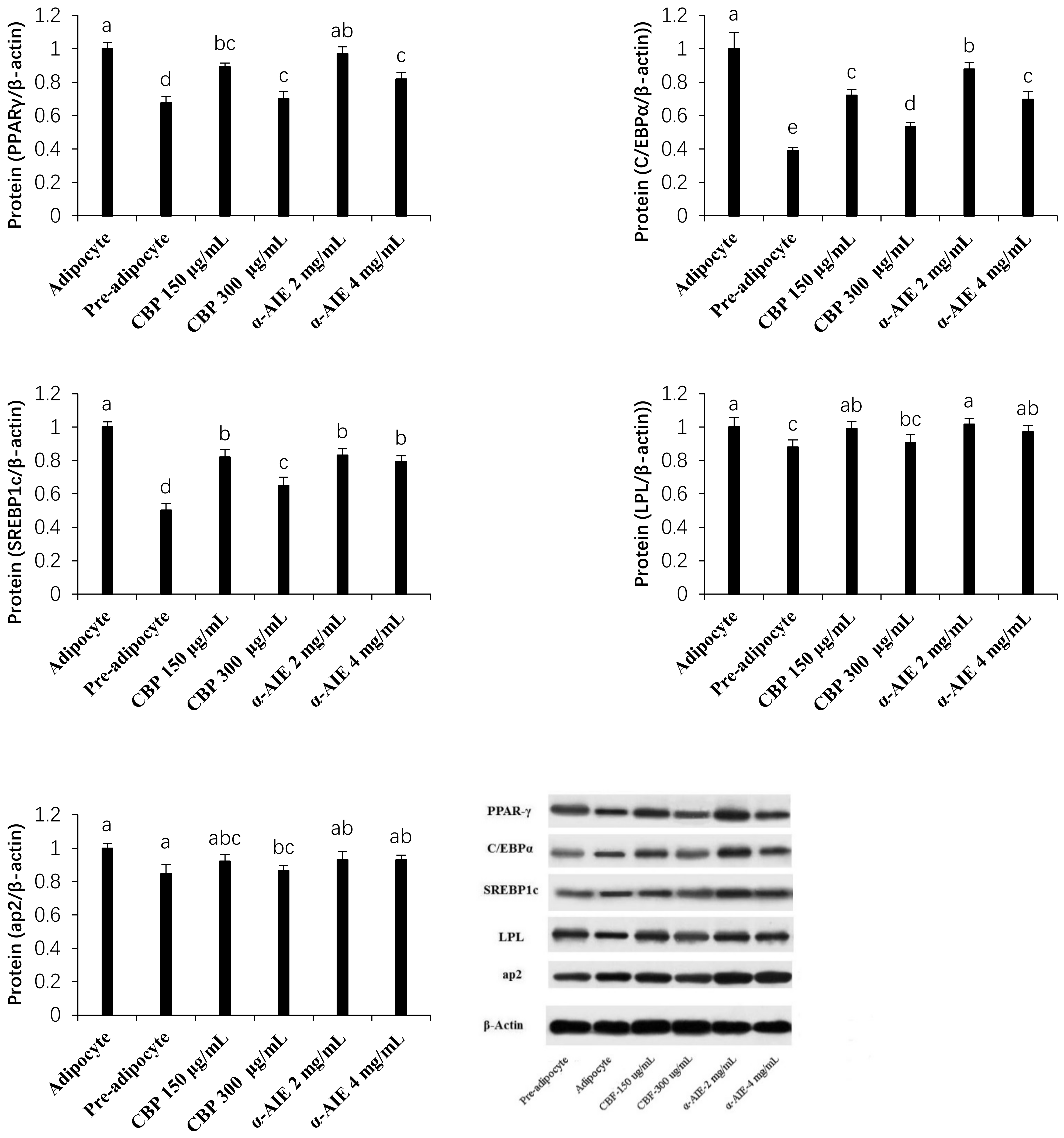
3.4. mRNA and Protein Expression of PPARγ, C/EBPα, and SREBP-1c
3.5. mRNA and Protein Expression of LPL and ap2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masa, J.F.; Pépin, J.-L.; Borel, J.-C.; Mokhlesi, B.; Murphy, P.B.; Sánchez-Quiroga, M.Á. Obesity hypoventilation syndrome. Eur. Respir. Rev. 2019, 28, 151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H.; Wang, Z.; Du, W.; Su, C.; Zhang, J.; Jiang, H.; Jia, X.; Huang, F.; Ouyang, Y. Prevalence and stabilizing trends in overweight and obesity among children and adolescents in China, 2011-2015. BMC Public Health 2018, 18, 571. [Google Scholar] [CrossRef] [Green Version]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Chen, X.; Kong, Q.; Cheng, H.; Cao, X.; Li, Y.; Li, C.; Liu, L.; Ding, Z. HSPA12A is required for adipocyte differentiation and diet-induced obesity through a positive feedback regulation with PPARγ. Cell Death Differ. 2019, 26, 2253–2267. [Google Scholar] [CrossRef]
- Siraj, F.M.; SathishKumar, N.; Kim, Y.J.; Kim, S.Y.; Yang, D.C. Ginsenoside F2 possesses anti-obesity activity via binding with PPARγ and inhibiting adipocyte differentiation in the 3T3-L1 cell line. J. Enzyme Inhib. Med. Chem. 2015, 30, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Park, J.-E.; Song, S.-B.; Cha, Y.-S. Effects of black adzuki bean (Vigna angularis) extract on proliferation and differentiation of 3T3-L1 preadipocytes into mature adipocytes. Nutrients 2015, 7, 277–292. [Google Scholar] [CrossRef] [Green Version]
- Tutunchi, H.; Saghafi-Asl, M.; Ostadrahimi, A. A systematic review of the effects of oleoylethanolamide, a high-affinity endogenous ligand of PPAR-α, on the management and prevention of obesity. Clin. Exp. Pharmacol. Physiol. 2020, 47, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Zingue, S.; do Carmo, Í.A.R.; Tchoumtchoua, J.; Tchoupang, E.N.; de Oliveira Souza Bratti, L.; Dal Mora, T.; Halabalaki, M.; Njamen, D.; Creczynski-Pasa, T.B.; Filippin-Monteiro, F.B. Millettia macrophylla (Fabaceae) phenolic fraction prevents differentiation of 3T3-L1 adipocytes and the increased risks of cardiovascular diseases in ovariectomized rats. J. Ethnopharmacol. 2018, 222, 87–98. [Google Scholar] [CrossRef]
- Zhu, H.; Ding, H.; Deng, J.; Pan, H.; Wang, L.; Li, N.; Wang, X.; Shi, Y.; Gong, F. Inhibition of preadipocyte differentiation and adipogenesis by zinc-α2-glycoprotein treatment in 3T3-L1 cells. J. Diabetes Investig. 2013, 4, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Toledo, M.E.O.; de Mejia, E.G.; Sivaguru, M.; Amaya-Llano, S.L. Common bean (Phaseolus vulgaris L.) protein-derived peptides increased insulin secretion, inhibited lipid accumulation, increased glucose uptake and reduced the phosphatase and tensin homologue activation in vitro. J. Funct. Foods 2016, 27, 160–177. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, Y.; Gao, Y.; Hu, Y.; Shi, Z.; Ren, G. Suppressive Effects of Barley β-Glucans with Different Molecular Weight on 3T3-L1 Adipocyte Differentiation. J. Food Sci. 2016, 81, H786–H793. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Gepts, P.; Debouck, D.G. Races of common bean (Phaseolus vulgaris, Fabaceae). Econ. Bot. 1991, 45, 379–396. [Google Scholar] [CrossRef]
- Thompson, H.; McGinley, J.; Neil, E.; Brick, M. Beneficial effects of common bean on adiposity and lipid metabolism. Nutrients 2017, 9, 998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neil, E.S.; McGinley, J.N.; Fitzgerald, V.K.; Lauck, C.A.; Tabke, J.A.; Streeter-McDonald, M.R.; Yao, L.; Broeckling, C.D.; Weir, T.L.; Foster, M.T. White Kidney Bean (Phaseolus vulgaris L.) Consumption Reduces Fat Accumulation in a Polygenic Mouse Model of Obesity. Nutrients 2019, 11, 2780. [Google Scholar] [CrossRef] [Green Version]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: A review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef]
- Guevara, M.; Tejera, E.; Granda-Albuja, M.G.; Iturralde, G.; Chisaguano-Tonato, M.; Granda-Albuja, S.; Jaramillo-Vivanco, T.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Chemical composition and antioxidant activity of the main fruits consumed in the western coastal region of Ecuador as a source of health-promoting compounds. Antioxidants 2019, 8, 387. [Google Scholar] [CrossRef] [Green Version]
- Layer, P.; Carlson, G.L.; Dimagno, E.P. Partially purified white bean amylase inhibitor reduces starch digestion in vitro and inactivates intraduodenal amylase in humans. Gastroenterology 1985, 88, 1895–1902. [Google Scholar] [CrossRef]
- Shi, Z.; Zhu, Y.; Teng, C.; Yao, Y.; Ren, G.; Richel, A. Anti-obesity effects of α-amylase inhibitor enriched-extract from white common beans (Phaseolus vulgaris L.) associated with the modulation of gut microbiota composition in high-fat diet-induced obese rats. Food Funct. 2020, 11, 1624–1634. [Google Scholar] [CrossRef]
- Choi, J.W.; Synytsya, A.; Capek, P.; Bleha, R.; Pohl, R.; Park, Y. Il Structural analysis and anti-obesity effect of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.). Carbohydr. Polym. 2016, 146, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Lao, W.W. Targeting Cellurlar Dysfunction with Green Tea Polyphenols to Treat the Underlying Basis of the Metabolic Complications of Obesity. Ph.D. Thesis, School of Life Sciences, University of Technology Sydney, Sydney, Australia, 2018. [Google Scholar]
- Kim, N.-H.; Jegal, J.; Kim, Y.N.; Heo, J.-D.; Rho, J.-R.; Yang, M.H.; Jeong, E.J. Chokeberry extract and its active polyphenols suppress adipogenesis in 3T3-L1 adipocytes and modulates fat accumulation and insulin resistance in diet-induced obese mice. Nutrients 2018, 10, 1734. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, J.; Liu, M.; Zhao, H.; Yaqoob, S.; Zheng, M.; Cai, D.; Liu, J. Antiobesity effects of ginsenoside Rg1 on 3T3-L1 preadipocytes and high fat diet-induced obese mice mediated by AMPK. Nutrients 2018, 10, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Yao, Y.; Zhu, Y.; Ren, G. Isoflavones in Chickpeas Inhibit Adipocyte Differentiation and Prevent Insulin Resistance in 3T3-L1 Cells. J. Agric. Food Chem. 2015, 63, 9696–9703. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yao, Y.; Zhu, Y.; Ren, G. Nutritional composition and biological activities of 17 Chinese adzuki bean (Vigna angularis) varieties. Food Agric. Immunol. 2017, 28, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Cheng, X.; Wang, L.; Wang, S.; Ren, G. Biological potential of sixteen legumes in China. Int. J. Mol. Sci. 2011, 12, 7048–7058. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.H.; Chen, C.L.; Jeng, T.L.; Sung, J.M. Comparisons of α-amylase inhibitors from seeds of common bean mutants extracted through three phase partitioning. Food Chem. 2011, 128, 1066–1071. [Google Scholar] [CrossRef]
- Yao, Y.; Hu, Y.; Zhu, Y.; Gao, Y.; Ren, G. Comparisons of phaseolin type and α-amylase inhibitor in common bean (Phaseolus vulgaris L.) in China. Crop J. 2016, 4, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, L.; Yang, J.; Liang, Z. Structure analysis of water-soluble polysaccharide CPPS3 isolated from Codonopsis pilosula. Fitoterapia 2010, 81, 157–161. [Google Scholar] [CrossRef]
- Yao, Y.; Xue, P.; Zhu, Y.; Gao, Y.; Ren, G. Antioxidant and immunoregulatory activity of polysaccharides from adzuki beans (Vigna angularis). Food Res. Int. 2015, 77, 251–256. [Google Scholar] [CrossRef]
- Jiang, X.; Li, T.; Liu, R.H. 2α-Hydroxyursolic acid inhibited cell proliferation and induced apoptosis in MDA-MB-231 human breast cancer cells through the p38/MAPK signal transduction pathway. J. Agric. Food Chem. 2016, 64, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A.; Rychlik, J.; Grajek, W. Cranberries (Oxycoccus quadripetalus) inhibit lipid metabolism and modulate leptin and adiponectin secretion in 3T3-L1 adipocytes. Food Chem. 2015, 185, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-Q.; Gan, R.-Y.; Ge, Y.-Y.; Zhang, D.; Corke, H. Ultrasonic treatment increases extraction rate of common bean (Phaseolus vulgaris L.) antioxidants. Antioxidants 2019, 8, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, J.; Suh, J.H.; Wang, Y. Effects of heat treatment of mandarin peel on flavonoid profiles and lipid accumulation in 3T3-L1 adipocytes. J. Food Drug Anal. 2019, 27, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Chaiittianan, R.; Chayopas, P.; Rattanathongkom, A.; Tippayawat, P.; Sutthanut, K. Anti-obesity potential of corn silks: Relationships of phytochemicals and antioxidation, anti-pre-adipocyte proliferation, anti-adipogenesis, and lipolysis induction. J. Funct. Foods 2016, 23, 497–510. [Google Scholar] [CrossRef]
- Aranaz, P.; Navarro-Herrera, D.; Zabala, M.; Miguéliz, I.; Romo-Hualde, A.; López-Yoldi, M.; Alfredo Martínez, J.; Vizmanos, J.L.; Milagro, F.I.; González-Navarro, C.J. Phenolic compounds inhibit 3T3-L1 adipogenesis depending on the stage of differentiation and their binding affinity to PPARγ. Molecules 2019, 24, 1045. [Google Scholar] [CrossRef] [Green Version]
- Santimone, M.; Koukiekolo, R.; Moreau, Y.; Le Berre, V.; Rougé, P.; Marchis-Mouren, G.; Desseaux, V. Porcine pancreatic α-amylase inhibition by the kidney bean (Phaseolus vulgaris) inhibitor (α-AI1) and structural changes in the α-amylase inhibitor complex. Biochim. Biophys. Acta (BBA)-Proteins Proteomics 2004, 1696, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Zou, W.; Schulz, B.L.; Tan, X.; Sissons, M.; Warren, F.J.; Gidley, M.J.; Gilbert, R.G. The role of thermostable proteinaceous α-amylase inhibitors in slowing starch digestion in pasta. Food Hydrocoll. 2019, 90, 241–247. [Google Scholar] [CrossRef]
- Liu, J.L.; Yang, L.C.; Zhu, X.J.; Wang, W.J.; Zheng, G.D. Combinational effect of pine needle polysaccharide and kudzu flavonoids on cell differentiation and fat metabolism in 3T3-L1 cells. Food Sci. Technol. Res. 2018, 24, 903–910. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, X.; Zhang, T.; Li, Q.; Zhang, J.; Zhao, J. Astragalus polysaccharide improves insulin sensitivity via AMPK activation in 3T3-L1 Adipocytes. Molecules 2018, 23, 2711. [Google Scholar] [CrossRef] [Green Version]
- Hadrich, F.; Sayadi, S. Apigetrin inhibits adipogenesis in 3T3-L1 cells by downregulating PPARγ and CEBP-α. Lipids Health Dis. 2018, 17, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, E.W.Y.; Ahmed, S.; Peshdary, V.; Atlas, E. Firemaster® 550 and its components isopropylated triphenyl phosphate and triphenyl phosphate enhance adipogenesis and transcriptional activity of peroxisome proliferator activated receptor (Pparγ) on the adipocyte protein 2 (aP2) promoter. PLoS ONE 2017, 12, e0175855. [Google Scholar] [CrossRef] [PubMed]
- Burak, M.F.; Dagtekin, N.; Bejo, P.; Vaillancourt, E.; Liu, L.; Gorgun, C.; Rimm, E.; Tuncman, G.; Hotamisligil, G. OR01-1 Leveraging Immunometabolic Control to Prevent and Treat Obesity Related Asthma. J. Endocr. Soc. 2019, 3, OR01-1. [Google Scholar] [CrossRef]


| Gene Name | Forward Primer | Reverse Primer | Accession No. |
|---|---|---|---|
| PPARγ | TTTTCAAGGGTGCCAGTTTC | AATCCTTGGCCCTCTGAGAT | NM_011146 |
| C/EBPα | TTACAACAGGCCAGGTTTCC | GGCTGGCGACATACAGTACA | NM_007678 |
| SREBP1c | ACAGACAAACTGCCCATCCA | GCAAGAAGCGGATGTAGTCG | NM 011480.3 |
| LPL | CATCGAGAGGATCCGAGTGAA | TGCTGAGTCCTTTCCCTTCTG | NM_008509 |
| ap2 | GGCCAAGCCCAACATGATC | CACGCCCAGTTTGAAGGAAA | NM_024406 |
| β-actin | CCACAGCTGAGAGGGAAATC | AAGGAAGGCTGGAAAAGAGC | X03672 |
| CBP | a-AIE | NSP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ferulic Acid (mg/g) | p-Coumaric Acid (mg/g) | Sinapic Acid (mg/g) | Chlorogenic Acid (μg/g) | Quercetin (μg/g) | TPC (mg GAE/g) | Total Protein (%) | Inhibitory Activity of α-AIE (U mg−1 Dry Weight) | Purity (%) | Molecular Weight (Da) |
| 0.31 ± 0.04 | 0.12 ± 0.03 | 0.11 ± 0.02 | 14.32 ± 1.45 | 6.46 ± 0.26 | 6.57 ± 0.24 | 68.97 ± 2.47 | 1027.1 ± 154.2 | 88.37 ± 2.12 | 6.27 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Zhang, X.; Zhu, Y.; Yao, Y.; Ren, G. Natural Extracts from White Common Bean (Phaseolus vulgaris L.) Inhibit 3T3-L1 Adipocytes Differentiation. Appl. Sci. 2021, 11, 167. https://doi.org/10.3390/app11010167
Shi Z, Zhang X, Zhu Y, Yao Y, Ren G. Natural Extracts from White Common Bean (Phaseolus vulgaris L.) Inhibit 3T3-L1 Adipocytes Differentiation. Applied Sciences. 2021; 11(1):167. https://doi.org/10.3390/app11010167
Chicago/Turabian StyleShi, Zhenxing, Xin Zhang, Yingying Zhu, Yang Yao, and Guixing Ren. 2021. "Natural Extracts from White Common Bean (Phaseolus vulgaris L.) Inhibit 3T3-L1 Adipocytes Differentiation" Applied Sciences 11, no. 1: 167. https://doi.org/10.3390/app11010167







