Brilliant Red HE-3B Dye Biosorption by Immobilized Residual Consortium Bacillus sp. Biomass: Fixed-Bed Column Studies
Abstract
:1. Introduction
- (1)
- passive—based on the property of the microorganism film formation. The supporting materials are in contact with the cellular suspension of a tested micro-organism for a period of time (before sterilization and inoculation with the starting microorganism suspension), after which a microorganism film is formed on the supporting surface. This technique offers a number of advantages over other immobilization methods, including the ease of immobilization (natural catching/fixing), no need for the addition of chemicals, high rate of mass transfer in particles, and ease of the immobilization technique extension. However, this technique can only be applied to microorganisms with a natural tendency to attach or aggregate on a solid support.
- (2)
- active—based on gel capture/entrapment and chemical cross-linking (covalent binding to vector compounds and reticulation). Gel capture/entrapment is the most widely used technique for immobilizing microorganisms within a polymeric matrix, made using natural polysaccharides (chitosan, agar, carrageenan or alginates), proteins (gelatin, collagen), or synthetic polymers (acrylamide, photo-reticulated resins, polyurethanes). However, chemical entrapment or cross-linking has greater disadvantages when micro-organisms are intended for immobilization, because chemical interaction (covalent binding or reticulation-involving glutaraldehyde or photocrossable resins) causes damage to the cell surface, drastically reducing cell viability.
2. Experimental Section
2.1. Materials
2.2. Methods Used for Quantitative Determinations
2.3. Dynamic Biosorption Procedure
2.3.1. Dynamic Biosorption Working Methodology
- (1)
- The feeding temperature and pH were stabilized at 25 °C and 3.0 based on the best results found in the batch adsorption study (static regime) [27];
- (2)
- Three different flow rates (Fvi = 2.9, 4.5 and 7.3 mL/min) of dye-containing solutions with the same concentration were passed through the fixed/packed biosorbent bed in the column, corresponding to the mass of biosorbent (m) equal to 3.800, 4.074 and 6.770 g, respectively.
- (3)
- All experiments were stopped when saturation was achieved and after further control (at least three samplings after the saturation point had equal or higher values than the initial dye concentration in the collected treated samples).
2.3.2. Biosorption Design in the Fixed/Packed Bed Column
2.4. Modeling of Experimental Biosorption Data in Dynamic Regime
3. Results and Discussion
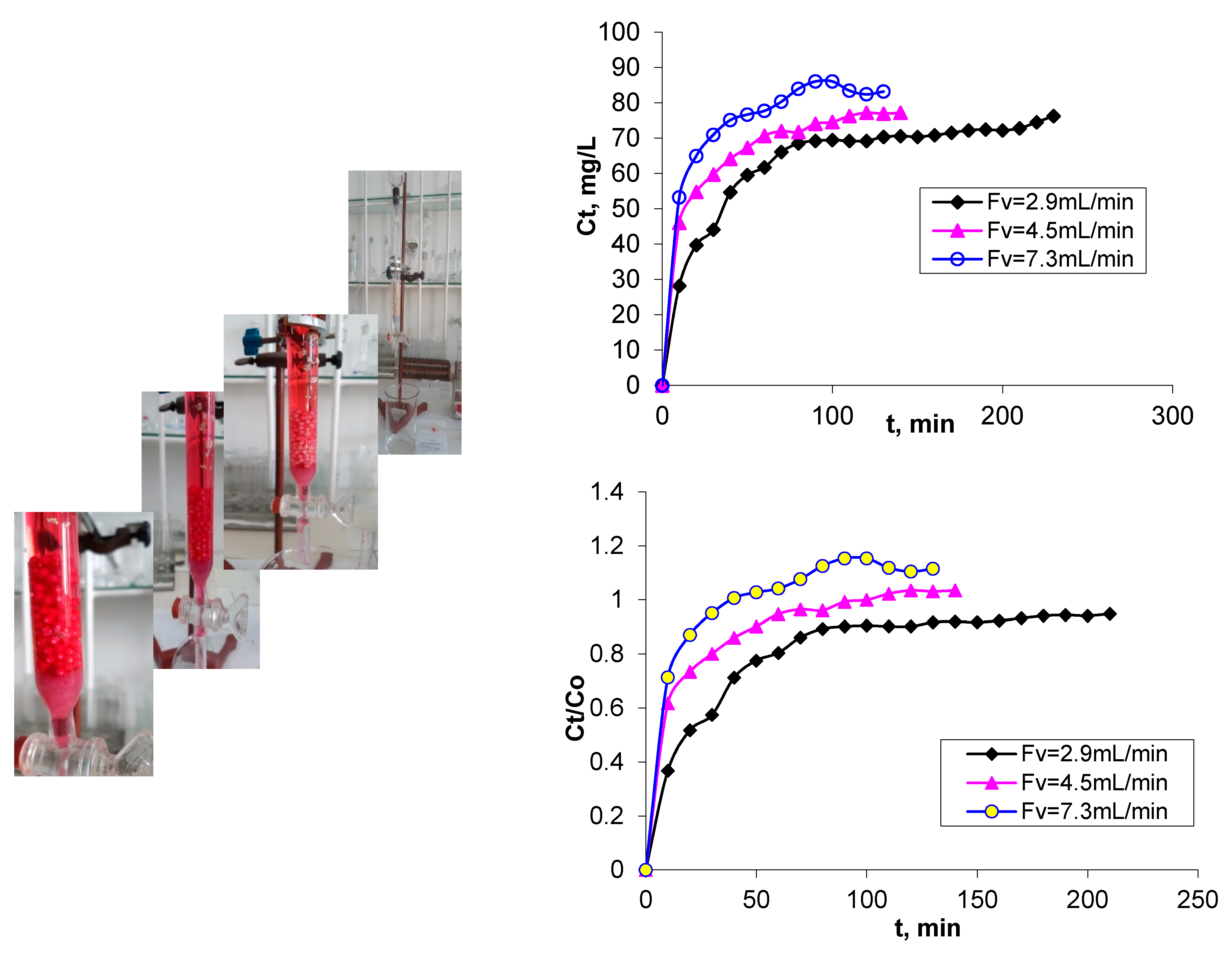
3.1. Breakthrough Curves
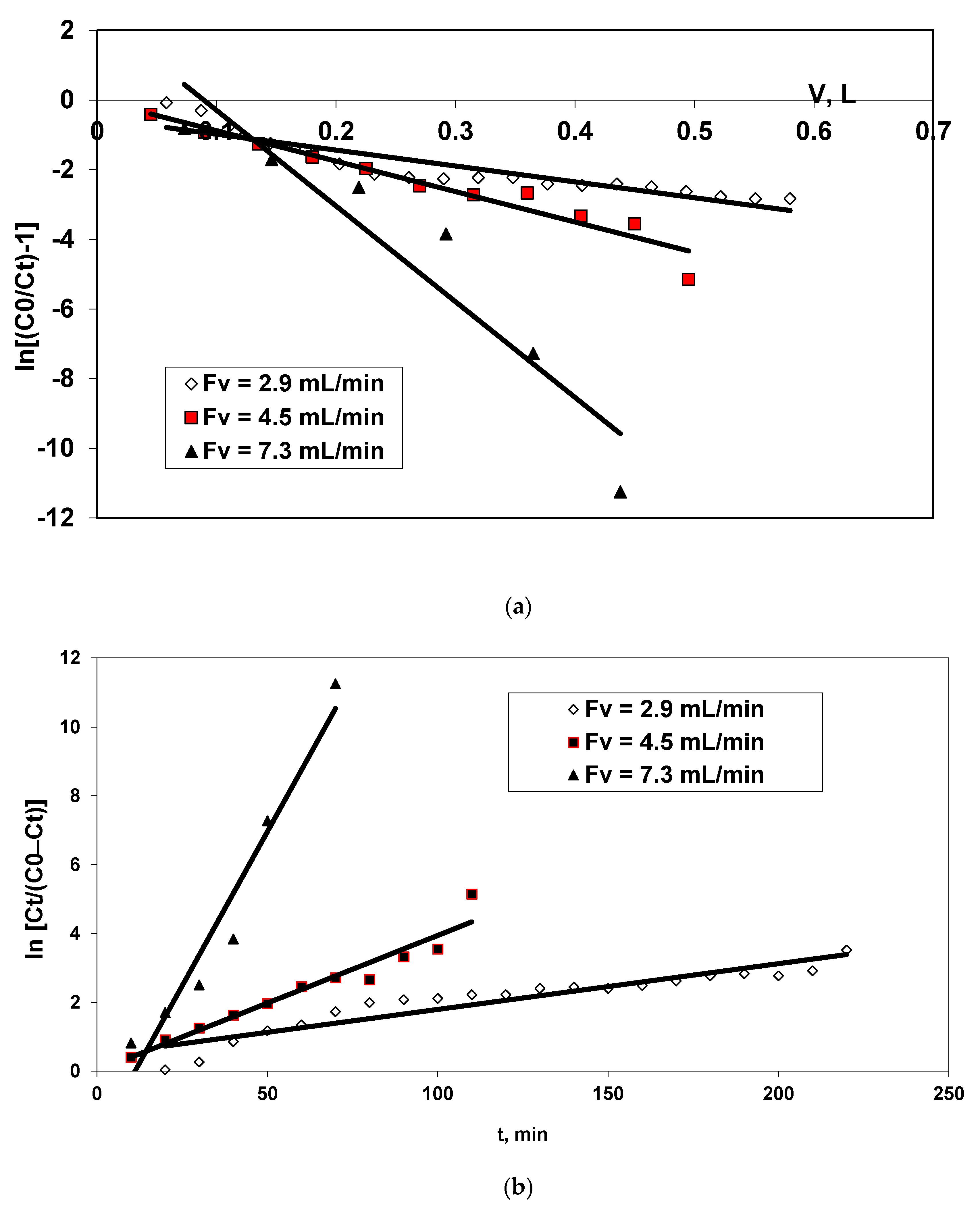
3.2. Experimental Biosorption Data Modeling in a Dynamic Regime
- Because the plot ln[(C0/Ct) − 1] versus V gives a straight line (Figure 3a) and the R2 is lower than 0.938 (equal for Fv = 4.5 mL/min), the viable suggestion is that the Thomas kinetic model is relatively well fitted for modeling of kinetic data, especially for Fv ≥ 4.5 mL/min and the maximum biosorption capacity is of 38.05 mg/g.
- The linearity of plots ln[Ct/(C0 − Ct)] versus t for all three tested flow rates and the high values of the correlation coefficient, R2 (e.g., 0.947 for Fv = 7.3 mL/min), suggest that the biosorption kinetic of BRed dye onto residual biomass follows the Yoon-Nelson kinetic model, and the maximum biosorption capacity is of around 34.742 mg/g.
- Both kinetic models (Thomson and Yoon-Nelson) can be used to describe to biosorption kinetics of BRed anionic dye onto immobilised residual biomass when is working with flow rates higher than 4.5 mL/min.
3.3. Biosorption Design in the Fixed/Packed Bed (Column) Reactor
| Biosorption (column) reactor (dynamic regime) characterized by Vads = 17.251 m3; Mads = 9945.7 kg | DR = 1.671 m | Hads = 2.507 m HR = 3.76 m Efficiency: low–satisfactory, qmax = (34.742–38.05) mg/g |
| Radial biosorption tank/reactor (static regime) characterized by VR = min 52.783 m3; variable biosorbent amounts can be used | DR = 4.10 m | HR = 4 m (with aeration by air dispersion); Huseful = 3.6 m Efficiency: moderate–high, qmax = 588.235 mg/g [27] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franjic, S. Importance of Environment Protection on the Global Level. Sci. J. Res. Rev. 2018, 1, 000506. [Google Scholar] [CrossRef]
- Dogaru, L. The Importance of Environmental Protection and Sustainable Development. Procedia Soc. Behav. Sci. 2013, 93, 1344–1348. [Google Scholar] [CrossRef] [Green Version]
- Kueffer, C.; Kinney, K. What is the importance of islands to environmental conservation? Environ. Conserv. 2017, 44, 311–322. [Google Scholar] [CrossRef]
- Nikiema, J.; Nkhalambayausi-Chirwa, E.M.; Andrès, Y. Quality and Pollution Control Technologies for Water, Air, and Soil. Int. J. Chem. Eng. 2010, 2010, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N. Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manag. 2017, 197, 732–749. [Google Scholar] [CrossRef]
- Munirasu, S.; Abu Haija, M.; Banat, F. Use of membrane technology for oil field and refinery produced water treatment—A review. Process. Saf. Environ. Prot. 2016, 100, 183–202. [Google Scholar] [CrossRef]
- Bhandari, V.M.; Ranade, V.V. (Eds.) Advanced physico-chemical methods of treatment for inbdustrial wastewaters. In Industrial Wastewater Treatment, Recycling and Reuse; Elsevier Ltd.: Oxford, UK, 2014; pp. 81–140. [Google Scholar]
- Rene, E.R.; Shu, L.; Jegatheesan, V. (Eds.) Environmentally Friendly (Bio) Technologies for Removal of Emerging Organic and Inorganic Pollutants from Waste; IWA Publishing: London, UK, 2019. [Google Scholar]
- Rene, E.R.; Shu, L.; Jegatheesan, V. Sustainable eco-technologies for water and wastewater treatment. J. Water Supply: Res. Technol. 2019, 68, 617–622. [Google Scholar] [CrossRef]
- Zhao, C.; Dong, Y.; Feng, Y.; Li, Y.; Dong, Y. Thermal desorption for remediation of contaminated soil: A review. Chemosphere 2019, 221, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.; Im, J.; Cho, G.-C. Introduction of Microbial Biopolymers in Soil Treatment for Future Environmentally-Friendly and Sustainable Geotechnical Engineering. Sustainability 2016, 8, 251. [Google Scholar] [CrossRef] [Green Version]
- Luengas, A.; Barona, A.; Hort, C.; Gallastegui, G.; Platel, V.; Elias, A. A review of indoor air treatment technologies. Rev. Environ. Sci. Bio/Technology 2015, 14, 499–522. [Google Scholar] [CrossRef]
- Ezeonu, C.S.; Tagbo, R.; Anike, E.N.; Oje, O.A.; Onwurah, I.N.E. Biotechnological Tools for Environmental Sustainability: Prospects and Challenges for Environments in Nigeria—A Standard Review. Biotechnol. Res. Int. 2012, 2012, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K. Biosorption and bioaccumulation—The prospects for practical applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Suteu, D.; Zaharia, C.; Blaga, A.C. Biosorption—Current bioprocess for wastewater treatment. In Current Topics, Concepts and Research Priorities in Environmental Chemistry; Zaharia, C., Ed.; Univ. A.I.Cuza Publishing House: Iasi, Romania, 2012; Volume I, pp. 221–244. [Google Scholar]
- Gavrilescu, M.; Chisti, Y. Biotechnology—A sustainable alternative for chemical industry. Biotechnol. Adv. 2005, 23, 471–499. [Google Scholar] [CrossRef]
- Agarwal, A.; Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M. A review on valorization of biomass in heavy metal removal from wastewater. J. Water Process. Eng. 2020, 38, 101602. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Suteu, D.; Zaharia, C.; Blaga, A.C. The action of microorganisms from organic pollutants in water, air, soil. In Current Topics, Concepts and Research Priorities in Environmental Chemistry; Zaharia, C., Ed.; Univ. A.I.Cuza Publishing House: Iasi, Romania, 2013; Volume II, pp. 259–274. [Google Scholar]
- Blaga, A.C.; Horciu, L.; Zaharia, C.; Rusu, L.; Grigoras, C.; Suteu, D. Biosorbents based on microorganisms. Bull. Polytech. Inst. Iasi 2020, in press. [Google Scholar]
- Todorovaa, K.; Velkovab, Z.; Stoytchevac, M.; Kirovab, G.; Kostadinovad, S.; Gochevd, V. Novel composite biosorbent from Bacillus cereus for heavy metals removal from aqueous solutions. Biotechnol. Biotechnol. Equip. 2019, 33, 730–738. [Google Scholar] [CrossRef] [Green Version]
- Mustapha, M.U.; Halimoon, N. Microorganisms and Biosorption of Heavy Metals in the Environment: A Review Paper. J. Microb. Biochem. Technol. 2015, 7, 253–256. [Google Scholar] [CrossRef]
- Moreno-Garrido, I. Microalgae immobilization: Current techniques and uses. Bioresour. Technol. 2008, 99, 3949–3964. [Google Scholar] [CrossRef] [PubMed]
- Galaction, A.I.; Lupasteanu, A.M.; Turnea, M.; Cascaval, D. Comparative analysis of mixing efficiency and distribution in-duced by radial impellers an bioreactors with stirred bed of immobilized cells. Chem. Ind. Chem. Eng. Quaterly 2010, 16, 47–64. [Google Scholar] [CrossRef]
- Cascaval, D.; Galaction, A.-I.; Rotaru, R.; Turnea, M. Study on the mixing efficiency in a basket bioreactor with immobilized yeasts cells. Environ. Eng. Manag. J. 2011, 10, 711–716. [Google Scholar] [CrossRef]
- Galaction, A.I.; Rotaru, R.; Caşcaval, D. Glucose Mass Transfer Under Substrate Inhibition Conditions in a Stationary Basket Bioreactor with Immobilized Yeast Cells. Int. J. Chem. React. Eng. 2012, 10, 1–13. [Google Scholar] [CrossRef]
- Horciu, I.L.; Blaga, A.C.; Rusu, L.; Zaharia, C.; Suteu, D. Biosorption of reactive dyes from aqueos media using the Bacillus sp. residual biomass. Des.Water Treat. 2020, 195, 353–360. [Google Scholar]
- Tofan, L.; Paduraru, C.; Teodosiu, C.; Toma, O. Fixed bed column study on the removal of chromium (III) ions from aqueous solutions by using hemp fibers with improved sorption performance. Cellul. Chem. Technol. 2015, 49, 219–229. [Google Scholar]
- Mazouz, R.; Filali, N.; Hattab, Z.; Guerfi, K. Valorization of granulated slag of Arcelor-Mittal (Algeria) in cationic dye ad-sorption from aqueous solution: Column studies. J. Water Reuse Desalination 2016, 6, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Suteu, D.; Nica, I.; Biliuta, G.; Zaharia, C.; Rusu, L.; Coseri, S. Fixed-bed-column studies for methylene blue removal by cellulose cellets. Environ. Eng. Manag. J. 2020, 19, 269–279. [Google Scholar] [CrossRef]
- Musteret, C.; Fighir, D.; Gavrilescu, D.; Zaharia, C.; Teodosiu, C. Tratarea si Epurarea Apelor: Aplicatíi Practice (In English: Treatment of Waters and Wastewaters: Practical Applications); Politehnium: Iasi, Romania, 2014. [Google Scholar]
- Chu, K.H. Breakthrough curve analysis by simplistic models of fixed bed adsorption: In defense of the century-old Bohart-Adams model. Chem. Eng. J. 2020, 380, 122513. [Google Scholar] [CrossRef]
- Zaharia, C.; Suteu, D. Industrial wasted materials as low cost sorbents for effluent treatment. In Current Topics, Concepts and Research Priorities in Environmental Chemistry; Zaharia, C., Ed.; Univ. A.I.Cuza Publishing House: Iasi, Romania, 2013; Volume II, pp. 189–212. [Google Scholar]
- Smaranda, C.; Popescu, M.-C.; Bulgariu, D.; Măluţan, T.; Gavrilescu, M. Adsorption of organic pollutants onto a Romanian soil: Column dynamics and transport. Process. Saf. Environ. Prot. 2017, 108, 108–120. [Google Scholar] [CrossRef]
- Tomczak, E.; Tosik, P. Waste Plant Material as a Potential Adsorbent of a Selected Azo Dye. Chem. Process Eng. 2017, 38, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Srivastava, S.K.; Tyagi, R. Design parameters for the treatment of phenolic wastes by carbon columns (obtained from fertilizer waste material). Water Res. 2000, 34, 1543–1550. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Zain, S.M.; Rashid, A.K.; Rafique, R.F.; Khalid, K. Breakthrough Curve Analysis for Column Dynamics Sorption of Mn(II) Ions from Wastewater by UsingMangostana garciniaPeel-Based Granular-Activated Carbon. J. Chem. 2012, 2013, 1–8. [Google Scholar] [CrossRef] [Green Version]
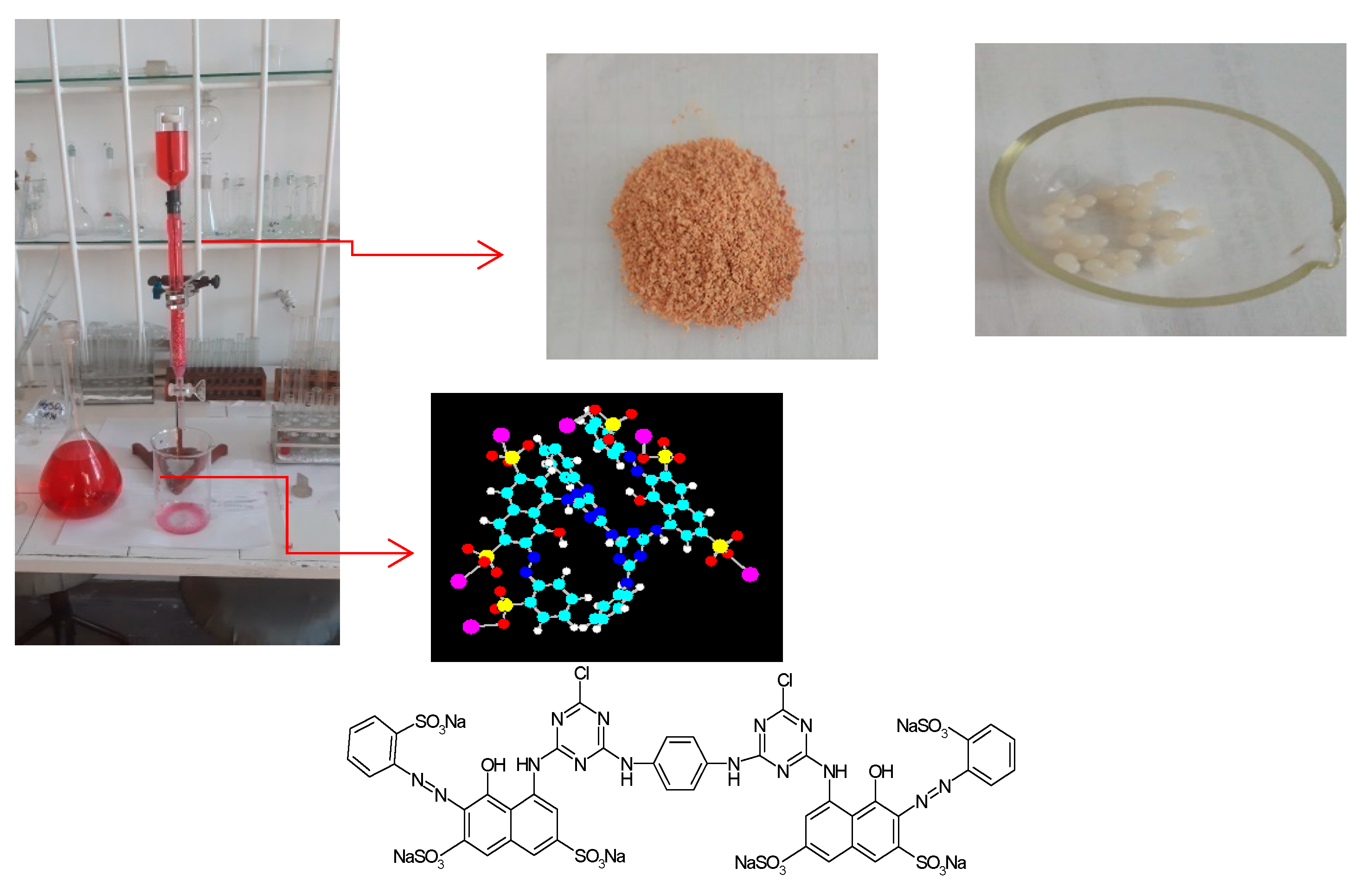
| Biosorbent Mass, [kg] | Biosorbent Volume, [m3] | Internal Diameter of Biosorption (Column) Reactor, [m] | Height of Packed Bed, [m] | Height of Biosorption (Column) Reactor, [m] |
|---|---|---|---|---|
β = 1–3 | (λ = 1–3) |
| Parameter | Significance and Characteristics | Experimental Values For Each Tested Flowrate (Fv), (mL/min) | ||
|---|---|---|---|---|
| 2.9 | 4.5 | 7.3 | ||
| Biosorbent packed bed height—h (cm) | Height in column of each added immobilized biosorbent amount bed | 4.0 | 7.0 | 3.8 |
| Breakthrough time—tb (min) | Time required for attaining the breakthrough point, when the dye concentration has the value of 0.1 C0 (Cb) | 7.0 | 4.0 | 1.5 |
| Saturation time—ts (min) | Time required for attaining the saturation point, where dye concentration has a value of 0.9 C0 (Cs) | 55.0 | 32.5 | 16.5 |
| Length of mass transfer zone—L(MTZ) (cm) | where h—the height of biosorbent bed (cm) | 3.49 | 6.138 | 3.45 |
| Breakthrough volume—Vb (mL) | Volume of treated effluent at breakthrough point, calculated as Vb = Fv × tb, where Fv is the flow rate (mL/min) | 20.3 | 18.0 | 10.95 |
| Saturation volume—Vs (mL) | Volume of treated effluent at saturation point, calculated as VS = Fv . tS, where Fv is the flow rate (mL/min) | 159.5 | 146.25 | 120.45 |
| Breakthrough capacity—qb (mg/g) | Amount of BRed dye retained per immobilized biosorbent mass at breakthrough point. where m—adsorbent mass, g. | 7.293 × 10−3 | 3.89 × 10−3 | 4.2 × 10−3 |
| Saturation capacity—qS (mg/g) | Amount of BRed dye retained per immobilized biosorbent mass at saturation point where m—adsorbent mass, g. | 57.307 | 34.81 | 46.17 |
| Rate of exhaustion—RAE (g/L) | Amount of exhausted immobilized biosorbent(g) per volume of treated effluent at the breakthrough point (L) | 0.2114 | 0.556 | 0.208 |
| Model | Fv, Initial Flow Rate (mL/min) | kT (L/min mg) | q0(T) (mg/g) | R2 | kYN (min−1) | t1/2 (min) | q0(YN) (mg/g) | R2 | q0(Langmuir) * (mg/g) |
|---|---|---|---|---|---|---|---|---|---|
| Thomas | 2.9 4.5 * 7.3 | 1.722 × 10−4 5.126 × 10−4 2.614 × 10−3 | 38.05 | 0.824 0.938 0.891 | 588.235 * [27] | ||||
| Yoon–Nelson | 2.9 4.5 7.3 * | 0.0133 0.0393 0.179 | 11.166 | 34.742 | 0.875 0.938 0.947 |
| Parameter | Significance and Characteristics | Experimental Values for Each Tested Flow Rate (Fv), (mL/min) | ||
|---|---|---|---|---|
| 2.9 | 4.5 | 7.3 | ||
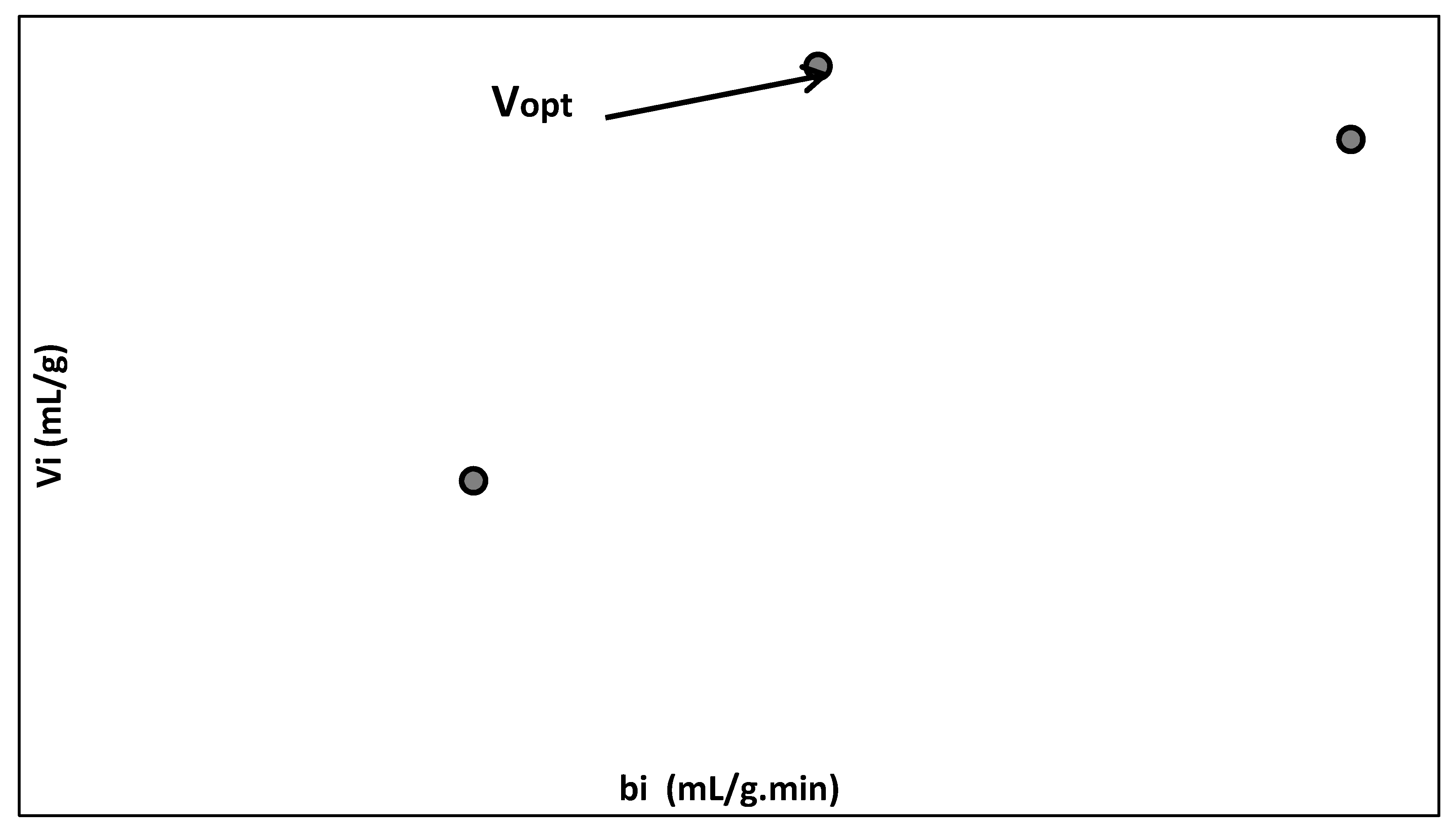
| Mean flow rate per immobilized biosorbent mass, bi (mL/g.min) | where Vn is the treated effluent volume passing through the packed bed of immobilized biosorbent (mL); ni is the number of analyzed samples; v is the volume of analyzed sample (v = 5 mL); m is the biosorbent mass (g) and tni is the total biosorption time (min). | 1.8757 | 0.6396 | 1.1248 |
| Mean volume of treated effluent passing through the packed bed of biosorbent (mL) per immobilized biosorbent mass (g), Vi (mL/g) | The biosorption time until maximum admissible concentration of residual dye in the treated effluent (tri, (min)): 10, 7.86 and 5.652 min. | 10.149 | 5.027 | 11.248 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horciu, L.I.; Zaharia, C.; Blaga, A.C.; Rusu, L.; Suteu, D. Brilliant Red HE-3B Dye Biosorption by Immobilized Residual Consortium Bacillus sp. Biomass: Fixed-Bed Column Studies. Appl. Sci. 2021, 11, 4498. https://doi.org/10.3390/app11104498
Horciu LI, Zaharia C, Blaga AC, Rusu L, Suteu D. Brilliant Red HE-3B Dye Biosorption by Immobilized Residual Consortium Bacillus sp. Biomass: Fixed-Bed Column Studies. Applied Sciences. 2021; 11(10):4498. https://doi.org/10.3390/app11104498
Chicago/Turabian StyleHorciu, Luiza Ioana, Carmen Zaharia, Alexandra Cristina Blaga, Lacramioara Rusu, and Daniela Suteu. 2021. "Brilliant Red HE-3B Dye Biosorption by Immobilized Residual Consortium Bacillus sp. Biomass: Fixed-Bed Column Studies" Applied Sciences 11, no. 10: 4498. https://doi.org/10.3390/app11104498
APA StyleHorciu, L. I., Zaharia, C., Blaga, A. C., Rusu, L., & Suteu, D. (2021). Brilliant Red HE-3B Dye Biosorption by Immobilized Residual Consortium Bacillus sp. Biomass: Fixed-Bed Column Studies. Applied Sciences, 11(10), 4498. https://doi.org/10.3390/app11104498









