Nanoencapsulation of Essential Oils as Natural Food Antimicrobial Agents: An Overview
Abstract
:1. Introduction
2. Synthetic and Natural Food Antimicrobial Agents
2.1. Synthetic Antimicrobial Agents
2.2. Natural Antimicrobial Agents
3. Essential Oils as Food Preservatives: Current Status and Challenges
3.1. Advances in Research on EOs as Antimicrobial Agent
3.2. Challenges in the Use of EOs as Food Antimicrobials
4. Nanoencapsulation versus Microencapsulation
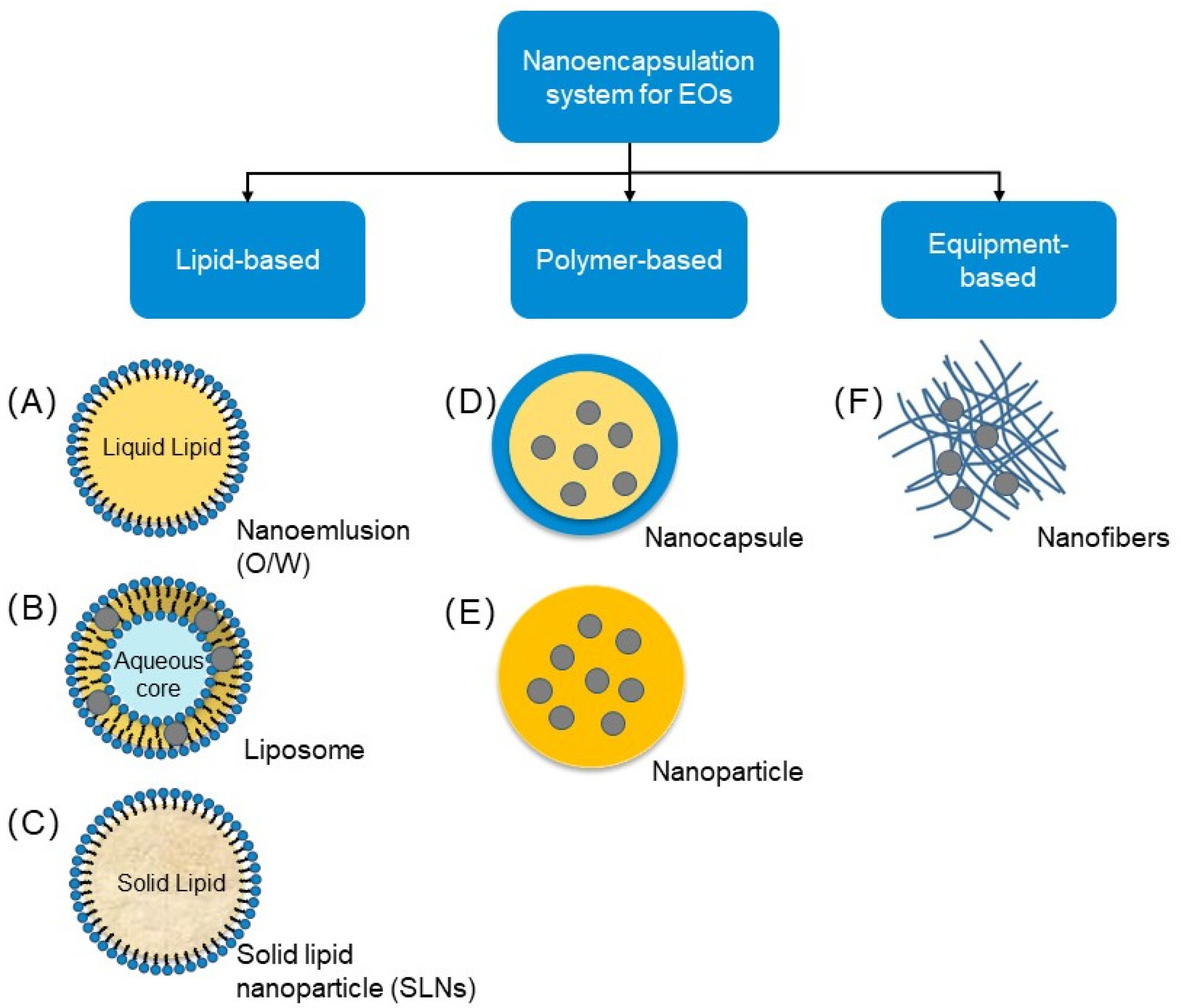
5. Main Strategies for Nanoencapsulation of Essential Oils
5.1. Biopolymer-Based Nanoencapsulation
5.1.1. Polysaccharide-Based Biopolymers
5.1.2. Protein-Based Biopolymers
5.1.3. Complexation of Biopolymers
5.2. Lipid-Based Nanoencapsulation
5.2.1. Nanoemulsions
5.2.2. Nanoliposomes
5.2.3. Solid Lipid Nanoparticles
5.3. Equipment-Based Nanoencapsulation
6. Effect of Nanoencapsulation on the Antimicrobial Activity of Essential Oils
| Delivery System | Bioactive Compounds | Encapsulating Material | Techniques | Size | Major Findings | References |
|---|---|---|---|---|---|---|
| Nanoemulsion (prepared by low-energy methods) | Thymol | Zein-Sodium caseinate | Emulsion polymerization | 65.8~87.6 nm | Compared with the control, the encapsulated thymol was more effective in lowering S. aureus counts during a period of 13 days | [141] |
| Vitamin E | Edible mustard oil with Tween-80 | Emulsion diffusion | ~86 nm | High encapsulation efficiency close to 100%; the antioxidant and antimicrobial activity of nanoemulsions was improved | [57] | |
| Cuminum cyminum oil | Chitosan-caffeic acid | Ionic gelation | <100 nm | When tested under unsealed condition, nanogel-containing oils showed better antimicrobial activity than the free oils against Aspergillus flavus | [29] | |
| Nanoemulsion (prepared by high-energy methods) | Peppermint oil | Medium-chain triacylglycerol | High pressure homogenization | <200 nm | Nanoemulsions containing EO showed improved antimicrobial activity against Listeria monocytogenes and Staphylococcus aureus | [112] |
| Eucalyptus oil | Tween 80 | Ultrasonication | 17.1 nm | Nanoemulsions containing eucalyptus showed to inactivate B. cereus at 0 min, S. aureus at 15 min, and E. coli at 1 h | [142] | |
| Essential oils (Lemongrass, clove, tea tree, thyme, geranium, marjoram, palmarosa, rosewood, sage or mint) | Sodium alginate and tween 80 | Microfluidization | <20 nm | The antibacterial activity depends on the type of essential oil used in the formulation, not their droplet size | [133] | |
| Thymus capitatus oil | Soybean oil and SDS (sodium dodecylsulfate) | High pressure micro-fluidizer | ~110 nm | Nanoencapsulated EO exhibited higher antibacterial inhibition diameters against Staphylococcus aureus compared to those formed by free EO | [143] | |
| SLNs | Nisin | Cetylpalmitate, Softisan 378, Softisan 154, Imwitor 900 and Witepsol E85 | High pressure homogenization | 119 nm | The antibacterial activity of nisin-loaded SLNs against L. monocytogenes and L. plantarum was up to 20 and 15 days, respectively, while the free nisin was only 1 day and 3 days, respectively. | [144] |
| Zataria multiflora oil | Glyceryl monostearat and Precirol® | High-shear homogenization and ultrasound | 255.5 nm | Z. multiflora essential-oil-loaded SLNs exhibited strong antifungal activity compared to free oil against Aspergillus ochraceus, Aspergillus niger | [123] | |
| Nanoliposome | Nisin | Soy and marine lecithin | Microfluidization | 151~181 nm | Lecithin-encapsulated nisin exhibited higher stability for 6 weeks and showed better antibacterial activity compared to free nisin | [145] |
| Rose essential oil | Phosphatidylcholine and cholesterol | Supercritical fluid technology | <100 nm | The liposomes formed by the supercritical process have high encapsulation efficiency and small particle size with a unimodal distribution | [146] | |
| Polysaccharide-based | Cardamom oil | Chitosan nano-particles | Ionic gelation | 50~100 nm | The encapsulation efficiency of chitosan nano-composites was more than 90%; it exhibited excellent anti-microbial potential against Escherichia coli and Staphylococcus aureus. | [147] |
| Lemon ironbark oil | Cashew gum | Spray dryer | 27.7~432.6 nm | Nanoencapsulated oil showed improved activity against Salmonela Enteritidis. | [148] | |
| Lime oil | Chitosan | Phase inversion emulsification | 100~300 nm | Nanoencapsulated lime EO exhibited enhanced antibacterial activity against Staphylococcus aureus, Listeria monocytogenes, Shigella dysenteriae, and Escherichia coli | [149] | |
| Protein-based | Thymol/carvacral | Zein | Emulsion diffusion | 263 nm /275 nm | The encapsulated EOs in zein nanoparticles can increase their solubility by up to 14 times without affecting their ability to scavenge free radicals or to control E. coli growth | [150] |
| Thymol | Sodium Caseinate | High shear homogenization | ~130 nm | Compared with thymol crystals, the encapsulated thymol exhibited significantly improved anti-Listeria activity in milk with different fat levels | [82] | |
| Biopolymer-based | Eugenol oil | Whey protein and maltodextrin | High-speed homogenizer | 100~300 nm | The nanoencapsulated eugenol showed improved antimicrobial activity against E. coli and L. monocytogenes than the free oil | [151] |
| Terpenes mixture and d-limonene | Starch and soy lecithin | High Pressure Homogenization | 100~400 nm | The addition of low dose of the nanoencapsulated terpenes can delay the microbial growth (1.0 g/L terpenes) or completely inactivate microorganisms such as Lactobacillus delbrueckii, Saccharomyces | [117] | |
| Equipment-based | Peppermint oil | Alginate biopolymer | Electrospinning and electrospraying | ~80 nm | The nanoencapsulated peppermint oil exhibited a high antimicrobial activity against E. coli and S. aureus bacteria | [128] |
7. Applications of Nanoencapsulated Natural Antimicrobial Agents
7.1. Aqueous Food Systems
7.2. Solid Food Systems
7.3. Active Food Packaging
8. Legislative Aspects Concerning the Use of Nanoparticles in Food Products
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Gustafsson, J.; Cederberg, C.; Sonesson, U.; Emanuelsson, A. The Methodology of the FAO Study: Global Food Losses and Food Waste-Extent, Causes and Prevention-FAO, 2011; SIK Institutet för Livsmedel och Bioteknik: Gothenburg, Sweden, 2013. [Google Scholar]
- Zhang, Y.; Chen, H.; Pan, K. Chapter 5—Nanoencapsulation of Food Antimicrobial Agents and Essential Oils. In Nanoencapsulation of Food Bioactive Ingredients; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 183–221. [Google Scholar]
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control 2018, 89, 1–11. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Encapsulation strategies to enhance the antibacterial properties of essential oils in food system. Food Control 2021, 123, 107856. [Google Scholar] [CrossRef]
- Shatalov, D.; Kedik, S.; Zhavoronok, E.; Aydakova, A.; Ivanov, I.; Evseeva, A.; Beliakov, S.; Biryulin, S.; Kovalenko, A.; Mikhailenko, E. The current state and development of perspectives of application of synthetic antimicrobial agents. Polym. Sci. Ser. D 2017, 10, 293–299. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Zeng, W.C.; He, Q.; Sun, Q.; Zhong, K.; Gao, H. Antibacterial activity of water-soluble extract from pine needles of Cedrus deodara. Int. J. Food Microbiol. 2012, 153, 78–84. [Google Scholar] [CrossRef]
- Gould, G.W. Mechanisms of Action of Food Preservation Procedures; Elsevier Applied Science: London, UK, 1989. [Google Scholar]
- Bagamboula, C.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; de Souza, C.R.F.; Oliveira, W.P. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Annunziata, M.; Vincensi, M.; Ferrari, G. Design of nanoemulsion-based delivery systems of natural antimicrobials: Effect of the emulsifier. J. Biotechnol. 2012, 159, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef]
- Schlüter, B.; Pflegel, P.; Lindequist, U.; Jülich, W. Aspects of the antimicrobial efficacy of grapefruit seed extract and its relation to preservative substances contained. Die Pharm. 1999, 54, 452–456. [Google Scholar]
- Levy, J.; Boyer, R.R.; Neilson, A.P.; O’Keefe, S.F.; Chu, H.S.S.; Williams, R.C.; Dorenkott, M.R.; Goodrich, K.M. Evaluation of peanut skin and grape seed extracts to inhibit growth of foodborne pathogens. Food Sci. Nutr. 2017, 5, 1130–1138. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.M.; Critzer, F.J.; Taylor, T.M. Naturally occurring antimicrobials for minimally processed foods. Annu. Rev. Food Sci. Technol. 2013, 4, 163–190. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.; Zivanovic, S. The use of natural antimicrobials. In Food Preservation Techniques; Elsevier: Amsterdam, The Netherlands, 2003; pp. 5–30. [Google Scholar]
- Elsser-Gravesen, D.; Elsser-Gravesen, A. Biopreservatives. In Biotechnology of Food and Feed Additives; Springer: Berlin, Germany, 2013; pp. 29–49. [Google Scholar]
- Bergholz, T.M.; Tang, S.; Wiedmann, M.; Boor, K.J. Nisin resistance of Listeria monocytogenes is increased by exposure to salt stress and is mediated via LiaR. Appl. Environ. Microbiol. 2013, 79, 5682–5688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Nagasawa, T. ε-Poly-L-lysine: Microbial production, biodegradation and application potential. Appl. Microbiol. Biotechnol. 2003, 62, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Sperotto, A.R.; Moura, D.J.; Péres, V.F.; Damasceno, F.C.; Caramão, E.B.; Henriques, J.A.; Saffi, J. Cytotoxic mechanism of Piper gaudichaudianum Kunth essential oil and its major compound nerolidol. Food Chem. Toxicol. 2013, 57, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Melaleuca alternifolia (tea tree) oil inhibits germ tube formation by Candida albicans. Med. Mycol. 2000, 38, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, X.; Cheng, S.; Wang, H.; Yu, D.; Mungai, C. The possible mechanism of antifungal action of tea tree oil on Botrytis cinerea. J. Appl. Microbiol. 2013, 114, 1642–1649. [Google Scholar] [CrossRef]
- Zhaveh, S.; Mohsenifar, A.; Beiki, M.; Khalili, S.T.; Abdollahi, A.; Rahmani-Cherati, T.; Tabatabaei, M. Encapsulation of Cuminum cyminum essential oils in chitosan-caffeic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crop Prod. 2015, 69, 251–256. [Google Scholar] [CrossRef]
- Tavakoli, H.R.; Mashak, Z.; Moradi, B.; Sodagari, H.R. Antimicrobial activities of the combined use of Cuminum cyminum L. essential oil, nisin and storage temperature against Salmonella Typhimurium and Staphylococcus aureus in vitro. Jundishapur J. Microbiol. 2015, 8, e24838. [Google Scholar] [CrossRef] [Green Version]
- Bisht, D.S.; Menon, K.; Singhal, M.K. Comparative Antimicrobial Activity of Essential oils of Cuminum cyminum L. and Foeniculum vulgare Mill. seeds against Salmonella typhimurium and Escherichia coli. J. Essent. Oil Bear. Plants 2014, 17, 617–622. [Google Scholar] [CrossRef]
- Aguirre, A.; Borneo, R.; León, A. Antimicrobial, mechanical and barrier properties of triticale protein films incorporated with oregano essential oil. Food Biosci. 2013, 1, 2–9. [Google Scholar] [CrossRef]
- Bhargava, K.; Conti, D.S.; da Rocha, S.R.; Zhang, Y. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 2015, 47, 69–73. [Google Scholar] [CrossRef]
- Kurekci, C.; Padmanabha, J.; Bishop-Hurley, S.L.; Hassan, E.; Al Jassim, R.A.; McSweeney, C.S. Antimicrobial activity of essential oils and five terpenoid compounds against Campylobacter jejuni in pure and mixed culture experiments. Int. J. Food Microbiol. 2013, 166, 450–457. [Google Scholar] [CrossRef]
- Tserennadmid, R.; Takó, M.; Galgóczy, L.; Papp, T.; Pesti, M.; Vágvölgyi, C.; Almássy, K.; Krisch, J. Anti yeast activities of some essential oils in growth medium, fruit juices and milk. Int. J. Food Microbiol. 2011, 144, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Yuste, J.; Fung, D. Inactivation of Listeria monocytogenes Scott A 49594 in apple juice supplemented with cinnamon. J. Food Prot. 2002, 65, 1663–1666. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Marshall, M.R.; Wei, C.-I. Antibacterial activity of some essential oil components against five foodborne pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Catherine, A.A.; Deepika, H.; Negi, P.S. Antibacterial activity of eugenol and peppermint oil in model food systems. J. Essent. Oil Res. 2012, 24, 481–486. [Google Scholar] [CrossRef]
- Du, E.; Gan, L.; Li, Z.; Wang, W.; Liu, D.; Guo, Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2015, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Seydim, A.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Bucar, F.; Wube, A.; Schmid, M. Natural product isolation—How to get from biological material to pure compounds. Nat. Prod. Rep. 2013, 30, 525–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Padilla, A.; Soto, K.M.; Hernández Iturriaga, M.; Mendoza, S. Food antimicrobials nanocarriers. Sci. World J. 2014, 2014, 837215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazana, M.T.; Codevilla, C.F.; de Menezes, C.R. Nanoencapsulation of Bioactive Compounds: Challenges and Perspectives. Curr. Opin. Food Sci. 2019, 26, 47–56. [Google Scholar] [CrossRef]
- Yada, R.Y.; Buck, N.; Canady, R.; DeMerlis, C.; Duncan, T.; Janer, G.; Juneja, L.; Lin, M.; McClements, D.J.; Noonan, G. Engineered nanoscale food ingredients: Evaluation of current knowledge on material characteristics relevant to uptake from the gastrointestinal tract. Compr. Rev. Food Sci. Food Saf. 2014, 13, 730–744. [Google Scholar] [CrossRef]
- Zhang, Y.; Hsu, B.Y.; Ren, C.; Li, X.; Wang, J. Silica-based nanocapsules: Synthesis, structure control and biomedical applications. Chem. Soc. Rev. 2014, 44, 315–335. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Fery, A.; Weinkamer, R. Mechanical properties of micro-and nanocapsules: Single-capsule measurements. Polymer 2007, 48, 7221–7235. [Google Scholar] [CrossRef] [Green Version]
- Shin, G.H.; Kim, J.T.; Park, H.J. Recent developments in nanoformulations of lipophilic functional foods. Trends Food Sci. Technol. 2015, 46, 144–157. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Paramasivam, N.; Vadivel, V. Essential oil based nanoemulsions to improve the microbial quality of minimally processed fruits and vegetables: A review. Food Res. Int. 2018, 111, 509–523. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef]
- de Souza Simões, L.; Madalena, D.A.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Ramos, O.L. Micro-and nano bio-based delivery systems for food applications: In vitro behavior. Adv. Colloid Interface Sci. 2017, 243, 23–45. [Google Scholar] [CrossRef] [Green Version]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Jia, Z.; Dumont, M.-J.; Orsat, V. Encapsulation of phenolic compounds present in plants using protein matrices. Food Biosci. 2016, 15, 87–104. [Google Scholar] [CrossRef]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Influence of surfactant type and thermal cycling on formation and stability of flavor oil emulsions fabricated by spontaneous emulsification. Food Res. Int. 2016, 89, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, N.; Ranjan, S.; Mundra, S.; Ramalingam, C.; Kumar, A. Fabrication of food grade vitamin E nanoemulsion by low energy approach, characterization and its application. Int. J. Food Prop. 2016, 19, 700–708. [Google Scholar] [CrossRef]
- Dalmolin, L.F.; Khalil, N.M.; Mainardes, R.M. Delivery of vanillin by poly (lactic-acid) nanoparticles: Development, characterization and in vitro evaluation of antioxidant activity. Mater. Sci. Eng. C 2016, 62, 1–8. [Google Scholar] [CrossRef]
- Aditya, N.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef]
- Ho, K.K.; Schroën, K.; San Martín-González, M.F.; Berton-Carabin, C.C. Physicochemical stability of lycopene-loaded emulsions stabilized by plant or dairy proteins. Food Struct. 2017, 12, 34–42. [Google Scholar] [CrossRef]
- Pérez-Masiá, R.; López-Nicolás, R.; Periago, M.J.; Ros, G.; Lagaron, J.M.; López-Rubio, A. Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chem. 2015, 168, 124–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Toole, M.G.; Henderson, R.M.; Soucy, P.A.; Fasciotto, B.H.; Hoblitzell, P.J.; Keynton, R.S.; Ehringer, W.D.; Gobin, A.S. Curcumin encapsulation in submicrometer spray-dried chitosan/Tween 20 particles. Biomacromolecules 2012, 13, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Busch, V.; Pereyra-Gonzalez, A.; Šegatin, N.; Santagapita, P.; Ulrih, N.P.; Buera, M. Propolis encapsulation by spray drying: Characterization and stability. LWT 2017, 75, 227–235. [Google Scholar] [CrossRef]
- Sanchez-Reinoso, Z.; Osorio, C.; Herrera, A. Effect of microencapsulation by spray drying on cocoa aroma compounds and physicochemical characterisation of microencapsulates. Powder Technol. 2017, 318, 110–119. [Google Scholar] [CrossRef]
- Bejrapha, P.; Min, S.-G.; Surassmo, S.; Choi, M.-J. Physicothermal properties of freeze-dried fish oil nanocapsules frozen under different conditions. Dry. Technol. 2010, 28, 481–489. [Google Scholar] [CrossRef]
- Yamashita, C.; Chung, M.M.S.; dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I.G. Microencapsulation of an anthocyanin-rich blackberry (Rubus spp.) by-product extract by freeze-drying. LWT 2017, 84, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Fioramonti, S.A.; Rubiolo, A.C.; Santiago, L.G. Characterisation of freeze-dried flaxseed oil microcapsules obtained by multilayer emulsions. Powder Technol. 2017, 319, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Chew, S.-C.; Nyam, K.-L. Microencapsulation of kenaf seed oil by co-extrusion technology. J. Food Eng. 2016, 175, 43–50. [Google Scholar] [CrossRef]
- Wang, W.; Waterhouse, G.I.; Sun-Waterhouse, D. Co-extrusion encapsulation of canola oil with alginate: Effect of quercetin addition to oil core and pectin addition to alginate shell on oil stability. Food Res. Int. 2013, 54, 837–851. [Google Scholar] [CrossRef]
- Khor, C.M.; Ng, W.K.; Kanaujia, P.; Chan, K.P.; Dong, Y. Hot-melt extrusion microencapsulation of quercetin for taste-masking. J. Microencapsul. 2017, 34, 29–37. [Google Scholar] [CrossRef]
- Penalva, R.; Esparza, I.; Agüeros, M.; Gonzalez-Navarro, C.J.; Gonzalez-Ferrero, C.; Irache, J.M. Casein nanoparticles as carriers for the oral delivery of folic acid. Food Hydrocoll. 2015, 44, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Arroyo-Maya, I.J.; McClements, D.J. Biopolymer nanoparticles as potential delivery systems for anthocyanins: Fabrication and properties. Food Res. Int. 2015, 69, 1–8. [Google Scholar] [CrossRef]
- Yuan, Y.; Kong, Z.-Y.; Sun, Y.-E.; Zeng, Q.-Z.; Yang, X.-Q. Complex coacervation of soy protein with chitosan: Constructing antioxidant microcapsule for algal oil delivery. LWT 2017, 75, 171–179. [Google Scholar] [CrossRef]
- Jain, A.; Thakur, D.; Ghoshal, G.; Katare, O.; Shivhare, U. Characterization of microcapsulated β-carotene formed by complex coacervation using casein and gum tragacanth. Int. J. Biol. Macromol. 2016, 87, 101–113. [Google Scholar] [CrossRef]
- Yao, Z.-C.; Chang, M.-W.; Ahmad, Z.; Li, J.-S. Encapsulation of rose hip seed oil into fibrous zein films for ambient and on demand food preservation via coaxial electrospinning. J. Food Eng. 2016, 191, 115–123. [Google Scholar] [CrossRef]
- Fernandez, A.; Torres-Giner, S.; Lagaron, J.M. Novel route to stabilization of bioactive antioxidants by encapsulation in electrospun fibers of zein prolamine. Food Hydrocoll. 2009, 23, 1427–1432. [Google Scholar] [CrossRef]
- Khoshakhlagh, K.; Koocheki, A.; Mohebbi, M.; Allafchian, A. Development and characterization of electrosprayed Alyssum homolocarpum seed gum nanoparticles for encapsulation of d-limonene. J. Colloid Interface Sci. 2017, 490, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Feng, K.; Wen, P.; Zong, M.-H.; Lou, W.-Y.; Wu, H. Enhancing oxidative stability of encapsulated fish oil by incorporation of ferulic acid into electrospun zein mat. LWT 2017, 84, 82–90. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complementary Altern. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef] [Green Version]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Pan, K.; Chen, H.; Davidson, P.M.; Zhong, Q.J. Thymol nanoencapsulated by sodium caseinate: Physical and antilisterial properties. J. Agric. Food Chem. 2014, 62, 1649–1657. [Google Scholar] [CrossRef]
- Karathanos, V.T.; Mourtzinos, I.; Yannakopoulou, K.; Andrikopoulos, N.K. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chem. 2007, 101, 652–658. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Min, S.-G.; You, S.-K.; Choi, M.-J.; Hong, G.-P.; Chun, J.-Y. Effect of β-cyclodextrin on physical properties of nanocapsules manufactured by emulsion–diffusion method. J. Food Eng. 2013, 119, 588–594. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Min, S.-G.; Bourgeois, S.; Choi, M.-J. Development of a novel nanocapsule formulation by emulsion-diffusion combined with high hydrostatic pressure. J. Microencapsul. 2009, 26, 122–129. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Dahmani, F.Z.; Yin, L.; Zhou, J.; Yao, J. Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials 2014, 35, 7654–7665. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Nanoencapsulation of Zataria multiflora essential oil preparation and characterization with enhanced antifungal activity for controlling Botrytis cinerea, the causal agent of gray mould disease. Innov. Food Sci. Emerg. Technol. 2015, 28, 73–80. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Azizi, M.H. Nanoencapsulation approach to improve antimicrobial and antioxidant activity of thyme essential oil in beef burgers during refrigerated storage. Food Bioprocess Technol. 2016, 9, 1187–1201. [Google Scholar] [CrossRef]
- Fathi, M.; Martin, A.; McClements, D.J. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Chin, S.F.; Pang, S.C.; Tay, S.H. Size controlled synthesis of starch nanoparticles by a simple nanoprecipitation method. Carbohydr. Polym. 2011, 86, 1817–1819. [Google Scholar] [CrossRef]
- Zimet, P.; Rosenberg, D.; Livney, Y.D. Re-assembled casein micelles and casein nanoparticles as nano-vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocoll. 2011, 25, 1270–1276. [Google Scholar] [CrossRef]
- Narayanan, S.; Pavithran, M.; Viswanath, A.; Narayanan, D.; Mohan, C.C.; Manzoor, K.; Menon, D. Sequentially releasing dual-drug-loaded PLGA–casein core/shell nanomedicine: Design, synthesis, biocompatibility and pharmacokinetics. Acta Biomater. 2014, 10, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, R. The interaction between casein micelles and gold nanoparticles. J. Colloid Interface Sci. 2009, 332, 265–269. [Google Scholar] [CrossRef]
- Sangeetha, J.; Philip, J. The interaction, stability and response to an external stimulus of iron oxide nanoparticle-casein nanocomplexes. Colloids Surf. A Physicochem. Eng. Asp. 2012, 406, 52–60. [Google Scholar] [CrossRef]
- Parris, N.; Cooke, P.H.; Hicks, K.B. Encapsulation of essential oils in zein nanospherical particles. J. Agric. Food Chem. 2005, 53, 4788–4792. [Google Scholar] [CrossRef]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Ghasemi, S.; Jafari, S.M.; Assadpour, E.; Khomeiri, M. Production of pectin-whey protein nano-complexes as carriers of orange peel oil. Carbohydr. Polym. 2017, 177, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.H.; Emam-Djomeh, Z.; Sabatino, P.; Van der Meeren, P. Nanocomplexes arising from protein-polysaccharide electrostatic interaction as a promising carrier for nutraceutical compounds. Food Hydrocoll. 2015, 50, 16–26. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, R.; Jafari, S.M.; Jafarpour, A.; Dehnad, D. Loading of fish oil into nanocarriers prepared through gelatin-gum Arabic complexation. Food Hydrocoll. 2019, 90, 291–298. [Google Scholar] [CrossRef]
- Fathi, M.; Mozafari, M.-R.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Solans, C.; Esquena, J.; Forgiarini, A.M.; Uson, N.; Morales, D.; Izquierdo, P.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions: Formation, properties, and applications. Surfactant Sci. Ser. 2003, 109, 525–554. [Google Scholar]
- Henry, J.V.; Fryer, P.J.; Frith, W.J.; Norton, I.T. The influence of phospholipids and food proteins on the size and stability of model sub-micron emulsions. Food Hydrocoll. 2010, 24, 66–71. [Google Scholar] [CrossRef]
- Galanakis, C.M. Food Waste Recovery: Processing Technologies and Industrial Techniques; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Koroleva, M.Y.; Yurtov, E.V. Nanoemulsions: The properties, methods of preparation and promising applications. Russ. Chem. Rev. 2012, 81, 21. [Google Scholar] [CrossRef]
- Donsì, F.; Sessa, M.; Ferrari, G. Effect of emulsifier type and disruption chamber geometry on the fabrication of food nanoemulsions by high pressure homogenization. Ind. Eng. Chem. Res. 2011, 51, 7606–7618. [Google Scholar] [CrossRef]
- Hashtjin, A.M.; Abbasi, S. Nano-emulsification of orange peel essential oil using sonication and native gums. Food Hydrocoll. 2015, 44, 40–48. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Mereddy, R.; Li, L.; Sultanbawa, Y. Formulation, characterisation and antibacterial activity of lemon myrtle and anise myrtle essential oil in water nanoemulsion. Food Chem. 2018, 254, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, H.D.; Cerqueira, M.Â.; Vicente, A.A. Nanoemulsions for food applications: Development and characterization. Food Bioprocess Technol. 2012, 5, 854–867. [Google Scholar] [CrossRef] [Green Version]
- Jun, H.E.; Yao, X.L.; Feng, G.R.; Wang, R.; Yang, B.L. Preparations of the Nano-emulsion Grapefruit Essential Oil and Its Quality Evaluation. Food Res. Dev. 2015, 36, 3–6. [Google Scholar]
- Xue, J.; Davidson, P.M.; Zhong, Q. Antimicrobial activity of thyme oil co-nanoemulsified with sodium caseinate and lecithin. Int. J. Food Microbiol. 2015, 210, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Physical properties and antimicrobial efficacy of thyme oil nanoemulsions: Influence of ripening inhibitors. J. Agric. Food Chem. 2012, 60, 12056–12063. [Google Scholar] [CrossRef] [PubMed]
- da Silva Malheiros, P.; Daroit, D.J.; Brandelli, A. Food applications of liposome-encapsulated antimicrobial peptides. Trends Food Sci. Technol. 2010, 21, 284–292. [Google Scholar] [CrossRef]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.; Huang, C.-M. Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Mozafari, M.R.; Flanagan, J.; Matia-Merino, L.; Awati, A.; Omri, A.; Suntres, Z.E.; Singh, H. Recent trends in the lipid-based nanoencapsulation of antioxidants and their role in foods. J. Sci. Food Agric. 2006, 86, 2038–2045. [Google Scholar] [CrossRef]
- Mozafari, M.R. Nanocarrier Technologies: Frontiers of Nanotherapy; Springer: Berlin, Germany, 2006. [Google Scholar]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Nieto, G.; Huvaere, K.; Skibsted, L.H. Antioxidant activity of rosemary and thyme by-products and synergism with added antioxidant in a liposome system. Eur. Food Res. Technol. 2011, 233, 11–18. [Google Scholar] [CrossRef]
- Bai, C.; Peng, H.; Xiong, H.; Liu, Y.; Zhao, L.; Xiao, X. Carboxymethylchitosan-coated proliposomes containing coix seed oil: Characterisation, stability and in vitro release evaluation. Food Chem. 2011, 129, 1695–1702. [Google Scholar] [CrossRef]
- Detoni, C.B.; de Oliveira, D.M.; Santo, I.E.; Pedro, A.S.; El-Bacha, R.; da Silva Velozo, E.; Ferreira, D.; Sarmento, B.; de Magalhães Cabral-Albuquerque, E.C. Evaluation of thermal-oxidative stability and antiglioma activity of Zanthoxylum tingoassuiba essential oil entrapped into multi-and unilamellar liposomes. J. Liposome Res. 2012, 22, 1–7. [Google Scholar] [CrossRef]
- Guan, P.; Lu, Y.; Qi, J.; Niu, M.; Lian, R.; Wu, W. Solidification of liposomes by freeze-drying: The importance of incorporating gelatin as interior support on enhanced physical stability. Int. J. Pharm. 2015, 478, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Rojas, D.F.; Souza, C.R.; Oliveira, W.P. Encapsulation of eugenol rich clove extract in solid lipid carriers. J. Food Eng. 2014, 127, 34–42. [Google Scholar] [CrossRef]
- Nasseri, M.; Golmohammadzadeh, S.; Arouiee, H.; Jaafari, M.R.; Neamati, H. Antifungal activity of Zataria multiflora essential oil-loaded solid lipid nanoparticles in-vitro condition. Iran. J. Basic Med. Sci. 2016, 19, 1231. [Google Scholar]
- Schwarz, C.; Mehnert, W.; Lucks, J.; Müller, R. Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J. Control. Release 1994, 30, 83–96. [Google Scholar] [CrossRef]
- Jourghanian, P.; Ghaffari, S.; Ardjmand, M.; Haghighat, S.; Mohammadnejad, M. Sustained release curcumin loaded solid lipid nanoparticles. Adv. Pharm. Bull. 2016, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Bhushani, J.A.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Heunis, T.D.J.; Botes, M.; Dicks, L.M.T. Encapsulation of Lactobacillus plantarum 423 and its Bacteriocin in Nanofibers. Probiotics Antimicrob. Proteins 2010, 2, 46–51. [Google Scholar] [CrossRef]
- Ghayempour, S.; Mortazavi, S. Antibacterial activity of peppermint fragrance micro–nanocapsules prepared with a new electrospraying method. J. Essent. Oil Res. 2014, 26, 492–498. [Google Scholar] [CrossRef]
- Jafari, S.M. Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Aguiar, J.J.; Sousa, C.P.; Araruna, M.K.; Silva, M.K.; Portelo, A.C.; Lopes, J.C.; Carvalho, V.R.; Figueredo, F.G.; Bitu, V.C.; Coutinho, H.D. Antibacterial and modifying-antibiotic activities of the essential oils of Ocimum gratissimum L. and Plectranthus amboinicus L. Eur. J. Integr. Med. 2015, 7, 151–156. [Google Scholar] [CrossRef]
- Pesavento, G.; Calonico, C.; Bilia, A.; Barnabei, M.; Calesini, F.; Addona, R.; Mencarelli, L.; Carmagnini, L.; Di Martino, M.; Nostro, A.L. Antibacterial activity of Oregano, Rosmarinus and Thymus essential oils against Staphylococcus aureus and Listeria monocytogenes in beef meatballs. Food Control 2015, 54, 188–199. [Google Scholar] [CrossRef]
- Hamed, S.F.; Sadek, Z.; Edris, A. Antioxidant and antimicrobial activities of clove bud essential oil and eugenol nanoparticles in alcohol-free microemulsion. J. Oleo Sci. 2012, 61, 641–648. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. 2015, 43, 547–556. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; He, Z.; Han, C.; Liu, S.; Li, Y. Influence of surfactant and oil composition on the stability and antibacterial activity of eugenol nanoemulsions. LWT Food Sci. Technol. 2015, 62, 39–47. [Google Scholar] [CrossRef]
- Esmaeili, A.; Asgari, A. In vitro release and biological activities of Carum copticum essential oil (CEO) loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2015, 81, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Vriesekoop, F.; Yuan, Q.; Liang, H. Effects of nisin on the antimicrobial activity of d-limonene and its nanoemulsion. Food Chem. 2014, 150, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. J. Biotechnol. 2010, 67, 1908–1914. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Moghimi, R.; Ghaderi, L.; Rafati, H.; Aliahmadi, A.; McClements, D.J. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016, 194, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Buranasuksombat, U.; Kwon, Y.J.; Turner, M.; Bhandari, B. Influence of emulsion droplet size on antimicrobial properties. Food Sci. Biotechnol. 2011, 20, 793–800. [Google Scholar] [CrossRef]
- Li, K.-K.; Yin, S.-W.; Yin, Y.-C.; Tang, C.-H.; Yang, X.-Q.; Wen, S.-H. Preparation of water-soluble antimicrobial zein nanoparticles by a modified antisolvent approach and their characterization. J. Food Eng. 2013, 119, 343–352. [Google Scholar] [CrossRef]
- Sugumar, S.; Nirmala, J.; Ghosh, V.; Anjali, H.; Mukherjee, A.; Chandrasekaran, N. Bio-based nanoemulsion formulation, characterization and antibacterial activity against food-borne pathogens. J. Basic Microbiol. 2013, 53, 677–685. [Google Scholar] [CrossRef]
- Jemaa, M.B.; Falleh, H.; Serairi, R.; Neves, M.A.; Snoussi, M.; Isoda, H.; Nakajima, M.; Ksouri, R. Nanoencapsulated Thymus capitatus essential oil as natural preservative. Innov. Food Sci. Emerg. Technol. 2018, 45, 92–97. [Google Scholar] [CrossRef]
- Prombutara, P.; Kulwatthanasal, Y.; Supaka, N.; Sramala, I.; Chareonpornwattana, S. Production of nisin-loaded solid lipid nanoparticles for sustained antimicrobial activity. Food Control 2012, 24, 184–190. [Google Scholar] [CrossRef]
- Imran, M.; Revol-Junelles, A.-M.; Paris, C.; Guedon, E.; Linder, M.; Desobry, S. Liposomal nanodelivery systems using soy and marine lecithin to encapsulate food biopreservative nisin. LWT Food Sci. Technol. 2015, 62, 341–349. [Google Scholar] [CrossRef]
- Wen, Z.; You, X.; Jiang, L.; Liu, B.; Zheng, Z.; Pu, Y.; Cheng, B. Liposomal incorporation of rose essential oil by a supercritical process. Flavour Fragr. J. 2011, 26, 27–33. [Google Scholar] [CrossRef]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H. Encapsulation of cardamom essential oil in chitosan nano-composites: In-vitro efficacy on antibiotic-resistant bacterial pathogens and cytotoxicity studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef] [PubMed]
- Herculano, E.D.; de Paula, H.C.; de Figueiredo, E.A.; Dias, F.G.; Pereira, V.D.A. Technology. Physicochemical and antimicrobial properties of nanoencapsulated Eucalyptus staigeriana essential oil. LWT Food Sci. Technol. 2015, 61, 484–491. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.E.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Corona-Rangel, M.L. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. LWT 2017, 77, 15–20. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Y.; Wang, Q. Antioxidant and antimicrobial properties of essential oils encapsulated in zein nanoparticles prepared by liquid–liquid dispersion method. LWT Food Sci. Technol. 2012, 48, 283–290. [Google Scholar] [CrossRef]
- Shah, B.; Davidson, P.M.; Zhong, Q. Nanodispersed eugenol has improved antimicrobial activity against Escherichia coli O157:H7 and Listeria monocytogenes in bovine milk. Int. J. Food Microbiol. 2013, 161, 53–59. [Google Scholar] [CrossRef]
- Donsi, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Neethirajan, S.; Jayas, D.S. Nanotechnology for the food and bioprocessing industries. Food Bioprocess Technol. 2011, 4, 39–47. [Google Scholar] [CrossRef]
- Xiao, D.; Davidson, P.M.; Zhong, Q. Spray-dried zein capsules with coencapsulated nisin and thymol as antimicrobial delivery system for enhanced antilisterial properties. J. Agric. Food Chem. 2011, 59, 7393–7404. [Google Scholar] [CrossRef]
- Gökmen, V.; Mogol, B.A.; Lumaga, R.B.; Fogliano, V.; Kaplun, Z.; Shimoni, E. Development of functional bread containing nanoencapsulated omega-3 fatty acids. J. Food Eng. 2011, 105, 585–591. [Google Scholar] [CrossRef]
- Degnan, A.J.; Luchansky, J.B. Influence of beef tallow and muscle on the antilisterial activity of pediocin AcH and liposome-encapsulated pediocin AcH. J. Food Prot. 1992, 55, 552–554. [Google Scholar] [CrossRef]
- Commission, E.; Regulation, E.C. No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC. Off. J. Eur. Union L. 2004, 338, 4–16. [Google Scholar]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Worley, S.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Pilon, L.; Spricigo, P.C.; Miranda, M.; de Moura, M.R.; Assis, O.B.G.; Mattoso, L.H.C.; Ferreira, M.D. Chitosan nanoparticle coatings reduce microbial growth on fresh-cut apples while not affecting quality attributes. Int. J. Food Sci. Technol. 2015, 50, 440–448. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Sessa, M.; Ferrari, G.; Donsì, F. Novel edible coating containing essential oil nanoemulsions to prolong the shelf life of vegetable products. Chem. Eng. Trans. 2015, 43, 55–60. [Google Scholar]
- Dudefoi, W.; Terrisse, H.; Richard-Plouet, M.; Gautron, E.; Popa, F.; Humbert, B.; Ropers, M.H. Criteria to define a more relevant reference sample of titanium dioxide in the context of food: A multiscale approach. Food Addit. Contam. Part A 2017, 34, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Dorier, M.; Béal, D.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Carriere, M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology 2017, 11, 751–761. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Deng, H.; Hwang, H. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef] [Green Version]



| Chemical Compound | Application Range | Mechanism of Action or Application Effect |
|---|---|---|
| Benzoic acid and benzoates | Particularly used in acidic foods | Destruction of bacterial cell membrane, inhibition of cell membrane absorption of amino acid and respiratory enzymes, blocking condensation of acetyl-CoA, etc. |
| Sorbic acid and sorbates | Dried meats and acidic foods | Binding to biological enzymes and inhibiting enzyme activity. |
| Paraben (Hydroxybenzoates) | Bakery products, soft drinks, cosmetic | Destruction of cell membranes, denaturation of intracellular proteins, inhibition of the activity of respiratory enzymes and electron transport enzymes. |
| Propionic acid and propionates | Baked products such as pastries, bread, etc. | Binding to biological enzymes and inhibiting their biological activity. They have a good inhibitory effect on molds and Gram-negative bacteria under acidic pH, especially to prevent the production of aflatoxins. |
| Dimethyl dicarbonate | Beverages | Passing through the cell membrane and interacting with enzymes in the microbial cells to block intracellular metabolism. |
| Sulfur dioxide and sulfites | Dried fruits, wine making | Hydrogen ions generated by the decomposition of sulfite can cause damage of bacterial surface proteins and nucleic acids to kill microorganisms. |
| Antimicrobial Compound | Main Source | Target Microorganism | Reference | ||
|---|---|---|---|---|---|
| Gram-Positive Bacteria | Gram-Negative Bacteria | Fungi | |||
| Plant Origin | |||||
| Essential oils | Thyme | Staphylococcus aureus Listeria monocytogenes | Shigella sonnei Shigella flexneri Escherichia coli O157:H7 E. coli Salmonella enteritidis Salmonella typhimurium | Botrytis cinerea Candida albicans Penicillium digitatum | [9,10] |
| Clove | S. aureus Bacillus cereus L. monocytogenes | E. coli S. enteritidis | C. albicans Trichophyton mentagrophytes | [10,11] | |
| Cinnamon | Lactobacillus delbrueckii L. monocytogenes S. aureus | E. coli | Saccharomyces cerevisiae | [12] | |
| Oregano | L. monocytogenes Bacillus subtilis S. aureus | E. coli Pseudomonas aeruginosa Salmonella enterica S. typhimurium | C. albicans P. digitatum S. cerevisiae | [10,13] | |
| Plant Extracts | Grape seed | L. monocytogenes | S. typhimurium E. coli O157:H7 | Candida maltosa | [14,15] |
| Olive leaves extracts | B. cereus L. monocytogenes | E. coli E. coli O157:H7 | Candida oleophila | [16] | |
| Rosemary | B. cereus L. monocytogenes S. aureus | E. coli S. enteritidis S. typhimurium | Aspergillus S. cerevisiae C. albicans | [17] | |
| Animal origin | |||||
| Lysozyme | Chicken eggs Vegetables Insects | Bacillus Clostridium L. monocytogenes L. spp. | Weak inhibitory effects | Aspergillus, Candida, Fusarium, Sporothrix, Paecilomyces, Penicillium, Saccharomyces. | [18] |
| Lactoferrin | Milk | B. cereus Bacillus stearothermophilus L. monocytogenes | E. coli S. enteritidis. Klebsiella spp. S. enteritidis | No effect | [18] |
| Chitosan | Shellfish | S. aureus L. monocytogenes B. cereus | S. typhimurium Yersinia enterocolitica | Aspergillus flavus S. cerevisiae Zygosaccharomyces bailii | [19] |
| Microbial origin | |||||
| Natamycin | Streptomyces natalensis | No effect | No effect | Penicillium candidum Aspergillus. flavus S. cerevisiae Byssochlamys nivea Hemerocallis fulva Zygosaccharomyces bailii | [20] |
| Nisin | Lactococcus lactis | C. botulinum (spores) L. monocytogenes S. aureus Lactobacillus. plantarum | No effect | No effect | [21] |
| Polylysine | Streptomyces. | S. aureus Lactococcus lactis B. subtilis | E. coli S. typhymurium | Aspergillus niger T. mentagrophytes | [22] |
| Essential Oils | Bacteriostatic Mechanism | Antimicrobial Activity |
|---|---|---|
| Cumin | Cuminaldehyde and cuminalcohol can destroy cell membrane | Antimicrobial activity against Aspergillus flavus (MIC: 650 μg/mL) [29]; cumin EOs (≥30 μL/100 mL) combined with nisin (≥0.5 μg/mL) can inhibit S. typhimurium; cumin EOs (≥15 μL/100 mL) in combination with nisin (≥0.5 μg/mL) inhibited S. aureus [30]; inhibition of S. typhimurium and E. coli (MIC: 0.125% and 0.250%, v/v) [31]. |
| Oregano | Caused by carvacrol and thymol | 2% (w/v) oregano EOs inhibited S. aureus, E. coli, and Pseudomonas aeruginosa (inhibition zone diameters: 342.36, 21.53, and 9.70 mm, respectively) [32]; antimicrobial activity against L. monocytogenes, S. typhimurium, E. coli, etc. [33]. |
| Lemon | Limonene inhibits cellular respiration | Antimicrobial activity against C. jejuni C338, C. coli, and B. cereus (inhibition zone diameter: 28.3, 35.3, and 23.7 mm, respectively) [34]; inhibition of S. cerevisiae MB-21, fission yeast MB-89, Geotrichum candidum MB-102, and Pichia pastoris MB-196 (MIC: 0.245~0.51, 0.06~0.25, 0.5~1.0, and 0.5~0.73 μL/mL, respectively) [35]. |
| Cinnamon | Cinnamaldehyde can act on enzymes and cell wall. It can alter cell membrane permeability, inhibit ATPase activity and amino acid synthesis, and deplete proton potential energy | 0.1–0.3% (w/v) cinnamon EO inhibited L. monocytogenes (6.0 log CFU/mL) [36]; 2.0% (w/v) cinnamon EO inhibited E. coli O157:H7 (2.0 log CFU/mL) [37]. |
| Marjoram | Ethanol steroids (protein denaturation and dehydration), terpin-4-opening (destruction of cell membrane results, leakage of intracellular substances) [38] | Antimicrobial activity against S. cerevisiae, Fission yeast, Geotrichum candidum, and Pichia pastoris (MIC: 0.5~1.0, 0.0625, 0.5 and 0.5 μL/mL, respectively) [35]. |
| Peppermint | Caused by menthone, menthol, menthyl acetate, limonene and β-pinene | Gaseous peppermint EOs inhibits E. coli O157:H7 (MIC: 0.625 μL/mL); inhibition of S. aureus, Bacillus cereu, E. coli MTCC108, and Yersinia colitis (0.10%: 1.3–4.8 log CFU/ mL, 0.20%: 1.8–5.7 log CFU/mL, 0.25%: 0.4–2.6 log CFU/mL and 0.15%: 1.1–5.2 log CFU/mL, respectively) [39]. |
| Clove | Eugenol can destroy the structure of cells | Antimicrobial activity against S. mutans (MIC: 1000 μg/mL) [40], L. monocytogenes (1.6–4.3 log CFU/mL), L. sinensis (0.2–0.8 log CFU/mL) [37]. |
| Garlic | Organic sulfur compounds destroy cell membrane and intracellular macromolecular structure | Inhibition of E. coli O157:H7, S. aureus, S. enteritidis, L. monocytogenes, and L. plantarum (inhibition zone diameter: 9.3–11.36, 11.36–13.45, 8.43–10.48, 9.89–11.96, and 6.13–9.21 mm, respectively)) [41]. |
| Clary sage | Ethanol steroids (protein denaturation and dehydration), linalool (destroying cell membrane structure, inhibiting Gram-negative bacteria), caryophyllene (inhibiting Gram-positive bacteria) [35] | Antimicrobial activity against S. cerevisiae, Fission yeast, Geotrichum candidum, and Pichia pastoris (MIC: 0.5–1.0, 0.375–0.875, 1.0, and 0.5–1.0 μL/mL, respectively) [35]; 20,000 μg/mL EOs prolonged the lag phase of E. coli (3.20~6.40 h) and inhibited L. monocytogenes [42]. |
| Rosemary | Camphor and eucalyptus oil have an oxidizing effect and enhance the antibacterial activity of terpenoids | Antimicrobial activity against S. aureus, L. monocytogenes, E. coli, and Vibrio cholerae [42]. |
| Encapsulation Method | Description | Nanoencapsulation | Microencapsulation |
|---|---|---|---|
| Emulsification | Emulsification is a process of mixing two immiscible solvents, and the resulting product is referred to as an emulsion. It can be divided into top-down approaches (high-shear stirring, high pressure homogenization, microfluidization, and ultrasonication) and bottom-up (phase inversion temperature, emulsion phase inversion, and spontaneous nanoemulsification) approaches. | Vitamin E encapsulated by Tween-80 [57]; vanillin encapsulated in poly (lactic-acid) nanoparticles [58] | Curcumin encapsulated by Tween 80 and polyglycerol polyricinoleate [59]; lycopene encapsulated in plant (soy and pea) or dairy (whey and sodium caseinate) proteins [60] |
| Spray drying | The basic theory of spray-drying is to feed the liquid into a drying chamber in the form of tiny droplets containing biologically active compounds, supplying hot air to the drying chamber, forming microcapsules in the drying chamber, and recovering them through a cyclone. | Folic acid encapsulated by whey proteins and resistant starch [61]; curcumin encapsulated by chitosan/Tween 20 [62] | Propolis extracts bioactive compounds encapsulated by maltodextrin matrices with or without nature gums [63]; cocoa volatile compounds encapsulated by maltodextrins and modified starch [64] |
| Freeze drying | The basic principle of freeze-drying is to freeze water contained in a solution or suspension and then evaporate the water molecules from the solution or suspension. | Fish oil encapsulated by poly-e-caprolactone and Pluronic F68 [65] | Blackberry by-product extract encapsulated by maltodextrins [66]; flaxseed oil encapsulated by sodium alginate, whey protein, and maltodextrin [67] |
| Extrusion | Extrusion technique involves the injection of a bio-based solution into another solution to promote gelation and produce a hard and dense encapsulation system. | Seed oils encapsulated by sodium alginate and high methoxyl pectin [68] | Canola oil encapsulated by alginate and high methoxyl pectin [69]; quercetin encapsulated by carnauba wax, shellac, or zein [70] |
| Complex coacervation | Coacervation is a well-known implemented technique to produce micro- and nanosystems. The basic mechanism is the formation of an emulsion by electrostatic attraction between oppositely charged molecules to produce the encapsulating structure. | Folic acid encapsulated by casein nanoparticles [71]; anthocyanins encapsulated by whey protein isolate and beet pectin [72] | Algal oil encapsulated by soy protein isolate and chitosan [73]; β-carotene encapsulated by casein and gum tragacanth [74] |
| Electro-spinning and electro-spraying | They are two modes of electrohydrodynamic processes that use a charged jet to rotate or spray a polymer solution to produce fibers or particles. | Rose hip seed oil encapsulated by zein prolamine fiber [75]; β-carotene encapsulated by zein prolamine fiber [76] | d-limonene encapsulated by seed gum and tween 20 [77]; fish oil encapsulated by a composite zein fiber [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, W.; Badri, W.; Dumas, E.; Ghnimi, S.; Elaissari, A.; Saurel, R.; Gharsallaoui, A. Nanoencapsulation of Essential Oils as Natural Food Antimicrobial Agents: An Overview. Appl. Sci. 2021, 11, 5778. https://doi.org/10.3390/app11135778
Liao W, Badri W, Dumas E, Ghnimi S, Elaissari A, Saurel R, Gharsallaoui A. Nanoencapsulation of Essential Oils as Natural Food Antimicrobial Agents: An Overview. Applied Sciences. 2021; 11(13):5778. https://doi.org/10.3390/app11135778
Chicago/Turabian StyleLiao, Wei, Waisudin Badri, Emilie Dumas, Sami Ghnimi, Abdelhamid Elaissari, Rémi Saurel, and Adem Gharsallaoui. 2021. "Nanoencapsulation of Essential Oils as Natural Food Antimicrobial Agents: An Overview" Applied Sciences 11, no. 13: 5778. https://doi.org/10.3390/app11135778






