Abstract
Banana puree, due to its nutritional composition, is a good substrate for fermentation in the development of probiotic products. The production of banana puree mainly consists of three phases, i.e., raw material pretreatment, heat treatment, and the addition of anti-browning agents. In this study, we conducted three experimental protocols to evaluate the effect of ripeness grade, heat treatment, and ascorbic acid addition on fermentation performance. At the end of each protocol, the substrate was subjected to the fermentation process (37 °C, 48 h), and then measurements of pH reduction, microbial growth, and lactic acid production were used as markers in the analysis of fermentation performance. Ripe bananas produced better results than unripe bananas whose fermentation appeared to be inhibited. Therefore, ripe bananas were used to test the effect of two different heat treatments (sterilization (121 °C, 20 min) versus tyndallization (70 °C, 30 min; 37 °C, 30 min; 70 °C, 30 min)) on banana puree fermentation, and no significant differences were observed. Finally, 500 or 1000 ppm of ascorbic acid, normally used as an anti-browning agent, was added to ripe tyndallized bananas. No differences in fermentation results were observed between the two tested conditions, though values obtained for growth and lactic acid production were significantly lower than those from fermentation of banana puree without ascorbic acid.
1. Introduction
Banana is one of the most important fruit crops in the world. The Cavendish cultivar is the most commercialized banana cultivar, representing half of the world’s total banana production [1]. Starting from banana as a raw material, industries produce several processed products, such as banana puree, banana pulp, and banana flour or powder. Banana puree, in particular, is one of the main processed banana products, manufactured for use by baby food, dairy, and bakery industries and widely used all over the world [2,3]. Banana is a rich source of antioxidant vitamins (A, C, and E), calcium, magnesium, potassium, carbohydrates, fiber, and water, and its composition is highly influenced by the species, growing area, and degree of ripeness. In fact, as bananas ripen, the starch content decreases while sugar concentrations increase due to the progressive hydrolysis of starch into soluble sugars [4]. Bananas are considered to be a good fermentation substrate because of their high level of soluble sugars and high presence of prebiotic components, such as fructooligosaccharides (FOS). In fact, sucrose, glucose, and fructose can be metabolized into lactic acid (which can act as a preservative, flavoring agent, and acidulant in the food industry) through lactic bacteria, as has been demonstrated in previous studies [5,6,7]. FOS, by their nature, have beneficial health effects on the human colon through stimulation of colonic lactic acid bacteria and by inhibiting putrefactive pathogens [8,9,10]. Thus, it is possible to obtain a synbiotic product via fermentation of a substrate, such as banana, through a probiotic strain. Industries are increasingly interested in the production of similar functional foods, responding to increased consumer interest in food and health. In addition, increasing attention is paid to non-dairy probiotic foods, such as cereals or legumes [11]. The development and production of new types of fermented product requires a thorough knowledge of both the raw material and the normal production process, in each single phase, to understand the possible influences of the process on fermentation. In particular, banana puree production involves the heat treatment of the raw material, followed by the draining; the next step is the removal of the peel and then the size reduction, as shown by Yap et al. [4]. The heat treatment can affect the raw material, reducing banana browning [12], but also modifying their nutritional quality [13] and, therefore, possibly influences the fermentation process. Browning is a problem to consider when fresh fruits are processed and, for this reason, banana puree production requires a further phase, i.e., the addition of an anti-browning agent. In some industries, for example, the baby food industry, the addition of food additives is not allowed and, therefore, only natural substances can be used, such as ascorbic acid [14,15]. However, the impact of adding ascorbic acid on fermentation parameters, such as probiotic growth or lactic acid production, must be precisely characterized. For this purpose, in this study, banana puree production was simulated at the lab-scale to verify if ripeness, heat treatment, and ascorbic acid addition have any influence on fermentation, with one variable being sequentially studied at a time. Firstly, we studied the impact of banana ripeness on bacterial growth, lactic acid production, and pH, testing second-grade (light green, breaking toward yellow) and sixth-grade (completely yellow) bananas as fermenting substrates. We identified the better raw material and used it in continued testing whereby two different heat treatments, sterilization and tyndallization, were evaluated. Finally, the better ripeness grade and heat treatment conditions were used to assess the potential effect of ascorbic acid addition on the same fermentation parameters. The purpose of this study was to understand if the conditions experienced (ripeness stage, heat treatment, and ascorbic acid), and therefore the industrial process, have any impact on the subsequent fermentation, carried out to obtain a semi-finished product that can be added as a functional ingredient to another food product, as fruit juice or puree.
2. Material and Methods
2.1. Banana Puree Pretreatment Protocols at Lab-Scale
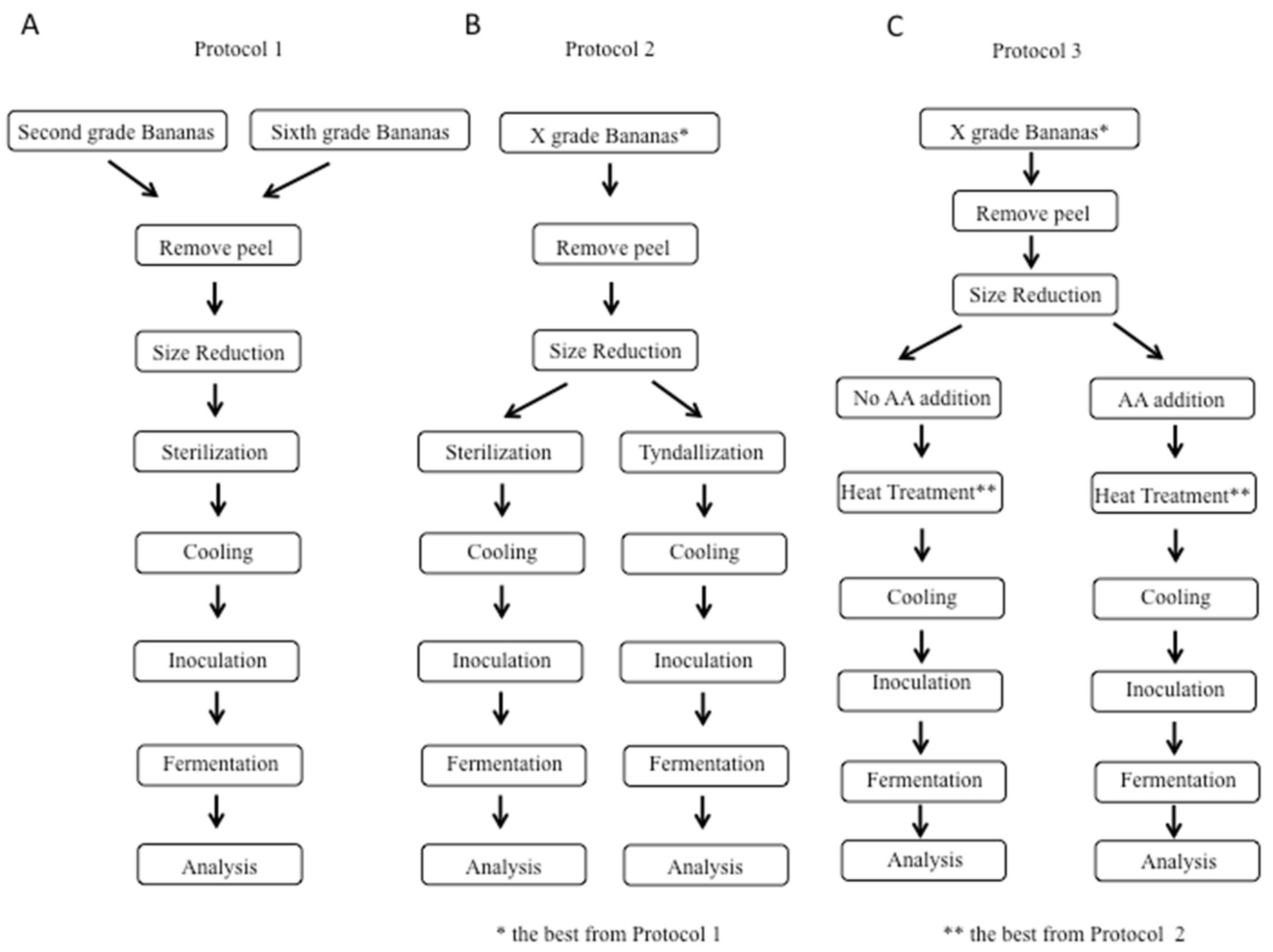
Three different protocols were used to study different variables and their effects on fermentation in a sequential manner, and these protocols, along with the overall process, are summarized in Figure 1.
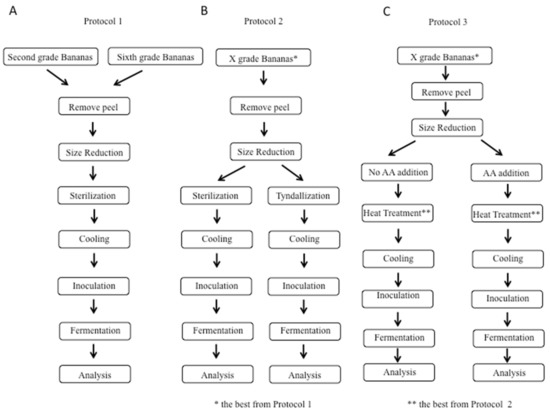
Figure 1.
Schematic representation of the three experimental protocols: (A) Protocol 1, comparison between two different raw materials: second-grade and sixth-grade bananas; (B) Protocol 2, comparison between two different heat treatments: sterilization and tyndallization; and (C) Protocol 3, comparison between samples without and with ascorbic acid addition: two different concentrations of ascorbic acid were tested (500 and 1000 ppm).
In Protocol 1, we focused on banana ripeness; in Protocol 2, we considered the heat treatment; and in Protocol 3, we studied the effect of ascorbic acid addition. Briefly, in Protocol 1, sixth-grade bananas were compared with second-grade bananas; both grades of bananas were subjected to peel removal and size reduction by using an immersion blender. The next phase was heat treatment, and sterilization was used. After that treatment, cooling for 4 h was performed. Finally, the two banana purees (one obtained by sixth-grade bananas and one obtained by second-grade bananas) were inoculated and then subjected to fermentation for 48 h at 37 °C, and the results were analyzed, as shown in Figure 1A. The bananas with the better ripeness grade for fermentation based on Protocol 1 were subjected to Protocol 2 (shown in Figure 1B) in which two different heat treatments were studied, i.e., sterilization and tyndallization, to understand if a heat treatment less intense than sterilization could have any impact on the fermentation. Finally, Protocol 3 was applied based on the bananas with the better ripeness grade and the better heat treatment evaluated from Protocols 1 and 2, respectively. Then, as shown in Figure 1C, the effect of ascorbic acid addition on fermentation was analyzed.
2.1.1. Raw Material
Banana samples, belonging to the Cavendish cultivar, were purchased in a local market. To study the effect of ripeness on fermentation parameters (during Protocol 1), second-grade (light green, breaking toward yellow) and sixth-grade (completely yellow) bananas were chosen. The ripeness grade was assigned according to the Banana Ripening Manual [16]. For each test, 600 g of banana was used.
2.1.2. Heat Treatment
The heat treatment is necessary to ensure the sterility of the puree. The heat treatments tested during Protocol 2 were sterilization and tyndallization. Specifically, sterilization was performed through autoclave treatment: banana puree was closed into an autoclavable bag and placed in the autoclave (121 °C for 20 min). Tyndallization was performed by using a thermostated bath: banana puree was treated into the same reactor used for the subsequent fermentation, with the circulation of heated water into the external jacket. Two heating cycles (70 °C for 30 min) were interspersed with one cooling cycle (37 °C for 30 min). This treatment was sufficient to eliminate any potential contamination, as demonstrated by preliminary tests (not shown).
2.1.3. Ascorbic Acid Addition
Ascorbic acid was added to the banana puree, only in Protocol 3 and prior to heat treatment. In particular, we tested the ascorbic acid concentration reported on the nutritional label of industrial banana puree (500 ppm) and double this concentration (1000 ppm). The ascorbic acid was provided by Kraft Heinz.
2.2. Lab-Scale Fermentation
2.2.1. Strain
The strain used as starter culture was Lactobacillus paracasei CBA L74, (Heinz Italia SpA), International Depository Accession Number LMG P-24778, which is a Gram-positive homofermentative, facultative anaerobic bacteria. It was stored in a freeze-dried form at −20 °C and revitalized in a 0.9% sodium chloride solution for 10 min before each fermentation test at 37 °C. The inoculum bacterial density was in the order of 108 CFU mL−1.
2.2.2. Lab-Scale Fermentation Apparatus
The experimental setup consisted of the following four components: a vessel, a mixing system, a system of thermal conditioning, and a system for pH and temperature measurements. The vessel was a Pyrex glass cylinder with an external jacket for circulating service fluid that was necessary for maintaining the constant temperature of the system. The height and diameter of the inner cylinder were 20 and 10 cm, respectively, while the dimensions of the external jacket were 18 and 14 cm, respectively. The vessel’s maximum capacity was 1.5 L. The mixing system consisted of a stainless-steel impeller with an inclined blade and a Rushton impeller connected to a motor (a three-phase asynchronous electric motor, 0.25 Hp, 0.18 kW, 1310 rpm with speed reductioner −170–−880 rpm), which allowed the stirring speed to be adjusted and respectively provided axial and radial mixing. Previous mixing tests were carried out, according to Gallo et al. [17], on the fermenting suspension, using a food dye to allow for the determination of the optimal speed rate (182 rpm in the present study) that guaranteed complete distribution of the dye in the medium within a maximum time of 10 min. For pH and temperature measurements, a Mettler Toledo probe was used (In Pro 3100, Mettler Toledo, Milano, Italy).
2.2.3. Lab-Scale Fermentation Protocol
Submerged fermentation was carried out in a 1.5 L fermenter. The first step was the sterilization of the fermenter and the mixing system at 121 °C for 20 min in autoclave. Then, 600 g of banana, pretreated as described above, and 300 mL of water were successively loaded into the reactor. The system was brought to 37 °C, and the strain was inoculated to start the fermentation process.
Banana puree was fermented for 48 h. Aseptic sampling was performed at different times (after inoculum (t0) and after 24 h (t24) and 48 h (t48) of processing). Only for Protocol 3 sampling times were increased from 3 to 8 (sampling times were: after inoculum (t0), after 4 h (t4), after 16 h (t16), after 20 h (t20), after 24 h (t24), after 40 h (t40), after 44 h (t44), and 48 h (t48) of processing); pH measurements, as well as microbiological and chemical analyses, were carried out for each sample.
2.3. Analysis
All culture media and kits were purchased from Oxoid (Oxoid, Rodano, Italy). Concerning the microbiological analysis, samples, after serial dilutions, were sown on Petri plates prepared with MRS agar; lactobacilli required an incubation of 48 h at 37 °C in anaerobic conditions, which could be guaranteed by using special anaerobic kits (Anaerogen Compact). To verify the presence of contaminants, samples were also sown on Petri plates prepared with PCA (bacteriological agar, yeast extract agar, Peptose Peptone, and glucose) for the total viable count of aerobic bacteria, MacConkey Agar for the isolation of Enterobacteriaceae, and Gelatin Peptone Agar for the growth of microorganisms other than lactobacilli. The pH analysis was performed by using an In Pro 3100 probe (Mettler Toledo, Milano, Italy). The amount of lactic acid produced during fermentation was determined by HPLC (Agilent Technologies 1100, Cernusco sul Naviglio, Italy), equipped with a column C18 (Agilent Zorbax C18, Cernusco sul Naviglio, Italy) and a UV detector set at 218 nm. The eluent was 0.1 M NH4H2PO4, at a flow rate of 0.8 mL min−1. Ascorbic acid was also detected by HPLC equipped with a column C18 (Agilent Zorbax C18, Cernusco sul Naviglio, Italy) and a UV detector set at 254 nm. The eluent was KH2PO4 (0.5% w/v) with dithiothreitol (0,1% w/v), at a flow rate of 0.5 mL min−1.
2.4. Statistical Analysis
Statistical analysis and graphics were obtained from GraphPad Prism (San Diego, CA, USA). Mean and standard deviation (n = 3) of the experimental data were calculated; their significance was evaluated by Student’s t-test, accepting only results that showed values of p < 0.05 as being significant.
3. Results
3.1. Protocol 1: Second-Grade Banana vs. Sixth-Grade Banana
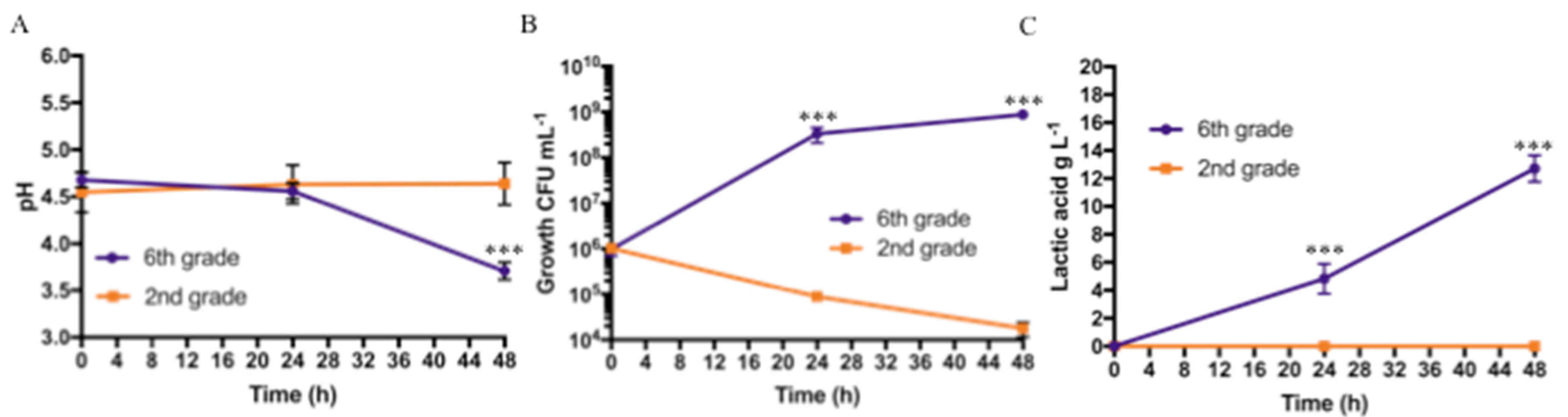
The pH values, Lactobacillus growth, and lactic acid production obtained during fermentation of bananas with two different degrees of ripeness (second-grade vs. sixth-grade bananas) are shown in Figure 2.
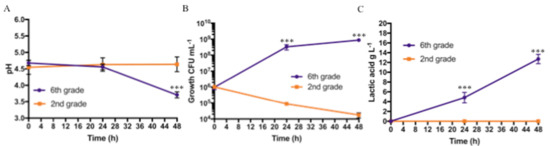
Figure 2.
Protocol 1 (2nd- vs. 6th-grade bananas) fermentation results at different sampling times (after inoculum (t0), and after 24 h (t24), and after 48 h, (t48): (A) pH values, (B) bacterial growth (CFU mL−1), and (C) Lactic acid production in g L−1. Bars represent standard deviation of three independent experiments. Student’s t-test. *** p < 0.001.
The pH values dropped from 4.7 ± 0.08 (t0) to a final value (t48) of 3.7 ± 0.09 for the puree obtained from sixth-grade bananas, while the pH value remained almost constant for the puree obtained from second-grade bananas, as shown in Figure 2A. A statistically significant difference was noted only at 48 h. For the microbiological results, the fermentation was started with an initial bacterial concentration of approximately 106 CFU mL−1 for both purees; bacterial concentrations of 3.3 × 108 ± 1.2 × 108 CFU mL−1 after 24 h, and 8.7 × 108 ± 1.0 × 108 CFU mL−1 after 48 h, were obtained for the sixth-grade banana puree samples. Otherwise, bacterial concentrations in the order of 105 CFU mL−1 after 24 h and 104 CFU mL−1 after 48 h were obtained for the fermentation of second-grade banana puree samples. Statistically significant differences were observed at 24 and 48 h, as shown in Figure 2B. Furthermore, in the PCA, no microbial growth was observed on MacConkey and Gelatine Peptone agar plates, demonstrating the absence of contaminants on both samples (not shown). In both tested conditions, lactic acid was not detected at t0; after 24 and 48 h, it reached a concentration of 4.8 ± 1.1 g L−1 and 12.7 ± 0.9 g L−1, respectively, for the sixth-grade banana puree as shown in Figure 2C. Only traces of lactic acid were detected on second-grade banana puree.
3.2. Protocol 2: Sterilization vs. Tyndallization
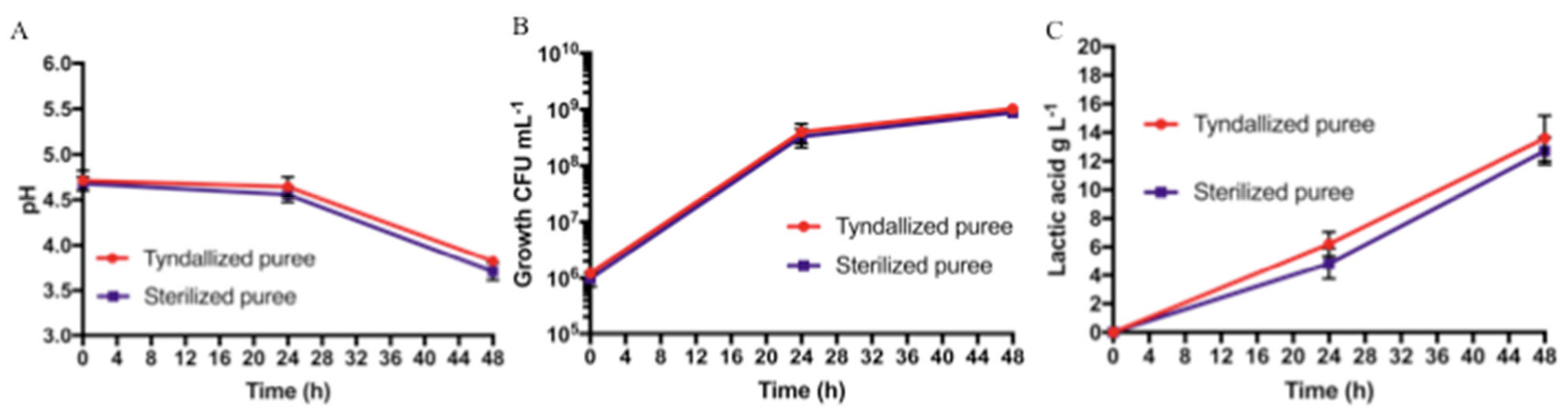
On the basis of the results obtained from Protocol 1, sixth-grade bananas were chosen as the raw material for Protocol 2. The pH values, L. paracasei growth, and lactic acid production for the two tested conditions (banana puree sterilized and banana puree tyndallized) are shown in Figure 3.
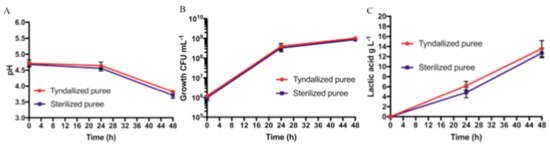
Figure 3.
Protocol 2 (sterilization vs. tyndallization) fermentation results at different sampling times (t0, t24, and t48): (A) pH values, (B) bacterial growth (CFU mL−1), and (C) lactic acid production in g L−1. Bars represent standard deviation of three independent experiments.
The pH values for the sterilized banana puree were the same for the sixth-grade bananas used in Protocol 1. As for the tyndallized banana puree, there was a pH reduction from 4.71 ± 0.11 to 3.82 ± 0.04 at 48 h (t48). No statistically significant difference was found, as shown in Figure 3A. For both treatment conditions, the fermentation started with an initial concentration in the order of 106 CFU mL−1, reaching, in both cases, concentrations in the order of 108 and 109 CFU mL−1, after 24 and 48 h of fermentation, respectively, and no statistically significant differences were found, as shown in Figure 3B. Furthermore, on PCA, McConkey and Gelatine Peptone agar plates no growth was observed, demonstrating the absence of contaminants on both samples (not shown). In both tested conditions, lactic acid was not detected at t0; after 24 h, it reached a concentration of 4.8 ± 1.1 g L−1 and 6.2 ± 0.9 g L−1 for the sterilized and tyndallized banana purees, respectively. At 48 h, we found lactic acid concentrations of 12.7 ± 0.9 g L−1 for the sterilized banana puree and 13.6 ± 1.6 g L-−1 for the tyndallized banana puree. No significant difference was noted, as shown in Figure 3C.
3.3. Protocol 3: Ascorbic Acid Addition
On the basis of the results obtained at the end of Protocol 1, sixth-grade bananas were chosen as the raw material. Regarding Protocol 2, no significative difference was noted between the two heat treatments and, thus, tyndallization was chosen for Protocol 3 because it was milder. Ascorbic acid was measured before and after the heat treatment, and the observed values are reported in Table 1.

Table 1.
Ascorbic acid concentrations measured before and after the heat treatment.
All results related to Protocol 3 are shown in Figure 4.
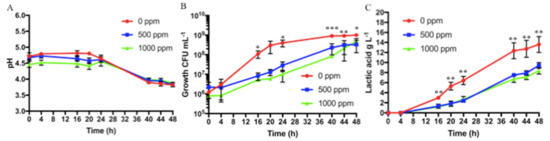
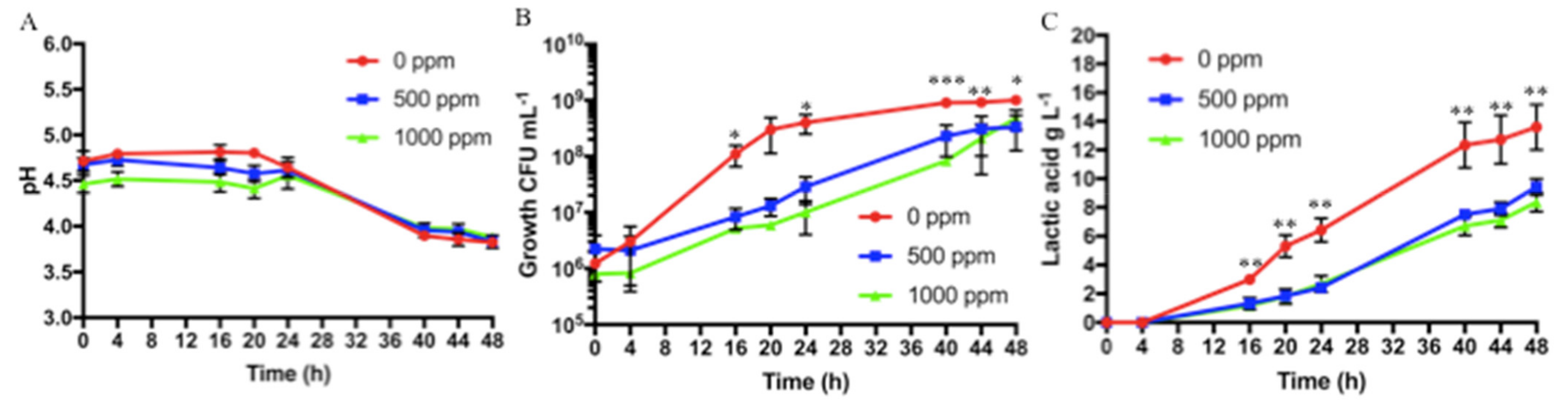
Figure 4.
Protocol 3 (ascorbic acid addition of 0, 500, or 1000 ppm) fermentation results at different sampling times (t0, t4, t16, t20, t24, t40, t44, and t48): (A) pH values, (B) bacterial growth (CFU mL−1), and (C) lactic acid production in g L−1. Bars represent standard deviation of three independent experiments. Student’s t-test. * p < 0.05, ** p < 0.01, *** p < 0.001.
As described in the Methods section, two different ascorbic acid concentrations were tested, and the results were compared with those obtained for the better condition identified for Protocol 2 (sixth-grade banana, tyndallized, and without ascorbic acid addition). A greater number of samplings was conducted, in order to study the effect of ascorbic acid more closely. The pH found at t0 was 4.67 ± 0.08 and 4.46 ± 0.09, when 500 and 1000 ppm of ascorbic acid were added, respectively. At 48 h, the pH reduced to final values of approximately 3.8 for both conditions and no statistically significant difference was noted, as shown in Figure 4A. For both the addition of 500 and of 1000 ppm of ascorbic acid, the fermentation started with an initial bacterial concentration of approximately 106 CFU mL−1, and at t24, it reached bacterial concentrations in the order of 107 CFU mL−1, with statistically significant differences as compared with banana puree without ascorbic acid (t24 bacterial concentration of 4.0 × 108 ± 1.5 × 108 CFU mL−1). After 48 h, both samples reached a concentration of approximately 4 × 108 CFU mL−1, of about one order of magnitude less than the bacterial concentration measured at the end for the banana puree with no addition of ascorbic acid. Differences in bacterial growth, obtained without and with addition of ascorbic acid (both 500 and 1000 ppm) were statistically significant starting from 24 h of fermentation to the end, as shown in Figure 4B. Furthermore, on PCA, McConkey and Gelatine Peptone agar plates no growth was observed, demonstrating the absence of contaminants on both samples (not shown). In all the tested conditions, lactic acid was not detected at the initial fermentation times. At 48 h, with the addition of ascorbic acid, lactic acid concentrations reached values of 9.4 ± 0.6 and of 8.3 ± 0.7 g L−1 when 500 and 1000 ppm of ascorbic acid were added, respectively, and the difference with no addition of ascorbic acid was statistically significant, as shown in Figure 4C.
4. Discussion
Industries are increasingly interested in functional foods such as probiotics (i.e., live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [18]) and postbiotics (i.e., preparation of inanimate microorganisms and/or their components that confers a health benefit on the host [19]). Most of these types of functional foods are dairy-based products [20,21,22], but products of a different nature are also on the rise. The ability of the bacterial strain that was used in the present study to ferment food matrices, such as cereals and legumes, has previously been demonstrated [17,23,24,25,26,27,28,29], and its probiotic activity has also been confirmed in previous studies [30,31,32,33]; in the present work, its ability to ferment an industrial product, such as banana puree, was investigated. Banana, both as a fresh or processed fruit, is one of the most consumed fruit crops worldwide, starting from infancy. Because of its nutritional composition, it seems to be a good substrate for fermentation, as previously studied [34,35]. Fermentation is a processing technology useful for increasing shelf life and improving organoleptic properties, and it is also a method to obtain functional foods. The present work was aimed at understanding the feasibility of fermenting an industrial product, i.e., banana puree, to obtain a potential functional food, paying attention to how the raw material (unripe or ripe banana) and the production process itself can impact fermentation. For this purpose, a banana-puree industrial process was reproduced at the lab-scale, with fermentation as the final step. The measurements of pH reduction, growth of L. paracasei, and production of lactic acid were used as indicators for the analysis of fermentation performance. Three different experimental protocols were tested. The first protocol was designed to determine whether unripe or ripe banana was a better raw material to be fermented. Significant differences were noted in fermentation performance, i.e., sixth-grade or ripe bananas gave better results in terms of L. paracasei growth, lactic acid production, and pH reduction as compared with second-grade bananas, for which an early bacterial death phase was observed. A similar result was observed by Aegerter and Dunlap [34], who studied the fermenting ability of five different strains on ripe and unripe banana puree. They showed a pH reduction (as a fermentation indicator) also for unripe banana puree, but only after several days. This is probably due to the difference in nutritional composition of second- and sixth-grade bananas. The ripening of bananas, in fact, leads to an increase in the content of soluble sugars, more accessibility to bacteria and, at the same time, a decrease in starch [4,36,37] and depolymerization of pectin [38]. Another factor that promotes fermentation in sixth-grade bananas could be the fact that fructan concentrations are higher in ripe bananas than those in unripe bananas [39], keeping in mind that fructans are considered to be prebiotic compounds for some bacterial strains [40]. After we determined the better raw material for fermentation (ripe bananas), we focused on the banana-puree production steps considered to be “more risky” for good fermentation, i.e., heat treatment and ascorbic acid addition, commonly used as an anti-browning agent. The heat treatment is considered risky for fermentation due to the possibility of reducing proteins, vitamins [13], or other elements useful for microbial metabolism. For our purposes, sterilization and tyndallization treatments were compared. We did not detect any difference between the treatments, suggesting that even the more intense heat treatment did not significantly alter the food substrate. Finally, in Protocol 3, we analyzed the effect of ascorbic acid on fermentation, since it is considered to be a critical phase in the fermentation process because of the antimicrobial properties of ascorbic acid [41]. In this case, sampling times were increased to study more closely the effect of ascorbic acid (which is an antimicrobial agent) on the bacterial fermentation. Two different concentrations were tested, i.e., 500 ppm (the concentration normally detected in the industrial processed product and reported on the label) and 1000 ppm. The decay of ascorbic acid due to heat treatment was measured to understand the actual concentration in contact with the microorganism during fermentation: a decay of about 50% was observed. No significant differences were noted between the experimental data obtained with these two concentrations in terms of bacterial growth, lactic acid production, and pH reduction, but both cases provided significantly different results as compared with the banana puree fermented without ascorbic acid addition, confirming the hypothesis regarding its inhibiting effect on microbial metabolism. In particular, considering bacterial growth, it seems that ascorbic acid, at the concentration used, did not absolutely inhibit it, but only slow it down. Notably, although the final bacterial growth (and, therefore, the production of lactic acid and the reduction of pH) was significantly reduced by ascorbic acid, a bacterial concentration higher than that considered sufficient to declare the banana product as probiotic (106 CFU mL−1 [42]) was achieved and a potential probiotic product was obtained. However, when ascorbic acid is added, the same bacterial and lactic acid concentrations of those observed without any addition could be reached at longer fermentation times and the process could be further optimized. Indeed, for banana puree fermented with no addition of ascorbic acid, we observed about a 3-log increase in bacterial concentration, which was higher than that obtained by Tsen et al. [43,44] on banana puree inoculated with free L. acidophilus. In these works, the optimization was carried out by encapsulating the strain in Ca alginate or k-carrageenan, and an increase of at least 3 log of the bacterial concentration was obtained, the same increase achieved in the present work without encapsulation.
To further optimize banana puree fermentation without the addition of ascorbic acid, though, at the same time, preserving the color, other approaches could be considered. Certainly, the use of different additives is a way forward, but we must also consider the impact they have on fermentation and if they are allowed by regulations for particular products, such as baby food.
5. Conclusions
The factors affecting fermentation during the production of banana puree were investigated. The choice of the raw material and the addition of ascorbic acid were the factors most capable of inhibiting/reducing bacterial growth and production of lactic acid. The degree of ripeness of the banana must be carefully evaluated to obtain a sufficient bacterial growth to declare a food “probiotic”, considering the inhibition effect of unripe bananas on the strain. In addition, regarding the addition of ascorbic acid, a sufficient bacterial concentration could be obtained even with the highest concentration tested (1000 ppm), although lower than that obtained in the absence of ascorbic acid. Therefore, it is possible to obtain a potentially probiotic banana puree through the conventional production process followed by L. paracasei CBA L-74 fermentation and the process could be further optimized in terms of bacterial concentration and lactic acid production. Further studies would be helpful to determine the most appropriate concentration of ascorbic acid to add to obtain both an anti-browning effect and the highest possible bacterial growth. Other anti-browning agents may also be considered; however, strict regulations regarding permitted additives for special foods, such as baby food, must be taken into account. Another necessary evaluation will be the sensory analysis to understand the impact of the whole process on the organoleptic properties of the banana puree.
Author Contributions
Conceptualization, M.G., F.P., P.S., A.B. and R.N.; methodology, M.G., F.P., P.S., A.E., R.C.C., F.N. and R.N.; validation, M.G., A.B. and R.N.; formal analysis, A.E., R.C.C. and F.N.; data curation, F.P., P.S., A.E., R.C.C. and F.N.; writing original draft, M.G. and F.P.; writing—review and editing, M.G. and F.P.; supervision, M.G. and R.N.; project administration, M.G. and R.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Andrea Budelli is currently employed by Heinz BV, Netherlands. He provided Lactobacillus paracasei CBA L74 and participated in the design of the study. He did not have any additional role in the data collection and analysis, decision to publish, or preparation of the manuscript. Heinz BV did not provide any financial support to the authors for the experimental activity and did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- FAO. The World Banana Economy 1985e2002. Retrieved 2011/2/20; FAO: Rome, Italy, 2003; Available online: http://www.fao.org/ (accessed on 6 January 2021).
- Pillay, M.; Tenkouano, A. Banana Breeding: Progress and Challenges; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Mohapatra, D.; Mishra, S.; Singh, C.B.; Jayas, D.S. Post-harvest Processing of Banana: Opportunities and Challenges. Food Bioprocess Technol. 2011, 4, 327–339. [Google Scholar] [CrossRef]
- Yap, M.; Fernando, W.; Brennan, C.S.; Jayasena, V.; Ranil Coorey, R. The effects of banana ripeness on quality indices for puree production. Food Sci. Technol. 2017, 80, 10–18. [Google Scholar] [CrossRef]
- Matamoros, J.J. Factibilidad Técnica de Fermentar Pulpa de Banana con L. casei y Posibilidades de Obtener un Producto Base. Master’s Thesis, Universidad de Costa Rica, San José, Costa Rica, 1981. [Google Scholar]
- De Porres, E. Minimización del tiempo requerido para reducir el pH del puré de banana mediante fermentación làctica. In Proceedings of the Segundo Congreso Latinoamericano y del Caribe de Tecnología de Alimentos, San José, Costa Rica; 1986. [Google Scholar]
- Chan-Blanco, Y.; Bonilla-Leiva, A.R.; Velμzquez, A.C. Using banana to generate lactic acid through batch process fermentation. Appl. Microbiol. Biotechnol. 2003, 63, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, H.; Eida, T.; Takizawa, T.; Tokanaga, T.; Tashiro, Y. Effects of fructooligosaccharides on intestinal flora and human health. Bifidobact. Microflora 1986, 5, 37–50. [Google Scholar] [CrossRef]
- Tomomatsu, H. Health effects of oligosaccharides. Food Technol. 1994, 48, 61–65. [Google Scholar]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Bunt, C.R.; Mason, S.L.; Hussain, M.A. Non-dairy probiotic food products: An emerging group of functional foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 2626–2641. [Google Scholar] [CrossRef]
- Sims, C.A.; Bates, R.P.; Arreola, A.G. Color, polyphenoloxidase, and sensory changes in banana juice as affected by heat and ultrafiltration. J. Food Qual. 2007, 5, 371–379. [Google Scholar] [CrossRef]
- Petruzzi, L.; Campaniello, D.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Thermal Treatments for Fruit and Vegetable Juices and Beverages: A Literature Overview. Compr. Rev. Food Sci. F. 2017, 16, 668–691. [Google Scholar] [CrossRef]
- Sapers, G.M.; Ziolkowski, M.A. Comparison of erythorbic and ascorbic acids as inhibitors of enzymatic browning in apple. J. Food Sci. 1987, 52, 1733–1747. [Google Scholar] [CrossRef]
- Guerrero, S.; Alzamora, S.M.; Gerschenson, L.N. Development of a Shelf-Stable Banana Puree by Combined Factors: Microbial Stability. J. Food Prot. 1994, 10, 902–907. [Google Scholar] [CrossRef]
- United Fruit Sales Corporation. Banana Ripening Manual; United Fruit Sales Corporation: Boston, MA, USA, 1964. [Google Scholar]
- Gallo, M.; Nigro, F.; Passannanti, F.; Salameh, D.; Schiattarella, P.; Budelli, A.; Nigro, R. Lactic fermentation of Cereal Flour: Feasibility Tests on Rice, Oat and Wheat. Appl. F. Biotechnol. 2019, 3. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 1–19. [Google Scholar] [CrossRef]
- Shahnawaz, U.K. Probiotics in dairy foods: A review. Nutr. Food Sci. 2014, 1, 71–88. [Google Scholar] [CrossRef]
- Civardi, E.; Garofoli, F.; Longo, S.; Mongini, M.E.; Grenci, B.; Mazzucchelli, I.; Angelini, M.; Castellazzi, A.; Fasano, F.; Grinzato, A.; et al. Safety, growth, and support to healthy gut microbiota by an infant formula enriched with functional compounds. Clin. Nutr. 2017, 36, 238–245. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Fakhri, O.; Farhoodi, A.; Kaboudari, A.; Pir-Mahalleh, S.F.R.; Tahapour, K.; Khayyati, M.; Chegin, R. A Review on Probiotic Dairy Products as Functional Foods Reported from Iran. Int. J. Food Nutr. Saf. 2015, 6, 1–12. [Google Scholar]
- Gallo, M.; Nigro, F.; Passannanti, F.; Salameh, D.; Budelli, A.; Marzocchella, A.; Nigro, R. Rice Fermentation by Lactobacillus paracasei CBAL74. Int. J. Rice Res. 2018, IJRR-103. [Google Scholar] [CrossRef]
- Gallo, M.; Passannanti, F.; Colucci Cante, R.; Nigro, F.; Salameh, D.; Schiattarella, P.; Schioppa, C.; Budelli, A.; Nigro, R. Effects of the Glucose Addition during Lactic Fermentation of Rice, Oat; Wheat Flours. Appl. Food Biotechnol. 2019, 1, 21–30. [Google Scholar] [CrossRef]
- Salameh, D.; Nigro, F.; Colucci Cante, R.; Passannanti, F.; Gallo, M.; Budelli, A.; Marzocchella, A.; Nigro, R. Fermentation of Rice Flour Supernatant Using Lactobacillus Paracasei Cba L74. Chem. Eng. Trans. 2019, 75, 289–294. [Google Scholar] [CrossRef]
- Colucci Cante, R.; Gallo, M.; Nigro, F.; Passannanti, F.; Salameh, D.; Budelli, A.; Nigro, R. Lactic Fermentation of cooked navy beans by Lactobacillus paracasei CBA L74 aimed at a potential production of functional legume-based foods. Can. J. Chem. Eng. 2020, 98. [Google Scholar] [CrossRef]
- Gallo, M.; Passannanti, F.; Cante, R.; Nigro, F.; Schiattarella, P.; Zappulla, S.; Budelli, A.; Nigro, R. Lactic fermentation of cereals aqueous mixture of oat and rice flours with and without glucose addition. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- Gallo, M.; Passannanti, F.; Schioppa, C.; Montella, S.; Cante, R.; Nigro, F.; Budelli, A.; Nigro, R. Enzymatic pre-treatment and lactic fermentation of wheat flour suspension at high solid content. J. Food Process. Preserv. 2020, 45, e15299. [Google Scholar] [CrossRef]
- Cante, R.; Gallo, M.; Nigro, F.; Passannanti, F.; Budelli, A.; Nigro, R. Mathematical modeling of Lactobacillus paracasei CBA L74 growth during rice flour fermentation performed with and without pH control. Appl. Sci. 2021, 11, 2921. [Google Scholar] [CrossRef]
- Nocerino, R.; Paparo, L.; Terrin, G.; Pezzella, V.; Amoroso, A.; Cosenza, L.; Cecere, G.; De Marco, G.; Micillo, M.; Albano, F.; et al. Cow’s milk and rice fermented with Lactobacillus paracasei CBA L74 prevent infectious diseases in children: A randomized controlled trial. Clin. Nutr. 2017, 1, 118–125. [Google Scholar] [CrossRef]
- Sarno, M.; Lania, G.; Cuomo, M.; Nigro, F.; Passannanti, F.; Budelli, A.; Fasano, F.; Troncone, R.; Auricchio, S.; Barone, M.V.; et al. Lactobacillus paracasei CBA L74 interferes with gliadin peptides entrance in Caco-2 cells. Int. J. Food Sci. Nutr. 2014, 65. [Google Scholar] [CrossRef]
- Labruna, G.; Nanayakkara, M.; Pagliuca, C.; Nunziato, M.; Iaffaldano, L.; D’Argenio, V.; Colicchio, R.; Budelli, A.L.; Nigro, R.; Salvatore, P.; et al. Celiac disease-associated Neisseria flavescens decreases mitochondrial respiration in CaCo-2 epithelial cells: Impact of Lactobacillus paracasei CBA L74 on bacterial-induced cellular imbalance. Cell Microbiol. 2019, 21. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Nigro, F.; Passannanti, F.; Nanayakkara, M.; Lania, L.; Parisi, F.; Salameh, D.; Budelli, A.; Barone, M.V.; Nigro, R. Effect of pH control during rice fermentation in preventing a gliadin P31-43 entrance in epithelial cells. Int. J. Food Sci. Nutr. 2019, 70. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, P.; Dunlap, C. Culture of Five Commonly Used Acid-Producing Bacteria on Banana Pulp. Appl. Environ. Microbiol. 1980, 5, 937–942. [Google Scholar] [CrossRef] [PubMed]
- De Porres, E.; de Arriola, M.C.; Garcia, R.; Rolz, C. Lactic acid fermentation of banana puree. Lebensm. Wiss. Technol. 1985, 18, 379–382. [Google Scholar]
- Emaga, T.; Andrianaivo, R.H.; Wathelet, B.; Tchango, J.; Paquot, M. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem. 2007, 103, 590–600. [Google Scholar] [CrossRef]
- Prabha, T.N.; Bhagyalakshmi, N. Carbohydrate metabolism in ripening banana fruit. Phytochemistry 1998, 6, 915–919. [Google Scholar] [CrossRef]
- Duan, X.; Cheng, G.; Yang, E.; Yi, C.; Ruenroengklin, N.; Lu, W.; Luo, Y.; Jiang, Y. Modification of pectin polysaccharides during ripening of postharvest banana fruit. Food Chem. 2008, 1, 144–149. [Google Scholar] [CrossRef]
- Shalini, R.; Antony, U. Fructan distribution in banana cultivars and effect of ripening and processing on Nendran banana. J. Food Sci. Technol. 2015, 12, 8244–8251. [Google Scholar] [CrossRef][Green Version]
- Shalini, R.; Abinaya, G.; Saranya, P.; Antony, U. Growth of selected probiotic bacterial strains with fructans from Nendran banana and garlic. Food Sci. Technol. 2017, 83, 68–78. [Google Scholar] [CrossRef]
- Tajkarimi, M.; Ibrahim, S.A. Antimicrobial activity of ascorbic acid alone or in combination with lactic acid on Escherichia coli O157:H7 in laboratory medium and carrot juice. Food Control 2011, 6, 801–804. [Google Scholar] [CrossRef]
- International Dairy Federation. Standards for Fermented Milks; International Dairy Federation: Brussels, Belgium, 1997; D-Doc. 316. [Google Scholar]
- Tsen, J.H.; Lin, Y.P.; King, V. Banana puree fermentation by Lactobacillus acidophilus immobilized in Ca-alginate. J. Gen. Appl. Microbiol. 2003, 49, 357–361. [Google Scholar] [CrossRef][Green Version]
- Tsen, J.H.; Lin, Y.P.; King, V. Fermentation of banana media by using kappa-carrageenan immobilized Lactobacillus acidophilus. Int. J. Food Microbiol. 2004, 91, 215–220. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).