Thermal and Kinetic Properties of Brazilian Coffea Arabica Beans
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Fat Extraction
2.4. Modulated Differential Scanning Calorimetry
2.5. Thermogravimetric Analysis
2.6. DSC Measurements
2.7. GC-FID Analysis of Fatty Acids Profile
2.8. Statistical Analysis
3. Results and Discussion
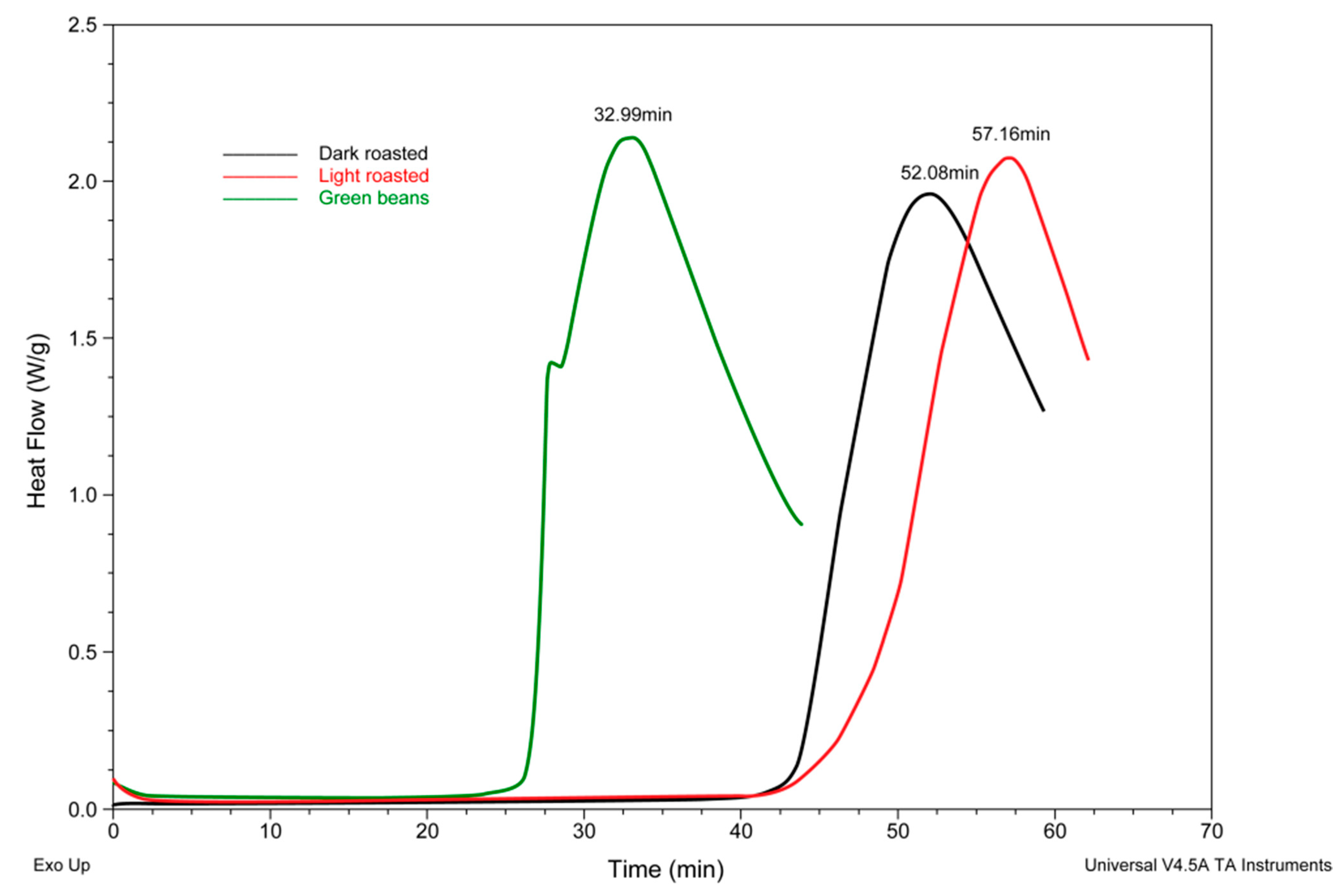
3.1. Determination of Glass Transition Temperature
3.2. Thermogravimetric Analysis
3.3. Oxidative Stability and Thermokinetic Parameters
3.4. Analysis of Fatty Acids Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Hečimović, I.; Belščak-Cvitanović, A.; Horžić, D.; Komes, D. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 2011, 129, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Fadai, N.T.; Melrose, J.; Please, C.P.; Schulman, A.; Van Gorder, R.A. A heat and mass transfer study of coffee bean roasting. Int. J. Heat Mass Transf. 2017, 104, 787–799. [Google Scholar] [CrossRef]
- Gloess, A.N.; Vietri, A.; Wieland, F.; Smrke, S.; Schönbächler, B.; López, J.A.S.; Petrozzi, S.; Bongers, S.; Koziorowski, T.; Yeretzian, C. Evidence of different flavour formation dynamics by roasting coffee from different origins: On-line analysis with PTR-ToF-MS. Int. J. Mass Spectrom. 2014, 365–366, 324–337. [Google Scholar] [CrossRef]
- Wang, H.Y.; Qian, H.; Yao, W.R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Trovato, V.; Curti, D.; Fischer, M. Effect of roasting on degradation and structural features of polysaccharides in Arabica coffee beans. Carbohydr. Res. 2002, 337, 421–431. [Google Scholar] [CrossRef]
- Liang, N.; Xue, W.; Kennepohl, P.; Kitts, D.D. Interactions between major chlorogenic acid isomers and chemical changes in coffee brew that affect antioxidant activities. Food Chem. 2016, 213, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; de Toledo Benassi, M. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Franca, A.S.; Mendonça, J.C.F.; Barros-Júnior, M.C. Proximate composition and fatty acids profile of green and roasted defective coffee beans. LWT Food Sci. Technol. 2006, 39, 235–239. [Google Scholar] [CrossRef]
- Speer, K.; Kölling-Speer, I. The lipid fraction of the coffee bean. Braz. J. Plant Physiol. 2006, 18, 201–216. [Google Scholar] [CrossRef]
- Ferrari, M.; Ravera, F.; De Angelis, E.; Liverani, F.S.; Navarini, L. Interfacial properties of coffee oils. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 365, 79–82. [Google Scholar] [CrossRef]
- Oliveira, A.L.; de Cruz, P.M.; Eberlin, M.N.; Cabral, F.A. Brazilian roasted coffee oil obtained by mechanical expelling: Compositional analysis by GC-MS. Ciência Tecnol. Aliment. 2005, 25, 677–682. [Google Scholar] [CrossRef]
- Gabbott, P. A practical introduction to Differential Scanning Calorimetry. In Principles and Applications of Thermal Analysis, 1st ed.; Gabbott, P., Ed.; Blackwell Publishing: Oxford, UK, 2008; pp. 1–49. [Google Scholar]
- Materazzi, S.; Gullifa, G.; Fabiano, M.A.; Frati, P.; Santurro, A.; Scopetti, M.; Fineschi, V.; Risoluti, R. New frontiers in thermal analysis: A TG/Chemometrics approach for postmortem interval estimation in vitreous humor. J. Therm. Anal. Calorim. 2017, 130, 549–657. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Górska, A.; Wirkowska-Wojdyła, M.; Bryś, J.; Dolatowska-Żebrowska, K.; Shamilowa, M.; Ratusz, K. Thermogravimetric characterization of dark and milk chocolates at different processing stages. J. Therm. Anal. Calorim. 2018, 134, 623–631. [Google Scholar] [CrossRef]
- Gill, P.S.; Sauerbrunn, S.R.; Reading, M. Modulated differential scanning calorimetry. J. Therm. Anal. 1993, 40, 931–939. [Google Scholar] [CrossRef]
- Zeng, J.-L.; Yu, S.-B.; Cao, Z.; Yang, D.-W.; Sun, L.-X.; Zhang, L.; Zhang, X.-F. Synthesize, Crystal structure, heat capacities and thermodynamic properties of a potential enantioselective catalyst. J. Therm. Anal. Calorim. 2011, 105, 961–968. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Yu, W. Evaluating the thermal stability of high performance fibers by TGA. J. Appl. Polym. Sci. 2006, 99, 937–944. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Ostrowska-Ligeza, E.; Gondek, E. Moisture sorption characteristics and glass transition temperature of apple puree powder. Int. J. Food. Sci. Technol. 2010, 45, 2515–2523. [Google Scholar] [CrossRef]
- Rahman, M.S.; Al-Marhubi, I.M.; Al-Mahrouqi, A. Measurement of glass transition temperature by mechanical (DMTA), thermal (DSC and MDSC), water diffusion and density methods: A comparison study. Chem. Phys. Lett. 2007, 440, 372–377. [Google Scholar] [CrossRef]
- Materazzi, S.; De Angelis Curtis, S.; Vecchio Ciprioti, S.; Risoluti, R.; Finamore, J. Thermogravimetric characterization of dark chocolate. J. Therm. Anal. Calorim. 2014, 116, 93–98. [Google Scholar] [CrossRef]
- Albis, A.; Ortiz, E.; Suárez, A.; Piñeres, I. TG/MS study of the thermal devolatization of Copoazú peels (Theobroma grandiflorum). J. Therm. Anal. Calorim. 2014, 115, 275–283. [Google Scholar] [CrossRef]
- Górska, A.; Ostrowska-Ligęza, E.; Wirkowska, M.; Bryś, J. Ocena parametrów utleniania kwasu linolowego z wykorzystaniem różnicowej kalorymetrii skaningowej. Zywn.-Nauka Technol. Jakosc 2011, 2, 106–114. [Google Scholar]
- Perdana, B.M.; Manihuruk, R.; Ashyar, R. Evaluation of the effect of roasting process on the energy transition and the crystalline structures of Arabica, Robusta, and Liberica coffee from Jambi Indonesia. IOP Conf. Ser. Mater. Sci. Eng. 2018, 345, 012021. [Google Scholar] [CrossRef]
- Rivera, W.; Velasco, X.; Gálvez, C.; Rincón, C.; Rosales, A.; Arango, P. Effect of the roasting process on glass transition and phase transition of Colombian Arabic coffee beans. Procedia Food Sci. 2011, 1, 385–390. [Google Scholar] [CrossRef]
- Frascareli, E.C.; Silva, V.M.; Tonon, R.V.; Hubinger, M.D. Determination of critical storage conditions of coffee oil microcapsules by coupling water sorption isotherms and glass transition temperature. Int. J. Food Sci. Technol. 2012, 47, 1044–1054. [Google Scholar] [CrossRef]
- Iaccheri, E.; Laghi, L.; Cevoli, C.; Berardinelli, A.; Ragni, L.; Romani, S.; Rocculi, P. Different analytical approaches for the study of water features in green and roasted coffee beans. J. Food Eng. 2015, 146, 28–35. [Google Scholar] [CrossRef]
- Pittia, P.; Nicoli, M.C.; Sacchetti, G. Effect of moisture and water activity on textural properties of raw and roasted coffee beans. J. Texture Stud. 2007, 38, 116–134. [Google Scholar] [CrossRef]
- Lapuerta, M.; Hernández, J.J.; Rodríguez, J. Comparison between the kinetics of devolatilisation of forestry and agricultural wastes from the middle-south regions of Spain. Biomass Bioenergy 2007, 31, 13–19. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Boonamnuayvitaya, V.; Sae-ung, S.; Tanthapanichakoon, W. Preparation of activated carbons from coffee residue for the adsorption of formaldehyde. Sep. Purif. Technol. 2005, 42, 159–168. [Google Scholar] [CrossRef]
- Chaiklangmuang, S.; Kurosawa, K.; Li, L.; Morishita, K.; Takarada, T. Thermal degradation behavior of coffee residue in comparison with biomasses and its product yields from gasification. J. Energy Inst. 2015, 88, 323–331. [Google Scholar] [CrossRef]
- Anese, M.; De Pilli, T.; Massini, R.; Lerici, C.R. Oxidative stability of the lipid fraction in roasted coffee. Ital. J. Food. Sci. 2000, 12, 457–462. [Google Scholar]
- Cämmerer, B.; Kroh, L.W. Antioxidant activity of coffee brews. Eur. Food Res. Technol. 2006, 223, 469–474. [Google Scholar] [CrossRef]
- Anese, M.; Nicoli, M.C. Antioxidant properties of ready-to-drink coffee brews. J. Agric. Food Chem. 2003, 51, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.; Wall, L. A quick direct method for determination of activation energy from thermo-gravimetric data. J. Polym. Sci. Pol. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetic analysis of derivative curves in thermal analysis. J. Therm. Anal. 1970, 2, 301–324. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Kowalski, B.; Wirkowska, M. Termokinetyczna analiza tłuszczu z kukurydzy z wykorzystaniem różnicowej kalorymetrii skaningowej. Zywn. Nauka Technol. Jakosc 2009, 1, 128–139. [Google Scholar]
- Litwinienko, G.; Daniluk, A.; Kasprzycka-Guttman, T. Study on autoxidation kinetics of fats by differential scanning calorimetry. 1. Saturated C12-C18 fatty acids and their esters. Am. Chem. Soc. 2000, 39, 7–12. [Google Scholar]
- Romano, R.; Santini, A.; Le Grottaglie, L.; Manzo, N.; Visconti, A.; Ritieni, A. Identification markers based on fatty acid composition to differentiate between roasted Arabica and Canephora (Robusta) coffee varieties in mixtures. J. Food Compos. Anal. 2014, 35, 1–9. [Google Scholar] [CrossRef]
- Feldman, E.B. The scientific evidence for a beneficial health relationship between walnuts and coronary heart disease. J. Nutr. 2002, 132, 1062S–1101S. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.J.; Pablos, F.; González, A.G.; Valdenebro, M.S.; León-Camacho, M. Fatty acid profiles as discriminant parameters for coffee varieties differentiation. Talanta 2001, 54, 291–297. [Google Scholar] [CrossRef]


| Coffee Sample | Tg onset (°C) | Tg midpoint (°C) | Tg endpoint (°C) | Water Activity aw |
|---|---|---|---|---|
| Green | −19.99 ± 1.17 a | −11.24 ± 0.84 a | −1.69 ± 0.24 a | 0.486 ± 0.007 c |
| Light | −19.89 ± 0.21 a | −11.93 ± 0.16 a | −2.49 ± 0.05 b | 0.399 ± 0.002 b |
| Dark | −19.81 ± 0.28 a | −11.87 ± 0.13 a | −2.48 ± 0.01 b | 0.388 ± 0.003 a |
| Heating Rate (°C min−1) | Ton (°C) | ||
|---|---|---|---|
| Green | Light Roasted | Dark Roasted | |
| 2.5 | 154.57 ± 0.86 | 168.8 ± 0.79 | 164.3 ± 0.79 |
| 4.0 | 161.74 ± 0.94 | 176.22 ± 1.33 | 170.86 ± 1.13 |
| 5.0 | 165.01 ± 0.76 | 180.81 ± 1.38 | 174.28 ± 0.96 |
| 7.5 | 172.56 ± 0.71 | 183.65 ± 0.69 | 180.91 ± 1.06 |
| 10.0 | 175.97 ± 1.23 | 191.61 ± 1.35 | 181.22 ± 1.19 |
| 12.5 | 181.07 ± 0.98 | 182.5 ± 0.98 | 190.31 ± 1.16 |
| Heating Rate (°C min−1) | Tmax (°C) | ||
|---|---|---|---|
| Green | Light Roasted | Dark Roasted | |
| 2.5 | 168.3 ± 0.90 | 180.28 ± 0.99 | 177.08 ± 0.66 |
| 4.0 | 181.95 ± 0.36 | 191.79 ± 0.87 | 188.55 ± 0.55 |
| 5.0 | 192.9 ± 0.45 | 193.25 ± 0.40 | 228.55 ± 1.32 |
| 7.5 | 228.5 ± 0.90 | 252.8 ± 0.56 | 241.72 ± 0.46 |
| 10.0 | 246.84 ± 1.73 | 252.19 ± 1.31 | 260.62 ± 1.53 |
| 12.5 | 253.6 ± 1.84 | 272.71 ± 1.64 | 287.91 ± 0.66 |
| Parameter | a | b | R2 | Ea | Z |
|---|---|---|---|---|---|
| Green coffee | |||||
| Value for Ton | −5169.5 | 12.5 | 0.99 | 94.11 | 5.63∙1010 |
| Value for Tmax | −1687.6 | 4.3 | 0.97 | 30.72 | 1.047∙103 |
| Light roasted | |||||
| Value for Ton | −5471.6 | 12.8 | 0.99 | 99.6 | 1.03∙1011 |
| Value for Tmax | −1797.6 | 4.3 | 0.99 | 32.7 | 1.210∙103 |
| Dark roasted | |||||
| Value for Ton | −5779.8 | 13.6 | 0.96 | 105.2 | 7.01∙1011 |
| Value for Tmax | −1589.8 | 3.9 | 0.99 | 28.9 | 5.36∙102 |
| Common Name | Lipid Number | % of Fatty Acids Peaks Area | ||
|---|---|---|---|---|
| Green Coffee | Light Roast | Dark Roast | ||
| Palmitic acid | 16:0 | 31.64 ± 0.14 a | 31.18 ± 0.18 a | 30.88 ± 0.22 a |
| Stearic acid | 18:0 | 8.71 ± 0.13 a | 8.72 ± 0.02 a | 8.8 ± 0.04 a |
| Oleic acid | 18:1(9) | 9.79 ± 0.10 a | 10.49 ± 0.01 b | 10.69 ± 0.01 b |
| Linoleic acid | 18:2(9,12) | 41.61 ± 0.17 a | 41.71 ± 0.02 a | 41.94 ± 0.07 a |
| α-Linolenic acid | 18:3(9,12,15) | 1.54 ± 0.04 a | 1.69 ± 0.02 b | 1.71 ± 0.01 b |
| Arachidic acid | 20:0 | 3.38 ± 0.03 a | 3.31 ± 0.07 a | 3.24 ± 0.05 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzezińska, R.; Górska, A.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Bryś, J. Thermal and Kinetic Properties of Brazilian Coffea Arabica Beans. Appl. Sci. 2021, 11, 6324. https://doi.org/10.3390/app11146324
Brzezińska R, Górska A, Wirkowska-Wojdyła M, Ostrowska-Ligęza E, Bryś J. Thermal and Kinetic Properties of Brazilian Coffea Arabica Beans. Applied Sciences. 2021; 11(14):6324. https://doi.org/10.3390/app11146324
Chicago/Turabian StyleBrzezińska, Rita, Agata Górska, Magdalena Wirkowska-Wojdyła, Ewa Ostrowska-Ligęza, and Joanna Bryś. 2021. "Thermal and Kinetic Properties of Brazilian Coffea Arabica Beans" Applied Sciences 11, no. 14: 6324. https://doi.org/10.3390/app11146324
APA StyleBrzezińska, R., Górska, A., Wirkowska-Wojdyła, M., Ostrowska-Ligęza, E., & Bryś, J. (2021). Thermal and Kinetic Properties of Brazilian Coffea Arabica Beans. Applied Sciences, 11(14), 6324. https://doi.org/10.3390/app11146324











