Performance Evaluation of an Inflatable Solar Dryer for Maize and the Effect on Product Quality Compared with Direct Sun Drying
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Inflatable Solar Dryer (ISD)
2.2. Drying Experiments
2.2.1. Experimental Site and Raw Material
2.2.2. Performance Evaluation
2.2.3. Absolute Humidity Calculation
2.2.4. Moisture Content Analysis
2.3. Quality Analysis
2.3.1. Yeasts and Moulds Enumeration
2.3.2. Total Aflatoxins Analysis
2.3.3. Colour Measurement
2.4. Statistical Data Analysis
3. Results
3.1. Performance Evaluation
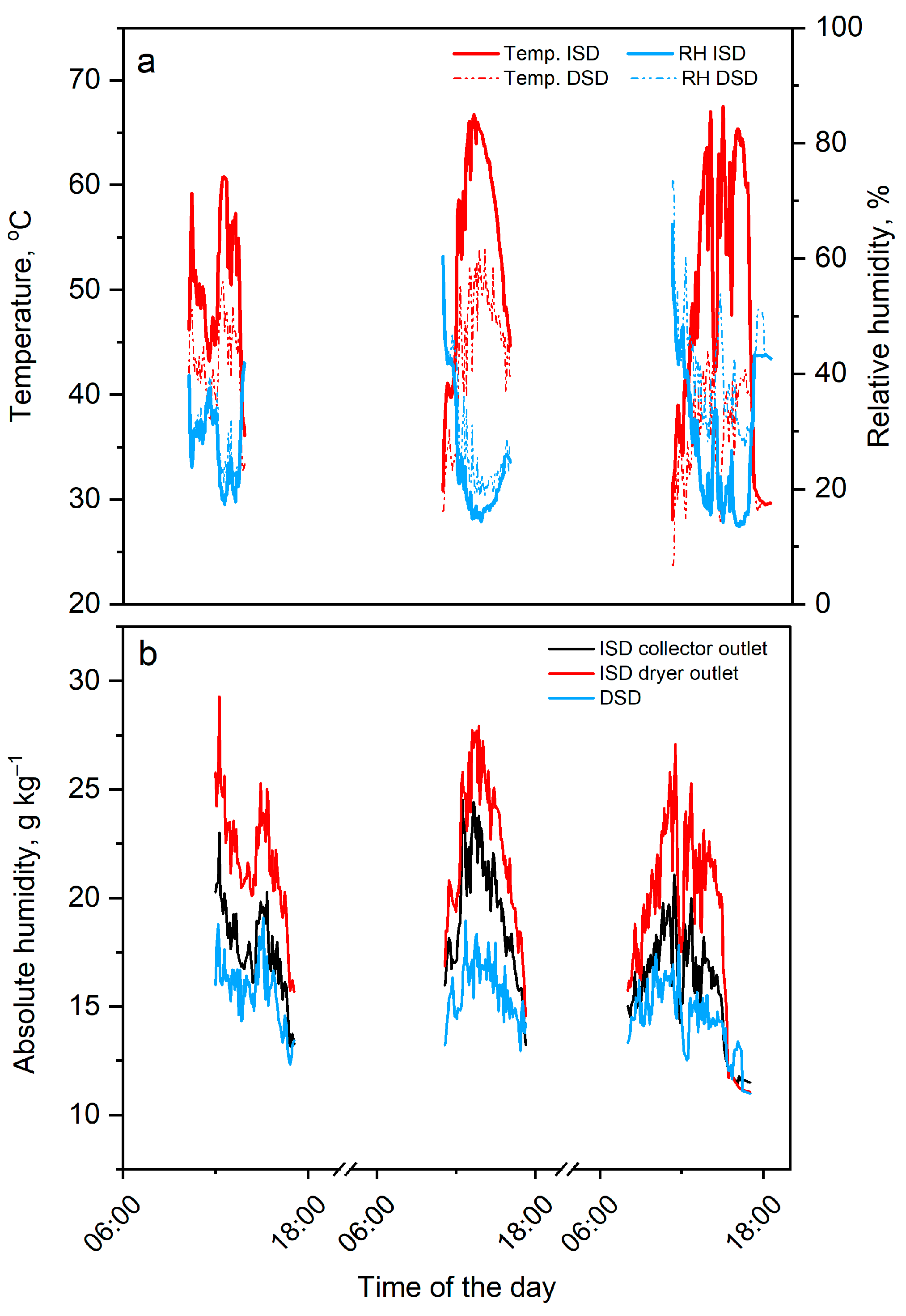
3.1.1. Temperature, Relative Humidity, and Water Content in Air
3.1.2. Drying Curves
3.2. Quality of the Dried Maize
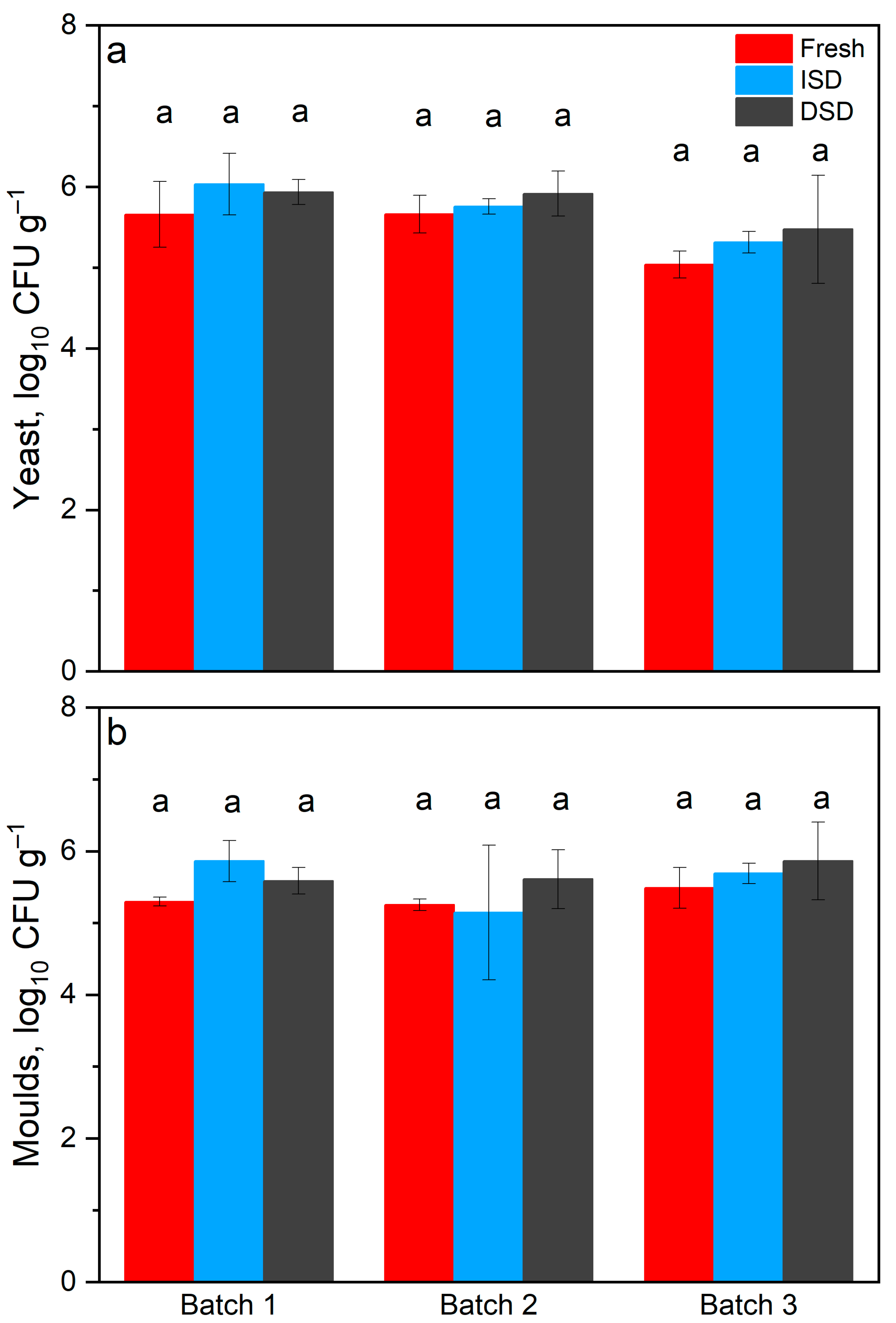
3.2.1. Yeasts and Moulds
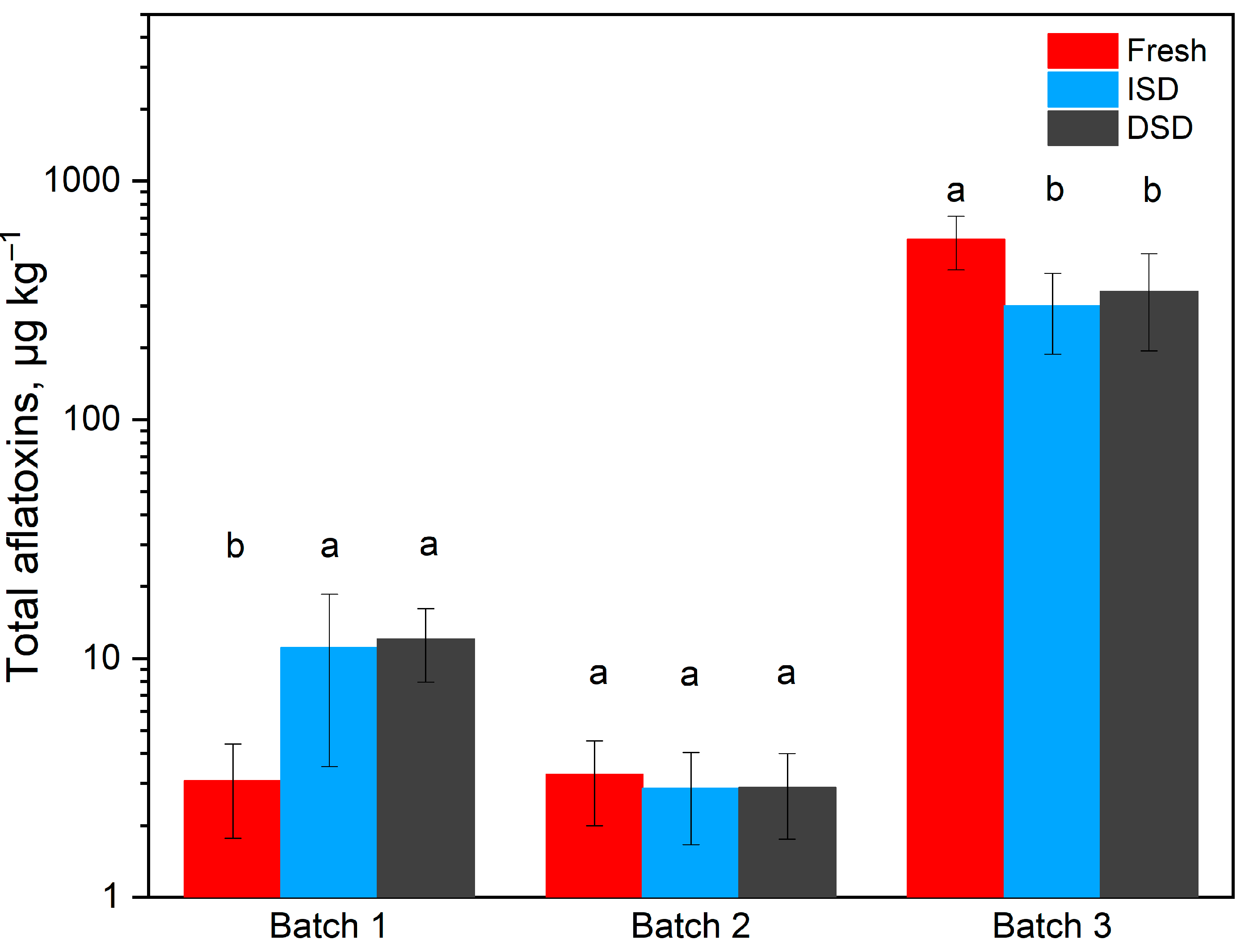
3.2.2. Total Aflatoxin Content
3.2.3. Colour of Dried Maize
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Uganda-Maize Production Quantity 2018. Available online: http://www.fao.org/faostat/en/#country/226 (accessed on 20 September 2019).
- Goletti, F.; Samman, E. Post-Harvest Systems in World Agriculture. In Crop Post-Harvest: Science and Technology, Volume 1: Principles and Practice; Golob, P., Farrell, G., Orchard, J.E., Eds.; Blackwell Science Ltd.: Oxford, UK, 2002; Volume 1. [Google Scholar]
- Bradford, K.J.; Dahal, P.; Van Asbrouck, J.; Kunusoth, K.; Bello, P.; Thompson, J.; Wu, F. The dry chain: Reducing postharvest losses and improving food safety in humid climates. Trends Food Sci. Technol. 2018, 71, 84–93. [Google Scholar] [CrossRef]
- Lukwago, F.B.; Mukisa, I.M.; Atukwase, A.; Kaaya, A.N.; Tumwebaze, S. Mycotoxins contamination in foods consumed in Uganda: A 12-year review (2006–18). Sci. Afr. 2019, 3, e00054. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D.M.; Payne, G.A. Factors Affecting Aspergillus flavus Group Infection and Aflatoxin Contamination of Crops. In The Toxicology of Aflatoxins; Academic Press: Cambridge, MA, USA, 1994; pp. 309–325. [Google Scholar] [CrossRef]
- Kaaya, A.N.; Kyamuhangire, W. The effect of storage time and agroecological zone on mould incidence and aflatoxin contamination of maize from traders in Uganda. Int. J. Food Microbiol. 2006, 110, 217–223. [Google Scholar] [CrossRef]
- EAC. Maize Grains Specification; EAS: Assam, India, 2013.
- European Commission. Commision Regulation (EC) Setting Maximum Levels for Certain Contaminants in Foodstuffs; 364/365-324; European Commission: Brussels, Belgium, 2006. [Google Scholar]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Bandyopadhyay, R.; Müller, J.; Vanlauwe, B. Mycotoxins in Sub-Saharan Africa: Present situation, socio-economic impact, awareness, and outlook. Food Control 2017, 72, 110–122. [Google Scholar] [CrossRef]
- Asiki, G.; Seeley, J.; Srey, C.; Baisley, K.; Lightfoot, T.; Archileo, K.; Agol, D.; Abaasa, A.; Wakeham, K.; Routledge, M.; et al. A pilot study to evaluate aflatoxin exposure in a rural Ugandan population. Trop. Med. Int. Health 2014, 19, 592–599. [Google Scholar] [CrossRef]
- Kang, M.-S.; Nkurunziza, P.; Muwanika, R.; Qian, G.; Tang, L.; Song, X.; Xue, K.; Nkwata, A.; Ssempebwa, J.; Lutalo, T.; et al. Longitudinal evaluation of aflatoxin exposure in two cohorts in south-western Uganda. Food Addit. Contam. Part A 2015, 32, 1322–1330. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: Boston, MA, USA, 2009; p. 520. [Google Scholar]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [Green Version]
- Squire, R. Ranking animal carcinogens: A proposed regulatory approach. Science 1981, 214, 877–880. [Google Scholar] [CrossRef]
- Prachayawarakorn, S.; Soponronnarit, S.; Wetchacama, S.; Chinnabun, K. Methodology for enhancing drying rate and improving maize quality in a fluidised-bed dryer. J. Stored Prod. Res. 2004, 40, 379–393. [Google Scholar] [CrossRef]
- FAO. Crop Calendar—An Information Tool for Seed Security. Available online: www.fao.org/agriculture/seed/cropcalendar/welcome.do (accessed on 29 October 2019).
- Wamatsembe, I.M.; Asea, G.; Haefele, S.M. A Survey: Potential Impact of Genetically Modified Maize Tolerant to Drought or Resistant to Stem Borers in Uganda. Agronomy 2017, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Mühlbauer, W.; Müller, J. Maize (Zea mays L.). In Drying Atlas; Mühlbauer, W., Müller, J., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 75–84. [Google Scholar]
- Kaminski, J.; Christiaensen, L. Post-harvest loss in sub-Saharan Africa—What do farmers say? Glob. Food Secur. 2014, 3, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Fudholi, A.; Sopian, K.; Ruslan, M.; Alghoul, M.; Sulaiman, M. Review of solar dryers for agricultural and marine products. Renew. Sustain. Energy Rev. 2010, 14, 1–30. [Google Scholar] [CrossRef]
- Udomkun, P.; Romuli, S.; Schock, S.; Mahayothee, B.; Sartas, M.; Wossen, T.; Njukwe, E.; Vanlauwe, B.; Müller, J. Review of solar dryers for agricultural products in Asia and Africa: An innovation landscape approach. J. Environ. Manag. 2020, 268, 110730. [Google Scholar] [CrossRef] [PubMed]
- Lutz, K.; Mühlbauer, W.; Müller, J.; Reisinger, G. Development of a multi-purpose solar crop dryer for arid zones. Sol. Wind Technol. 1987, 4, 417–424. [Google Scholar] [CrossRef]
- Lutz, K.; Mühlbauer, W. Solar tunnel dryer with integrated collector. Dry. Technol. 1986, 4, 583–603. [Google Scholar] [CrossRef]
- Djokoto, I.K.; Maurer, R.; Mühlbauer, W. A solar tunnel dryer for drying paddy. Agric. Mech. Asia Afr. Lat. Am. 1989, 20, 41–43. [Google Scholar]
- Salvatierra-Rojas, A.; Nagle, M.; Gummert, M.; de Bruin, T.; Müller, J. Development of an inflatable solar dryer for improved postharvest handling of paddy rice in humid climates. Int. J. Agric. Biol. Eng. 2017, 10, 269–282. [Google Scholar] [CrossRef]
- Romuli, S.; Schock, S.; Somda, M.K.; Müller, J. Drying Performance and Aflatoxin Content of Paddy Rice Applying an Inflatable Solar Dryer in Burkina Faso. Appl. Sci. 2020, 10, 3533. [Google Scholar] [CrossRef]
- Asemu, A.M.; Habtu, N.G.; Delele, M.A.; Subramanyam, B.; Alavi, S. Drying characteristics of maize grain in solar bubble dryer. J. Food Process. Eng. 2019, 43, e13312. [Google Scholar] [CrossRef]
- Mühlbauer, W. Handbuch der Getreidetrocknung: Grundlagen und Verfahren; Agrimedia: Clenze, Germany, 2009; p. 523. [Google Scholar]
- International Organization for Standardization. Microbiology of Food and Animal Feeding Stuffs—General Requirements and Guidance for Microbiological Examinations; EN ISO 7218; International Organization for Standardization: Geneva, Switzerland, 2007; Volume 66. [Google Scholar]
- International Organization for Standardization. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeast and Moulds; ISO 21527-2; International Organization for Standardization: Geneva, Switzerland, 2008; pp. 1–9. [Google Scholar]
- Argyropoulos, D.; Müller, J. Kinetics of change in colour and rosmarinic acid equivalents during convective drying of lemon balm (Melissa officinalis L.). J. Appl. Res. Med. Aromat. Plants 2014, 1, e15–e22. [Google Scholar] [CrossRef]
- Sanghi, A.; Ambrose, R.P.K.; Maier, D. CFD simulation of corn drying in a natural convection solar dryer. Dry. Technol. 2017, 36, 859–870. [Google Scholar] [CrossRef]
- Floyd, C.D.; Rooney, L.W.W.; Bockholt, A.J.; Bockholt, A.J. Measuring Desirable and Undesirable Color in White and Yellow Food Corn. Cereal Chem. 1995, 72, 488–490. [Google Scholar]
- Gunasekaran, S.; Paulsen, M.R. Breakage Resistance of Corn as a Function of Drying Rates. Trans. ASAE 1985, 28, 2071–2076. [Google Scholar] [CrossRef]
- Sacilik, K.; Keskin, R.; Elicin, A.K. Mathematical modelling of solar tunnel drying of thin layer organic tomato. J. Food Eng. 2006, 73, 231–238. [Google Scholar] [CrossRef]
- Müller, J.; Mühlbauer, W. Solar Drying. In Modern Drying Technology, 1st ed.; Tsotsas, E., Mujumdar, A.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 199–243. [Google Scholar]
- Weinberg, Z.; Yan, Y.; Chen, Y.; Finkelman, S.; Ashbell, G.; Navarro, S. The effect of moisture level on high-moisture maize (Zea mays L.) under hermetic storage conditions—In vitro studies. J. Stored Prod. Res. 2008, 44, 136–144. [Google Scholar] [CrossRef]
- Schemminger, J.; Mbuge, D.; Hofacker, W. Ambient air cereal grain drying—Simulation of the thermodynamic and microbial behavior. Therm. Sci. Eng. Prog. 2019, 13, 100382. [Google Scholar] [CrossRef]
- Raters, M.; Matissek, R. Thermal stability of aflatoxin B1 and ochratoxin A. Mycotoxin Res. 2008, 24, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Osuret, J.; Musinguzi, G.; Mukama, T.; Halage, A.A.; Nati, A.K.; Ssempebwa, J.C.; Wang, J.-S. Aflatoxin Contamination of Selected Staple Foods Sold for Human Consumption in Kampala Markets, Uganda. J. Biol. Sci. 2015, 16, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Rustom, I.Y. Aflatoxin in food and feed: Occurrence, legislation and inactivation by physical methods. Food Chem. 1997, 59, 57–67. [Google Scholar] [CrossRef]
- Battilani, P.; Leggieri, M.C.; Rossi, V.; Giorni, P. AFLA-maize, a mechanistic model for Aspergillus flavus infection and aflatoxin B1 contamination in maize. Comput. Electron. Agric. 2013, 94, 38–46. [Google Scholar] [CrossRef]


| Batch 1 | Batch 2 | Batch 3 | |
|---|---|---|---|
| MCw.b% ± SD | MCw.b% ± SD | MCw.b% ± SD | |
| ISD | 11.9 ± 0.4 a | 13.2 ± 0.3 a | 12.5 ± 0.5 a |
| DSD | 11.9 ± 0.1 a | 12.3 ± 0.3 a | 11.0 ± 0.0 b |
| L* | a* | b* | C* | h° | ∆E* | |
|---|---|---|---|---|---|---|
| Fresh | 63.4 ± 0.5 a | 1.9 ± 0.3 a | 15.3 ± 1.3 a | 15.4 ± 1.3 a | 1.5 ± 0.0 a | - |
| ISD | 61.6 ± 2.4 a | 2.2 ± 0.1 a | 15.5 ± 0.6 a | 15.7 ± 0.6 a | 1.4 ± 0.0 a | 2.8 ± 1.73 a |
| DSD | 66.0 ± 5.3 a | 2.0 ± 0.2 a | 16.1 ± 2.3 a | 16.1 ± 2.3 a | 1.4 ± 0.0 a | 5.1 ± 3.0 a |
| LSD | 6.59 | 0.48 | 2.54 | 2.55 | 0.023 | 11.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntwali, J.; Schock, S.; Romuli, S.; Chege, C.G.K.; Banadda, N.; Aseru, G.; Müller, J. Performance Evaluation of an Inflatable Solar Dryer for Maize and the Effect on Product Quality Compared with Direct Sun Drying. Appl. Sci. 2021, 11, 7074. https://doi.org/10.3390/app11157074
Ntwali J, Schock S, Romuli S, Chege CGK, Banadda N, Aseru G, Müller J. Performance Evaluation of an Inflatable Solar Dryer for Maize and the Effect on Product Quality Compared with Direct Sun Drying. Applied Sciences. 2021; 11(15):7074. https://doi.org/10.3390/app11157074
Chicago/Turabian StyleNtwali, Janvier, Steffen Schock, Sebastian Romuli, Christine G. Kiria Chege, Noble Banadda, Gloria Aseru, and Joachim Müller. 2021. "Performance Evaluation of an Inflatable Solar Dryer for Maize and the Effect on Product Quality Compared with Direct Sun Drying" Applied Sciences 11, no. 15: 7074. https://doi.org/10.3390/app11157074