Ultrasound-Guided Detection and Segmentation of Photoacoustic Signals from Bone Tissue In Vivo
Abstract
:1. Introduction
2. Materials and Methods
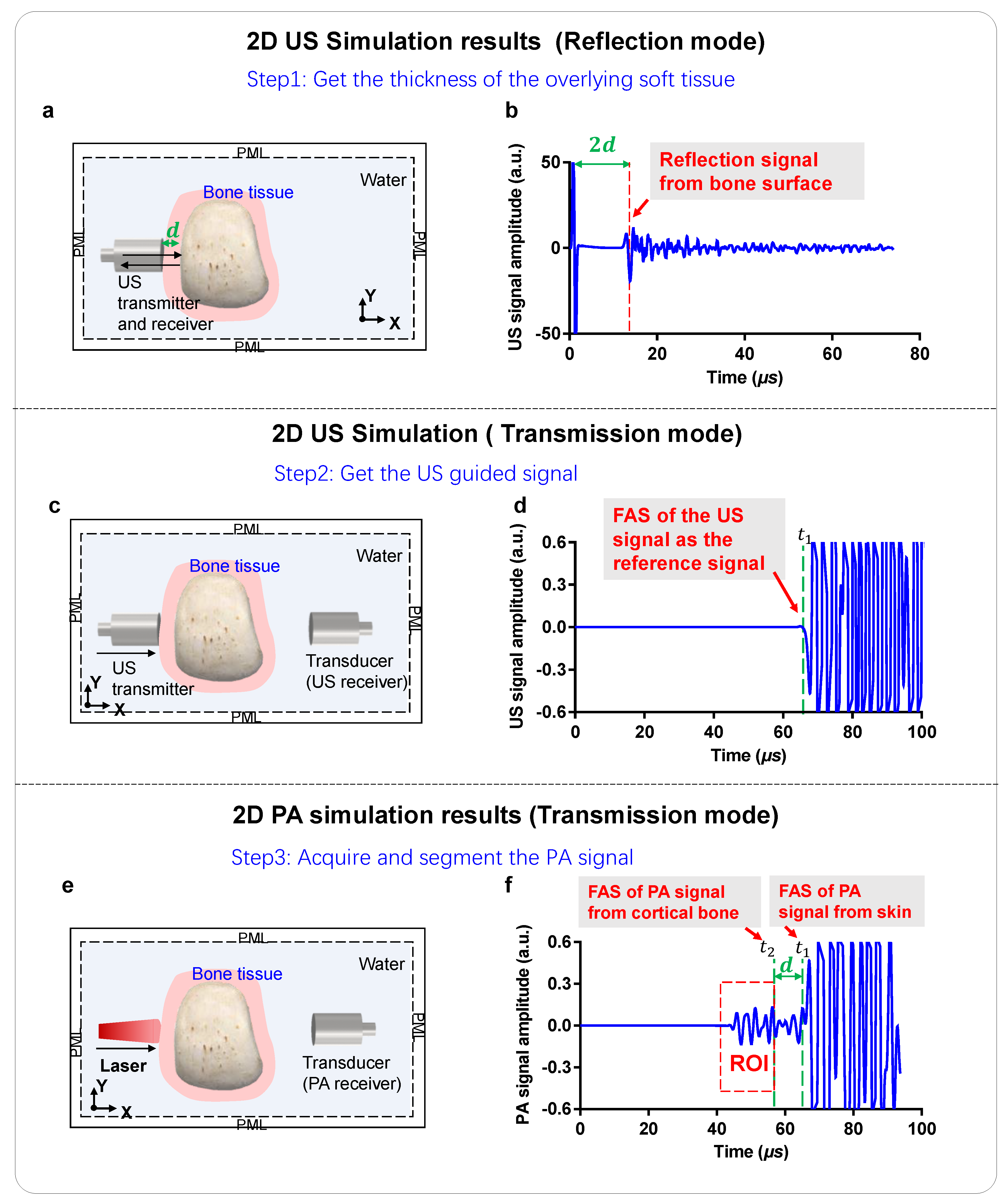
2.1. 2D Numerical US and PA Simulation Models
2.2. 2D Simulations of US Signal Propagation
2.3. 2D Simulations of PA Signal Generation and Propagation
2.4. Experimental Setup
3. Results and Discussion
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.F.; Yeung, D.K.W.; Antonio, G.E.; Lee, F.K.H.; Hong, A.W.L.; Wong, S.Y.S.; Lau, E.M.C.; Leung, P.C. Vertebral Bone Mineral Density, Marrow Perfusion, and Fat Content in Healthy Men and Men with Osteoporosis: Dynamic Contrast-enhanced MR Imaging and MR Spectroscopy. Radiology 2005, 236, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, E.P.; Gamsjaeger, S.; Klaushofer, K. Vibrational Spectroscopic Techniques to Assess Bone Quality. Osteoporos. Int. 2017, 28, 2275–2291. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.F.; Yeung, D.K.W.; Antonio, G.E.; Wong, S.Y.S.; Kwok, T.C.Y.; Woo, J.; Leung, P.C. Vertebral Marrow Fat Content and Diffusion and Perfusion Indexes in Women with Varying Bone Density: MR Evaluation. Radiology 2006, 241, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Patsch, J.M.; Burghardt, A.J.; Kazakia, G.; Majumdar, S. Noninvasive Imaging of Bone Microarchitecture. Ann. N. Y. Acad. Sci. 2011, 77, 1240. [Google Scholar] [CrossRef] [Green Version]
- Kanis, J.A. Pocket Reference to Osteoporosis; Springer: Berlin/Heidelberg, Germany, 2019; pp. 11–20. [Google Scholar]
- Marcocci, C.; Saponaro, F. Multidisciplinary Approach to Osteoporosis: From Assessment to Treatment; Springer: Berlin/Heidelberg, Germany, 2018; pp. 45–57. [Google Scholar]
- Raum, K.; Leguerney, I.; Chandelier, F.; Bossy, E.; Talmant, M.; Saïed, A.; Peyrin, F.; Laugier, P. Bone Microstructure and Elastic Tissue Properties Are Reflected in QUS Axial Transmission Measurements. Ultrasound Med. Biol. 2005, 31, 1225–1235. [Google Scholar] [CrossRef]
- Laugier, P. Bone Quantitative Ultrasound; Springer: Berlin/Heidelberg, Germany, 2011; pp. 47–71. [Google Scholar]
- Wear, K.A. Mechanisms of Interaction of Ultrasound with Cancellous Bone: A Review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2019, 67, 454–482. [Google Scholar] [CrossRef]
- Töyräs, J.; Nieminen, M.; Kröger, H.; Jurvelin, J. Bone Mineral Density, Ultrasound Velocity, and Broadband Attenuation Predict Mechanical Properties of Trabecular Bone Differently. Bone 2002, 31, 503–507. [Google Scholar] [CrossRef]
- Liu, C.; Ta, D.; Fujita, F.; Hachiken, T.; Matsukawa, M.; Mizuno, K.; Wang, W. The Relationship between Ultrasonic Backscatter and Trabecular Anisotropic Microstructure in Cancellous Bone. J. Appl. Phys. 2014, 115, 64906. [Google Scholar] [CrossRef]
- Njeh, C.F.; Boivin, C.M.; Langton, C.M. The Role of Ultrasound in the Assessment of Osteoporosis: A Review. Osteoporos. Int. 1997, 7, 7–22. [Google Scholar] [CrossRef]
- Pifferi, A.; Torricelli, A.; Taroni, P.; Bassi, A.; Chikoidze, E.; Giambattistelli, E.; Cubeddu, R. Optical Biopsy of Bone Tissue: A Step toward the Diagnosis of Bone Pathologies. J. Biomed. Opt. 2004, 9, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Schulmerich, M.V.; Dooley, K.A.; Morris, M.D.; Vanasse, T.M.; Goldstein, S.A. Transcutaneous Fiber Optic Raman Spectroscopy of Bone Using Annular Illumination and a Circular Array of Collection Fibers. J. Biomed. Opt. 2006, 11, 060502. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.D.; Mandair, G.S. Raman Assessment of Bone Quality. Clin. Orthop. Relat. Res. 2010, 469, 2160–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draper, E.R.C.; Morris, M.D.; Camacho, N.P.; Matousek, P.; Towrie, M.; Parker, A.W.; Goodship, A.E. Novel Assessment of Bone Using Time-Resolved Transcutaneous Raman Spectroscopy. J. Bone Miner. Res. 2005, 20, 1968–1972. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pang, Y.; Ku, G.; Xie, X.; Stoica, G.; Wang, L.V. Noninvasive Laser-Induced Photoacoustic Tomography for Structural and Functional in Vivo Imaging of the Brain. Nat. Biotechnol. 2003, 21, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Feng, T.; Cheng, Q.; Wang, X.; Du, S.; Sato, N.; Cheng, Q.; Singh, M.K.A. Towards Clinical Translation of LED-Based Photoacoustic Imaging: A Review. Sensors 2020, 20, 2484. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Photoacoustic Imaging in Biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Oraiqat, I.; Lei, H.; Carson, P.L.; El Naqa, I.; Wang, X. Dual-Modality X-Ray-Induced Radiation Acoustic and Ultrasound Imaging for Real-Time Monitoring of Radiotherapy. BME Front. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Tian, C.; Pei, M.; Shen, K.; Liu, S.; Hu, Z.; Feng, T. Impact of System Factors on the Performance of Photoacoustic Tomography Scanners. Phys. Rev. Appl. 2020, 13, 014001. [Google Scholar] [CrossRef] [Green Version]
- Cox, B.T.; Laufer, J.G.; Arridge, S.R.; Beard, P.C. Quantitative Spectroscopic Photoacoustic Imaging: A Review. J. Biomed. Opt. 2012, 17, 0612021–06120222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lashkari, B.; Yang, L.; Mandelis, A. The Application of Backscattered Ultrasound and Photoacoustic Signals for Assessment of Bone Collagen and Mineral Contents. Quant. Imaging Med. Surg. 2015, 5, 46–56. [Google Scholar] [PubMed]
- Yang, L.; Lashkari, B.; Mandelis, A.; Tan, J.W.Y. Bone Composition Diagnostics: Photoacoustics Versus Ultrasound. Int. J. Thermophys. 2015, 36, 862–867. [Google Scholar] [CrossRef]
- Feng, T.; Perosky, J.E.; Kozloff, K.M.; Xu, G.; Cheng, Q.; Du, S.; Cheng, Q.; Deng, C.X.; Wang, X. Characterization of Bone Microstructure Using Photoacoustic Spectrum Analysis. Opt. Express 2015, 23, 25217–25224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, T.; Kozloff, K.M.; Tian, C.; Perosky, J.E.; Hsiao, Y.-S.; Du, S.; Cheng, Q.; Deng, C.X.; Wang, X. Bone Assessment via Thermal Photo-Acoustic Measurements. Opt. Lett. 2015, 40, 1721–1724. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Zhang, W.; Mordovanakis, A.; Wang, X.; Paulus, Y.M. Noninvasive Chorioretinal Imaging in Living Rabbits Using Integrated Photoacoustic Microscopy and Optical Coherence Tomography. Opt. Express 2017, 25, 15947–15955. [Google Scholar] [CrossRef]
- Steinberg, I.; Shiloh, L.; Gannot, G.; Eyal, A. First-in-Human Study of Bone Pathologies Using Low-Cost and Compact Dual-Wavelength Photoacoustic System. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–8. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, Z.; Feng, T.; Cheng, Q.; Wang, X.; Ding, Y.; Zhan, H.; Yuan, J. Bone Mineral Density Value Evaluation Based on Photoacoustic Spectral Analysis Combined with Deep Learning Method. Chin. Opt. Lett. 2020, 18, 041701. [Google Scholar] [CrossRef]
- Feng, T.; Zhu, Y.; Morris, R.; Kozloff, K.M.; Wang, X. Functional Photoacoustic and Ultrasonic Assessment of Osteoporosis: A Clinical Feasibility Study. BME Front. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Liu, C.; Ta, D.; Hu, B.; Le, L.H.; Wang, W. The Analysis and Compensation of Cortical Thickness Effect on Ultrasonic Backscatter Signals in Cancellous Bone. J. Appl. Phys. 2014, 116, 124903. [Google Scholar] [CrossRef]
- Liu, C.; Han, H.; Ta, D.; Wang, W. Effect of Selected Signals of Interest on Ultrasonic Backscattering Measurement in Cancellous Bones. Sci. China Ser. G Phys. Mech. Astron. 2013, 56, 1310–1316. [Google Scholar] [CrossRef]
- Haltmeier, M.; Scherzer, O.; Burgholzer, P.; Paltauf, G. Thermoacoustic Computed Tomography with Large Planar Receivers. Inverse Probl. 2004, 20, 1663–1673. [Google Scholar] [CrossRef]
- Kim, M.; Jeng, G.-S.; O’Donnell, M.; Pelivanov, I. Correction of Wavelength-Dependent Laser Fluence in Swept-Beam Spectroscopic Photoacoustic Imaging with a Hand-Held Probe. Photoacoustics 2020, 19, 100192. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, L.V. Photoacoustic Tomography and Sensing in Biomedicine. Phys. Med. Biol. 2009, 54, R59–R97. [Google Scholar] [CrossRef] [PubMed]
- Jermyn, M.; Ghadyani, H.; Mastanduno, M.A.; Turner, W.; Davis, S.C.; Dehghani, H.; Pogue, B.W. Fast Segmentation and High-Quality Three-Dimensional Volume Mesh Creation from Medical Images for Diffuse Optical Tomography. J. Biomed. Opt. 2013, 18, 086007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghani, H.; Eames, M.E.; Yalavarthy, P.K.; Davis, S.C.; Srinivasan, S.; Carpenter, C.M.; Pogue, B.W.; Paulsen, K.D. Near Infrared Optical Tomography Using NIRFAST: Algorithm for Numerical Model and Image Reconstruction. Commun. Numer. Methods Eng. 2008, 25, 711–732. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical Properties of Biological tissues: A review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef]





| Bone Mineral | Bone Marrow | |
|---|---|---|
| Density (kg/m3) | 1960 | 1000 |
| First Lamé coefficient (GPa) | 14.8 | 2 |
| Second Lamé coefficient (GPa) | 8.3 | 0 |
| Longitudinal wave SOS (m/s) | 4002.6 | 1500 |
| Shear wave SOS (m/s) | 2057.8 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, T.; Zhu, Y.; Liu, C.; Du, S.; Ta, D.; Cheng, Q.; Yuan, J. Ultrasound-Guided Detection and Segmentation of Photoacoustic Signals from Bone Tissue In Vivo. Appl. Sci. 2021, 11, 19. https://doi.org/10.3390/app11010019
Feng T, Zhu Y, Liu C, Du S, Ta D, Cheng Q, Yuan J. Ultrasound-Guided Detection and Segmentation of Photoacoustic Signals from Bone Tissue In Vivo. Applied Sciences. 2021; 11(1):19. https://doi.org/10.3390/app11010019
Chicago/Turabian StyleFeng, Ting, Yunhao Zhu, Chengcheng Liu, Sidan Du, Dean Ta, Qian Cheng, and Jie Yuan. 2021. "Ultrasound-Guided Detection and Segmentation of Photoacoustic Signals from Bone Tissue In Vivo" Applied Sciences 11, no. 1: 19. https://doi.org/10.3390/app11010019







